Ability of the Right Ventricle to Serve as a Systemic Ventricle in Response to the Volume Overload at the Neonatal Stage
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Surgery
