Nuclear Lamins: Key Proteins for Embryonic Development
Abstract
:Simple Summary
Abstract
1. Introduction
2. Structure of Lamins
3. The Consequence of Lamin Genes Disruption
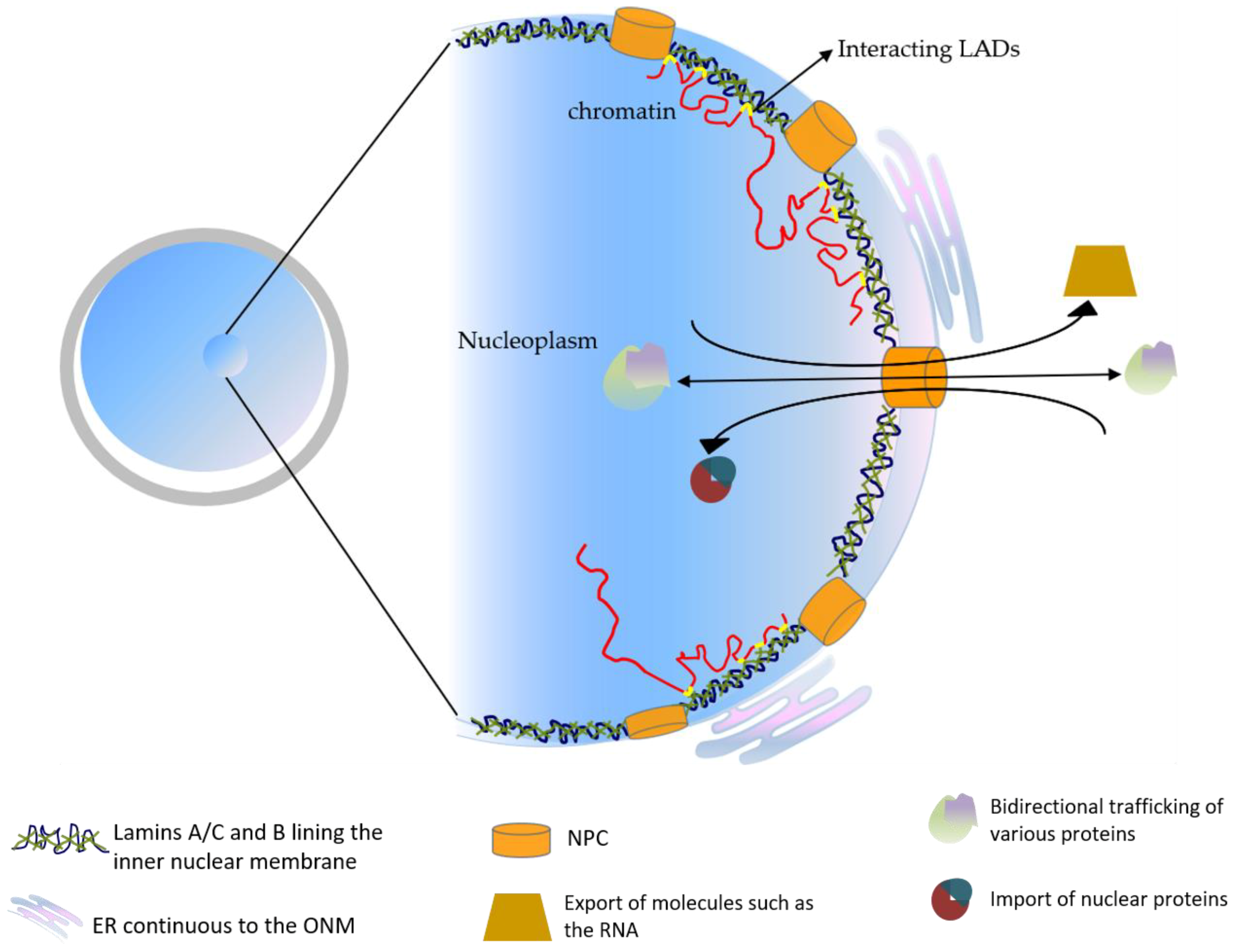
4. The Cellular Functions of the Lamins in Development
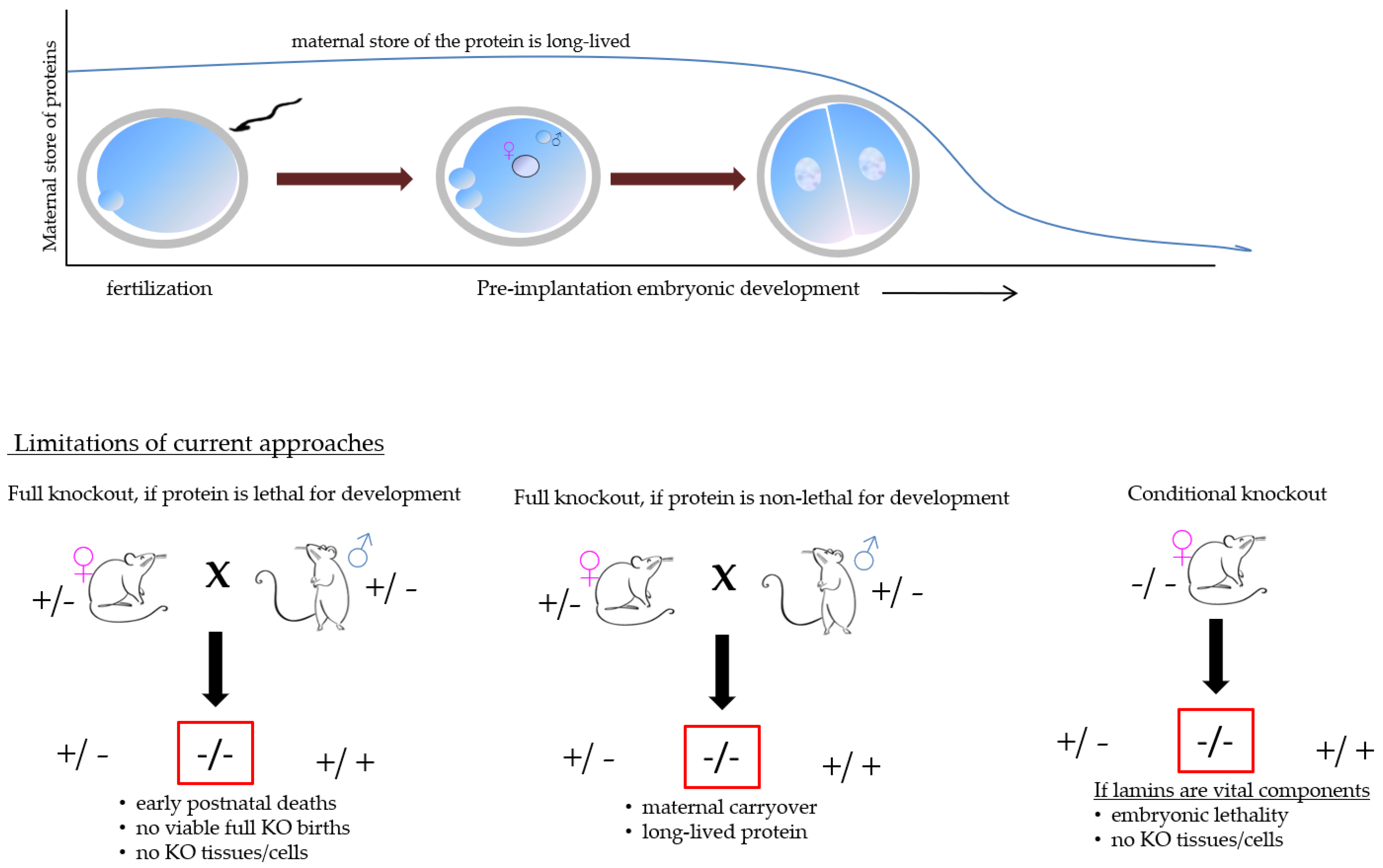
5. Lamin Function in Early Mammalian Embryos
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jorgensen, P.; Edgington, N.P.; Schneider, B.L.; Rupeš, I.; Tyers, M.; Futcher, B. The Size of the Nucleus Increases as Yeast Cells Grow. Mol. Biol. Cell 2007, 18, 3523–3532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillooly, J.F.; Hein, A.; Damiani, R. Nuclear DNA Content Varies with Cell Size across Human Cell Types. Cold Spring Harb. Perspect. Biol. 2015, 7, a019091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sexton, T.; Schober, H.; Fraser, P.; Gasser, S. Gene regulation through nuclear organization. Nat. Struct. Mol. Biol. 2007, 14, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jiménez, N.; Pradillo, M. The role of the nuclear envelope in the regulation of chromatin dynamics during cell division. J. Exp. Bot. 2020, 71, 5148–5159. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Zhang, H.; Qin, P.; Deng, L. Nuclear envelope integrity, DNA replication, damage repair and genome stability. Genome Instab. Dis. 2021, 2, 102–114. [Google Scholar] [CrossRef]
- Rowat, A.C.; Jaalouk, D.E.; Zwerger, M.; Ung, W.L.; Eydelnant, I.A.; Olins, D.E.; Olins, A.L.; Herrmann, H.; Weitz, D.A.; Lammerding, J. Nuclear Envelope Composition Determines the Ability of Neutrophil-type Cells to Passage through Micron-scale Constrictions. J. Biol. Chem. 2013, 288, 8610–8618. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, M.A.; Gomez-Cavazos, J.S.; Mei, A.; Lackner, D.H.; Hetzer, M.W. A Change in Nuclear Pore Complex Composition Regulates Cell Differentiation. Dev. Cell 2012, 22, 446–458. [Google Scholar] [CrossRef] [Green Version]
- De Las Heras, J.I.; Meinke, P.; Batrakou, D.G.; Srsen, V.; Zuleger, N.; Kerr, A.R.; Schirmer, E.C. Tissue specificity in the nuclear envelope supports its functional complexity. Nucleus 2013, 4, 460–477. [Google Scholar] [CrossRef] [Green Version]
- Watson, M.L. The nuclear envelope: Its structure and relation to cytoplasmic membranes. J. Biophys. Biochem. Cytol. 1955, 1, 257. [Google Scholar] [CrossRef] [Green Version]
- Dingwall, C.; Laskey, R. The nuclear membrane. Science 1992, 258, 942–947. [Google Scholar] [CrossRef]
- Fisher, D.Z.; Chaudhary, N.; Blobel, G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc. Natl. Acad. Sci. USA 1986, 83, 6450–6454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aebi, U.; Cohn, J.; Buhle, L.; Gerace, L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature 1986, 323, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Butin-Israeli, V.; Adam, S.A.; Goldman, A.E.; Goldman, R.D. Nuclear lamin functions and disease. Trends Genet. 2012, 28, 464–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rankin, J.; Ellard, S. The laminopathies: A clinical review. Clin. Genet. 2006, 70, 261–274. [Google Scholar] [CrossRef]
- Vigouroux, C.; Bonne, G. Laminopathies: One gene, two proteins, five diseases. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Kind, J.; van Steensel, B. Genome–nuclear lamina interactions and gene regulation. Curr. Opin. Cell Biol. 2010, 22, 320–325. [Google Scholar] [CrossRef]
- Puckelwartz, M.J.; Depreux, F.F.; McNally, E.M. Gene expression, chromosome position and lamin A/C mutations. Nucleus 2011, 2, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Constantinescu, D.; Gray, H.L.; Sammak, P.J.; Schatten, G.P.; Csoka, A.B. Lamin A/C Expression Is a Marker of Mouse and Human Embryonic Stem Cell Differentiation. Stem Cells 2006, 24, 177–185. [Google Scholar] [CrossRef]
- Liu, S.; Pellman, D. The coordination of nuclear envelope assembly and chromosome segregation in metazoans. Nucleus 2020, 11, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Kuga, T.; Nie, H.; Kazami, T.; Satoh, M.; Matsushita, K.; Nomura, F.; Maeshima, K.; Nakayama, Y.; Tomonaga, T. Lamin B2 prevents chromosome instability by ensuring proper mitotic chromosome segregation. Oncogenesis 2014, 3, e94. [Google Scholar] [CrossRef] [Green Version]
- Dittmer, T.A.; Misteli, T. The lamin protein family. Genome Biol. 2011, 12, 222. [Google Scholar] [CrossRef] [Green Version]
- Ciska, M.; De La Espina, S.M.D. The intriguing plant nuclear lamina. Front. Plant Sci. 2014, 5, 166. [Google Scholar] [CrossRef] [Green Version]
- Ciska, M.; Hikida, R.; Masuda, K.; De La Espina, S.M.D. Evolutionary history and structure of nuclear matrix constituent proteins, the plant analogues of lamins. J. Exp. Bot. 2019, 70, 2651–2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machiels, B.M.; Zorenc, A.H.; Endert, J.M.; Kuijpers, H.J.; van Eys, G.J.; Ramaekers, F.C.; Broers, J.L. An Alternative Splicing Product of the Lamin A/C Gene Lacks Exon 10. J. Biol. Chem. 1996, 271, 9249–9253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, K.; Inagaki, H.; Hotta, Y. Identification and Cloning of an mRNA Coding for a Germ Cell-Specific A-Type Lamin in Mice. Exp. Cell Res. 1994, 212, 426–430. [Google Scholar] [CrossRef]
- Koncicka, M.; Cervenka, J.; Jahn, D.; Sucha, R.; Vodicka, P.; Gad, A.; Alsheimer, M.; Susor, A. Expression of lamin C2 in mammalian oocytes. PLoS ONE 2020, 15, e0229781. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Hotta, Y. cDNA cloning of a germ cell specific lamin B3 from mouse spermatocytes and analysis of its function by ectopic expression in somatic cells. EMBO J. 1993, 12, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Stick, R. The gene structure ofXenopus nuclear lamin A: A model for the evolution of A-type from B-type lamins by exon shuffling. Chromosoma 1992, 101, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zheng, X.; Zheng, Y. Proliferation and differentiation of mouse embryonic stem cells lacking all lamins. Cell Res. 2013, 23, 1420–1423. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, N.; Blobel, G. A modified procedure for the isolation of a pore complex-lamina fraction from rat liver nuclei. J. Cell Biol. 1976, 70, 581–591. [Google Scholar] [CrossRef]
- Riemer, D.; Dodemont, H.; Weber, K. A nuclear lamin of the nematode Caenorhabditis elegans with unusual structural features; cDNA cloning and gene organization. Eur. J. Cell Biol. 1993, 62, 214–223. [Google Scholar]
- Ben-Harush, K.; Wiesel, N.; Frenkiel-Krispin, D.; Moeller, D.; Soreq, E.; Aebi, U.; Herrmann, H.; Gruenbaum, Y.; Medalia, O. The supramolecular organization of the C. elegans nuclear lamin filament. J. Mol. Biol. 2009, 386, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.W.; Huttenlauch, I.; Hutchison, C.J.; Stick, R. Filaments made from A- and B-type lamins differ in structure and organization. J. Cell Sci. 2008, 121, 215–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turgay, Y.; Eibauer, M.; Goldman, A.E.; Shimi, T.; Khayat, M.; Ben-Harush, K.; Dubrovsky-Gaupp, A.; Sapra, K.T.; Goldman, R.D.; Medalia, O. The molecular architecture of lamins in somatic cells. Nature 2017, 543, 261–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuurman, N.; Heins, S.; Aebi, U. Nuclear Lamins: Their Structure, Assembly, and Interactions. J. Struct. Biol. 1998, 122, 42–66. [Google Scholar] [CrossRef]
- Kitten, G.T.; Nigg, E. The CaaX motif is required for isoprenylation, carboxyl methylation, and nuclear membrane association of lamin B2. J. Cell Biol. 1991, 113, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Adam, S.A.; Sengupta, K.; Goldman, R.D. Regulation of Nuclear Lamin Polymerization by Importin α. J. Biol. Chem. 2008, 283, 8462–8468. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Okumura, N.; Kose, S.; Takao, T.; Imamoto, N. Identification of Cargo Proteins Specific for Importin-β with Importin-α Applying a Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC)-based in vitro Transport System. J. Biol. Chem. 2013, 288, 24540–24549. [Google Scholar] [CrossRef] [Green Version]
- Verstraeten, V.L.R.M.; Broers, J.L.V.; Ramaekers, F.C.S.; Steensel, M.A.M.V. The nuclear envelope, a key structure in cellular integrity and gene expression. Curr. Med. Chem. 2007, 14, 1231–1248. [Google Scholar] [CrossRef]
- AKaminski, A.; Fedorchak, G.R.; Lammerding, J. The cellular mastermind (?)—Mechanotransduction and the nucleus. Prog. Mol. Biol. Transl. Sci. 2014, 126, 157–203. [Google Scholar]
- Jimenez-Escrig, A.; Gobernado, I.; Garcia-Villanueva, M.; Antonio Sanchez-Herranz, B.S. Autosomal recessive Emery-Dreifuss muscular dystrophy caused by a novel mutation (R225Q) in the lamin A/C gene identified by exome sequencing. Muscle Nerve 2011, 45, 605–610. [Google Scholar] [CrossRef]
- Sullivan, T.; Escalante-Alcalde, D.; Bhatt, H.; Anver, M.; Bhat, N.; Nagashima, K.; Stewart, C.L.; Burke, B. Loss of A-Type Lamin Expression Compromises Nuclear Envelope Integrity Leading to Muscular Dystrophy. J. Cell Biol. 1999, 147, 913–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muchir, A.; Bonne, G.; van der Kooi, A.J.; van Meegen, M.; Baas, F.; Bolhuis, P.A.; de Visser, M.; Schwartz, K. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum. Mol. Genet. 2000, 9, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Muchir, A.; Shan, J.; Bonne, G.; Worman, H.J. Mitogen-Activated Protein Kinase Inhibitors Improve Heart Function and Prevent Fibrosis in Cardiomyopathy Caused by Mutation in Lamin A/C Gene. Circulation 2011, 123, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Bione, S.; Maestrini, E.; Rivella, S.; Mancini, M.; Regis, S.; Romeo, G.; Toniolo, D. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat. Genet. 1994, 8, 323–327. [Google Scholar] [CrossRef]
- Bonne, G.; Di Barletta, M.R.; Varnous, S.; Bécane, H.-M.; Hammouda, E.-H.; Merlini, L.; Muntoni, F.; Greenberg, C.R.; Gary, F.; Urtizberea, J.-A. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Gen. 1999, 21, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; Fryns, J.-P.; Auchus, R.J.; Garg, A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum. Mol. Genet. 2003, 12, 1995–2001. [Google Scholar] [CrossRef] [PubMed]
- Sahebalzamani, A.; Aryani, O. Mandibuloacral Dysplasia with Type A Lipodystrophy (MADA) in A 16 year-old Iranian Girl. SMU Med. J. 2016, 3, 13–20. [Google Scholar]
- Navarro, C.L.; Cadinanos, J.; Sandre-Giovannoli, A.D.; Bernard, R.; Courrier, S.; Boccaccio, I.; Boyer, A.; Kleijer, W.J.; Wagner, A.; Giuliano, F. Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of Lamin A precursors. Hum. Mol. Genet. 2005, 14, 1503–1513. [Google Scholar] [CrossRef]
- Moiseeva, O.; Lopes-Paciencia, S.; Huot, G.; Lessard, F.; Ferbeyre, G. Permanent farnesylation of lamin A mutants linked to progeria impairs its phosphorylation at serine 22 during interphase. Aging 2016, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, M.; Brown, W.T.; Gordon, L.B.; Glynn, M.W.; Singer, J.; Scott, L.; Erdos, M.R.; Robbins, C.M.; Moses, T.Y.; Berglund, P. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature 2003, 423, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.R.; Fain, P.R.; Sinagra, G.; Robinson, M.L.; Robertson, A.D.; Carniel, E.; Di Lenarda, A.; Bohlmeyer, T.J.; Ferguson, D.A.; Brodsky, G.L. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J. Am. Coll. Cardiol. 2003, 41, 771–780. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Rathee, V.; Krishnaswamy, R.; Bhattacharjee, P.; Ray, P.; Sood, A.K.; Sengupta, K. Viscoelastic Behavior of Human Lamin A Proteins in the Context of Dilated Cardiomyopathy. PLoS ONE 2013, 8, e83410. [Google Scholar] [CrossRef] [Green Version]
- Tazir, M.; Azzedine, H.; Assami, S.; Sindou, P.; Nouioua, S.; Zemmouri, R.; Hamadouche, T.; Chaouch, M.; Feingold, J.; Vallat, J.-M. Phenotypic variability in autosomal recessive axonal Charcot-Marie-Tooth disease due to the R298C mutation in lamin A/C. Brain 2004, 127, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Hegele, R.A. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum. Mol. Genet. 2000, 9, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, Y.; Li, J.; Bai, R.; Wang, J.; Peng, T.; Chen, L.; Wang, J.; Liu, Y.; Tian, T.; et al. LMNB1-Related Adult-Onset Autosomal Dominant Leukodystrophy Presenting as Movement Disorder: A Case Report and Review of the Literature. Front. Neurosci. 2019, 13, 1030. [Google Scholar] [CrossRef] [Green Version]
- Parry, D.A.; Martin, C.-A.; Greene, P.; Marsh, J.A.; Blyth, M.; Cox, H.; Donnelly, D.; Greenhalgh, L.; Greville-Heygate, S.; Harrison, V. Heterozygous lamin B1 and lamin B2 variants cause primary microcephaly and define a novel laminopathy. Genet. Med. 2020, 23, 408–414. [Google Scholar] [CrossRef]
- Damiano, J.A.; Afawi, Z.; Bahlo, M.; Mauermann, M.; Misk, A.; Arsov, T.; Oliver, K.L.; Dahl, H.-H.M.; Shearer, A.E.; Smith, R.J. Mutation of the nuclear lamin geneLMNB2in progressive myoclonus epilepsy with early ataxia. Hum. Mol. Genet. 2015, 24, 4483–4490. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Li, Y.; Fu, X.; Luo, X. A Chinese patient with acquired partial lipodystrophy caused by a novel mutation with LMNB2 gene. J. Pediatr. Endocrinol. Metab. 2012, 25, 375–377. [Google Scholar] [CrossRef]
- Röber, R.A.; Weber, K.; Osborn, M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: A developmental study. Development 1989, 105, 365–378. [Google Scholar] [CrossRef]
- Stewart, C.; Burke, B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell 1987, 51, 383–392. [Google Scholar] [CrossRef]
- Harborth, J.; Elbashir, S.M.; Bechert, K.; Tuschl, T.; Weber, K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J. Cell Sci. 2001, 114, 4557–4565. [Google Scholar] [CrossRef]
- Vergnes, L.; Peterfy, M.; Bergo, M.O.; Young, S.; Reue, K. Lamin B1 is required for mouse development and nuclear integrity. Proc. Natl. Acad. Sci. USA 2004, 101, 10428–10433. [Google Scholar] [CrossRef] [Green Version]
- Coffinier, C.; Chang, S.Y.; Nobumori, C.; Tu, Y.; Farber, E.A.; Toth, J.I.; Fong, L.G.; Young, S.G. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc. Natl. Acad. Sci. USA 2010, 107, 5076–5081. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.H.; Chang, S.Y.; Yin, L.; Tu, Y.; Hu, Y.; Yoshinaga, Y.; de Jong, P.J.; Fong, L.G.; Young, S.G. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum. Mol. Genet. 2011, 20, 3537–3544. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Sharov, A.A.; McDole, K.; Cheng, M.; Hao, H.; Fan, C.-M.; Gaiano, N.; Ko, M.S.; Zheng, Y. Mouse B-Type Lamins Are Required for Proper Organogenesis But Not by Embryonic Stem Cells. Science 2011, 334, 1706–1710. [Google Scholar] [CrossRef] [Green Version]
- Lourim, D.; Krohne, G. Membrane-associated lamins in Xenopus egg extracts: Identification of two vesicle populations. J. Cell Biol. 1993, 123, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Benavente, R.; Krohne, G.; Franke, W.W. Cell type-specific expression of nuclear lamina proteins during development of Xenopus laevis. Cell 1985, 41, 177–190. [Google Scholar] [CrossRef]
- Newport, J.W.; Wilson, K.L.; Dunphy, W.G. A lamin-independent pathway for nuclear envelope assembly. J. Cell Biol. 1990, 111, 2247–2259. [Google Scholar] [CrossRef] [Green Version]
- Meier, J.; Campbell, K.; Ford, C.; Stick, R.; Hutchison, C. The role of lamin LIII in nuclear assembly and DNA replication, in cell-free extracts of Xenopus eggs. J. Cell Sci. 1991, 98, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-Y.; Wang, S.; Heidinger, J.M.; Shumaker, D.K.; Adam, S.A.; Goldman, R.D.; Zheng, Y. A Mitotic Lamin B Matrix Induced by RanGTP Required for Spindle Assembly. Science 2006, 311, 1887–1893. [Google Scholar] [CrossRef] [Green Version]
- Pochukalina, G.N.; Ilicheva, N.V.; Podgornaya, O.I.; Voronin, A.P. Nucleolus-like body of mouse oocytes contains lamin A and B and TRF2 but not actin and topo II. Mol. Cytogenet. 2016, 9, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schatten, G.; Maul, G.G.; Schatten, H.; Chaly, N.; Simerly, C.; Balczon, R.; Brown, D.L. Nuclear lamins and peripheral nuclear antigens during fertilization and embryogenesis in mice and sea urchins. Proc. Natl. Acad. Sci. USA 1985, 82, 4727–4731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prather, R.S.; Sims, M.M.; Maul, G.G.; First, N.L.; Schatten, G. Nuclear Lamin Antigens are Developmentally Regulated during Porcine and Bovine Embryogenesis1. Biol. Reprod. 1989, 41, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.; Cooney, M.A.; Shanahan, P.; Tecirlioglu, R.T.; Ruddock, N.T.; French, A.J. Nuclear lamin antigen and messenger RNA expression in bovine in vitro produced and nuclear transfer embryos. Mol. Reprod. Dev. 2005, 72, 471–482. [Google Scholar] [CrossRef]
- Tunnah, D.; Sewry, C.A.; Vaux, D.; Schirmer, E.C.; Morris, G.E. The apparent absence of lamin B1 and emerin in many tissue nuclei is due to epitope masking. J. Mol. Histol. 2005, 36, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Pfeiffer, M.J.; Drexler, H.C.A.; Fuellen, G.; Boiani, M. Proteomic Analysis of Mouse Oocytes Identifies PRMT7 as a Reprogramming Factor that Replaces SOX2 in the Induction of Pluripotent Stem Cells. J. Proteome Res. 2016, 15, 2407–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israel, S.; Casser, E.; Drexler, H.C.; Fuellen, G.; Boiani, M. A framework for TRIM21-mediated protein depletion in early mouse embryos: Recapitulation of Tead4 null phenotype over three days. BMC Genom. 2019, 20, 755. [Google Scholar] [CrossRef] [Green Version]
- Ogushi, S.; Fulka, J.; Miyano, T. Germinal vesicle materials are requisite for male pronucleus formation but not for change in the activities of CDK1 and MAP kinase during maturation and fertilization of pig oocytes. Dev. Biol. 2005, 286, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Houliston, E.; Guilly, M.; Courvalin, J.; Maro, B. Expression of nuclear lamins during mouse preimplantation development. Development 1988, 102, 271–278. [Google Scholar] [CrossRef]
- Maul, G.G.; Schatten, G.; Jimenez, S.; Carrera, A.E. Detection of nuclear lamin B epitopes in oocyte nuclei from mice, sea urchins, and clams using a human autoimmune serum. Dev. Biol. 1987, 121, 368–375. [Google Scholar] [CrossRef]
- Prentice-Biensch, J.; Singh, J.; Alfoteisy, B.; Anzar, M. A simple and high-throughput method to assess maturation status of bovine oocytes: Comparison of anti-lamin A/C-DAPI with an aceto-orcein staining technique. Theriogenology 2012, 78, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.D.; Gruenbaum, Y.; Moir, R.D.; Shumaker, D.K.; Spann, T.P. Nuclear lamins: Building blocks of nuclear architecture. Genes Dev. 2002, 16, 533–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dechat, T.; Adam, S.A.; Taimen, P.; Shimi, T.; Goldman, R.D. Nuclear Lamins. Cold Spring Harb. Perspect. Biol. 2010, 2, a000547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennig, W. Heterochromatin. Chromosoma 1999, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ferrai, C.; de Castro, I.J.; Lavitas, L.; Chotalia, M.; Pombo, A. Gene Positioning. Cold Spring Harb. Perspect. Biol. 2010, 2, a000588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takizawa, T.; Meaburn, K.; Misteli, T. The Meaning of Gene Positioning. Cell 2008, 135, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Van Steensel, B.; Henikoff, S. Identification of in vivo DNA targets of chromatin proteins using tethered Dam methyltransferase. Nat. Biotechnol. 2000, 18, 424–428. [Google Scholar] [CrossRef]
- Barras, F.; Marinus, M. The great GATC: DNA methylation in E. coli. Trends Genet. 1989, 5, 139–143. [Google Scholar] [CrossRef]
- Meuleman, W.; Peric-Hupkes, D.; Kind, J.; Beaudry, J.-B.; Pagie, L.; Kellis, M.; Reinders, M.; Wessels, L.; van Steensel, B. Constitutive nuclear lamina–genome interactions are highly conserved and associated with A/T-rich sequence. Genome Res. 2012, 23, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Kind, J.; Van Steensel, B. Stochastic genome-nuclear lamina interactions. Nucleus 2014, 5, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Kind, J.; Pagie, L.; de Vries, S.S.; Nahidiazar, L.; Dey, S.S.; Bienko, M.; Zhan, Y.; Lajoie, B.; de Graaf, C.A.; Amendola, M.; et al. Genome-wide Maps of Nuclear Lamina Interactions in Single Human Cells. Cell 2015, 163, 134–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobecki, M.; Souaid, C.; Boulay, J.; Guerineau, V.; Noordermeer, D.; Crabbe, L. MadID, a Versatile Approach to Map Protein-DNA Interactions, Highlights Telomere-Nuclear Envelope Contact Sites in Human Cells. Cell Rep. 2018, 25, 2891–2903.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koziol, M.J.; Bradshaw, C.R.; Allen, G.E.; Costa, A.; Frezza, C.; Gurdon, J. Identification of methylated deoxyadenosines in vertebrates reveals diversity in DNA modifications. Nat. Struct. Mol. Biol. 2016, 23, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhu, Y.; Luo, G.-Z.; Wang, X.; Yue, Y.; Wang, X.; Zong, X.; Chen, K.; Yin, H.; Fu, Y.; et al. Abundant DNA 6mA methylation during early embryogenesis of zebrafish and pig. Nat. Commun. 2016, 7, 13052. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.P.; Wang, T.; Seetin, M.G.; Lai, Y.; Zhu, S.; Lin, K.; Liu, Y.; Byrum, S.D.; Mackintosh, S.G.; Zhong, M.; et al. DNA methylation on N6-adenine in mammalian embryonic stem cells. Nature 2016, 532, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kind, J.; Pagie, L.; Ortabozkoyun, H.; Boyle, S.; de Vries, S.S.; Janssen, H.; Amendola, M.; Nolen, L.D.; Bickmore, W.A.; van Steensel, B. Single-Cell Dynamics of Genome-Nuclear Lamina Interactions. Cell 2013, 153, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Noordermeer, D.; Leleu, M.; Schorderet, P.; Joye, E.; Chabaud, F.; Duboule, D. Temporal dynamics and developmental memory of 3D chromatin architecture at Hox gene loci. eLife 2014, 3, e02557. [Google Scholar] [CrossRef]
- Vieux-Rochas, M.; Fabre, P.; Leleu, M.; Duboule, D.; Noordermeer, D. Clustering of mammalian Hox genes with other H3K27me3 targets within an active nuclear domain. Proc. Natl. Acad. Sci. USA 2015, 112, 4672–4677. [Google Scholar] [CrossRef] [Green Version]
- Jachowicz, J.W.; Santenard, A.; Bender, A.; Muller, J.; Torres-Padilla, M.-E. Heterochromatin establishment at pericentromeres depends on nuclear position. Genes Dev. 2013, 27, 2427–2432. [Google Scholar] [CrossRef] [Green Version]
- Borsos, M.; Perricone, S.M.; Schauer, T.; Pontabry, J.; de Luca, K.L.; de Vries, S.S.; Ruiz-Morales, E.R.; Torres-Padilla, M.-E.; Kind, J. Genome–lamina interactions are established de novo in the early mouse embryo. Nature 2019, 569, 729–733. [Google Scholar] [CrossRef]
- Ward, W.S. Function of sperm chromatin structural elements in fertilization and development. Mol. Hum. Reprod. 2009, 16, 30–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLay, D.W.; Clarke, H.J. Remodelling the paternal chromatin at fertilization in mammals. Reproduction 2003, 125, 625. [Google Scholar] [CrossRef] [PubMed]
- Adenot, P.; Mercier, Y.; Renard, J.; Thompson, E. Differential H4 acetylation of paternal and maternal chromatin precedes DNA replication and differential transcriptional activity in pronuclei of 1-cell mouse embryos. Development 1997, 124, 4615–4625. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.; Torres-Padilla, M.-E. Chromatin dynamics in the regulation of cell fate allocation during early embryogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.; Torres-Padilla, M.-E. Epigenetic reprogramming and development: A unique heterochromatin organization in the preimplantation mouse embryo. Brief. Funct. Genom. 2010, 9, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Puschendorf, M.; Terranova, R.; Boutsma, E.; Mao, X.; Isono, K.; Brykczynska, U.; Kolb, C.; Otte, A.P.; Koseki, H.; Orkin, S.H.; et al. PRC1 and Suv39h specify parental asymmetry at constitutive heterochromatin in early mouse embryos. Nat. Genet. 2008, 40, 411–420. [Google Scholar] [CrossRef]
- Aguirre-Lavin, T.; Adenot, P.; Bonnet-Garnier, A.; Lehmann, G.; Fleurot, R.; Boulesteix, C.; Debey, P.; Beaujean, N. 3D-FISH analysis of embryonic nuclei in mouse highlights several abrupt changes of nuclear organization during preimplantation development. BMC Dev. Biol. 2012, 12, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solovei, I.; Wang, A.S.; Thanisch, K.; Schmidt, C.S.; Krebs, S.; Zwerger, M.; Cohen, T.V.; Devys, D.; Foisner, R.; Peichl, L.; et al. LBR and Lamin A/C Sequentially Tether Peripheral Heterochromatin and Inversely Regulate Differentiation. Cell 2013, 152, 584–598. [Google Scholar] [CrossRef] [Green Version]
- Harr, J.C.; Schmid, C.D.; Muñoz-Jiménez, C.; Romero-Bueno, R.; Kalck, V.; Gonzalez-Sandoval, A.; Hauer, M.H.; Padeken, J.; Askjaer, P.; Mattout, A. Loss of an H3K9me anchor rescues laminopathy-linked changes in nuclear organization and muscle function in an Emery-Dreifuss muscular dystrophy model. Genes Dev. 2020, 34, 560–579. [Google Scholar] [CrossRef] [Green Version]
- Cohen, T.V.; Gnocchi, V.F.; Cohen, J.E.; Phadke, A.; Liu, H.; Ellis, J.A.; Foisner, R.; Stewart, C.L.; Zammit, P.S.; Partridge, T.A. Defective skeletal muscle growth in lamin A/C-deficient mice is rescued by loss of Lap2α. Hum. Mol. Genet. 2013, 22, 2852–2869. [Google Scholar] [CrossRef] [Green Version]
| Disease | Lamin Mutations |
|---|---|
| Emery-Dreifuss muscular dystrophy (EDMD) | |
| Mandibuloacral dysplasia and partial lipodystrophy | Homozygous missense mutation, Arg527His, in LMNA gene. Muation in ZMPSTE24 [47] |
| Mandibuloacral dysplasia with type A lipodystrophy (MADA) | Homozygous mutation in R527H in the LMNA gene [48] |
| Restrictive dermopathy (RD) |
|
| Hutchinson- Gilford progeria syndrome (HGPS) | |
| Limb Girdle muscular dystrophy type 1B (LGMD1B) | Mutation linked to the chromosome 1q11-q21 of LMNA gene [43] |
| Dilated cardiomyopathy (DCM) | R89L, 959delT, R337H, S573L mutation in LMNA [52,53] |
| Autosomal recessive axonal Charcot-Marie-Tooth type 2 (CMT2) | R298C mutation in lamin A/C [54] |
| Dunnigan type familial partial lipodystrophy (FPLD) | R482Q mutation in lamin A/C, mutation in the gene mapped to chromosome 1q21-22 encoding for the LMNA gene [55] |
| Adult autosomal dominant leukodystrophy (movement disorder) | Associated with increase or accumulation of lamin B1 [56] |
| Primary microcephaly (neuro—developmental disorder) | Heterozygous dominant pathogenic variants in both lamin B1 and lamin B2 [57] |
| Progressive myoclonus epilepsy including the early identification of ataxia | Rare and novel homozygous missense p.His157Tyr mutation in the alpha- helical rod of the lamin B2 protein [58] |
| Acquired partial lipodystrophy (APL) | Mutation in the LMNB2 gene on 19p13.3 might be the cause of this disease [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, J.C.; Fulka, H. Nuclear Lamins: Key Proteins for Embryonic Development. Biology 2022, 11, 198. https://doi.org/10.3390/biology11020198
Paul JC, Fulka H. Nuclear Lamins: Key Proteins for Embryonic Development. Biology. 2022; 11(2):198. https://doi.org/10.3390/biology11020198
Chicago/Turabian StylePaul, Jasper Chrysolite, and Helena Fulka. 2022. "Nuclear Lamins: Key Proteins for Embryonic Development" Biology 11, no. 2: 198. https://doi.org/10.3390/biology11020198
APA StylePaul, J. C., & Fulka, H. (2022). Nuclear Lamins: Key Proteins for Embryonic Development. Biology, 11(2), 198. https://doi.org/10.3390/biology11020198






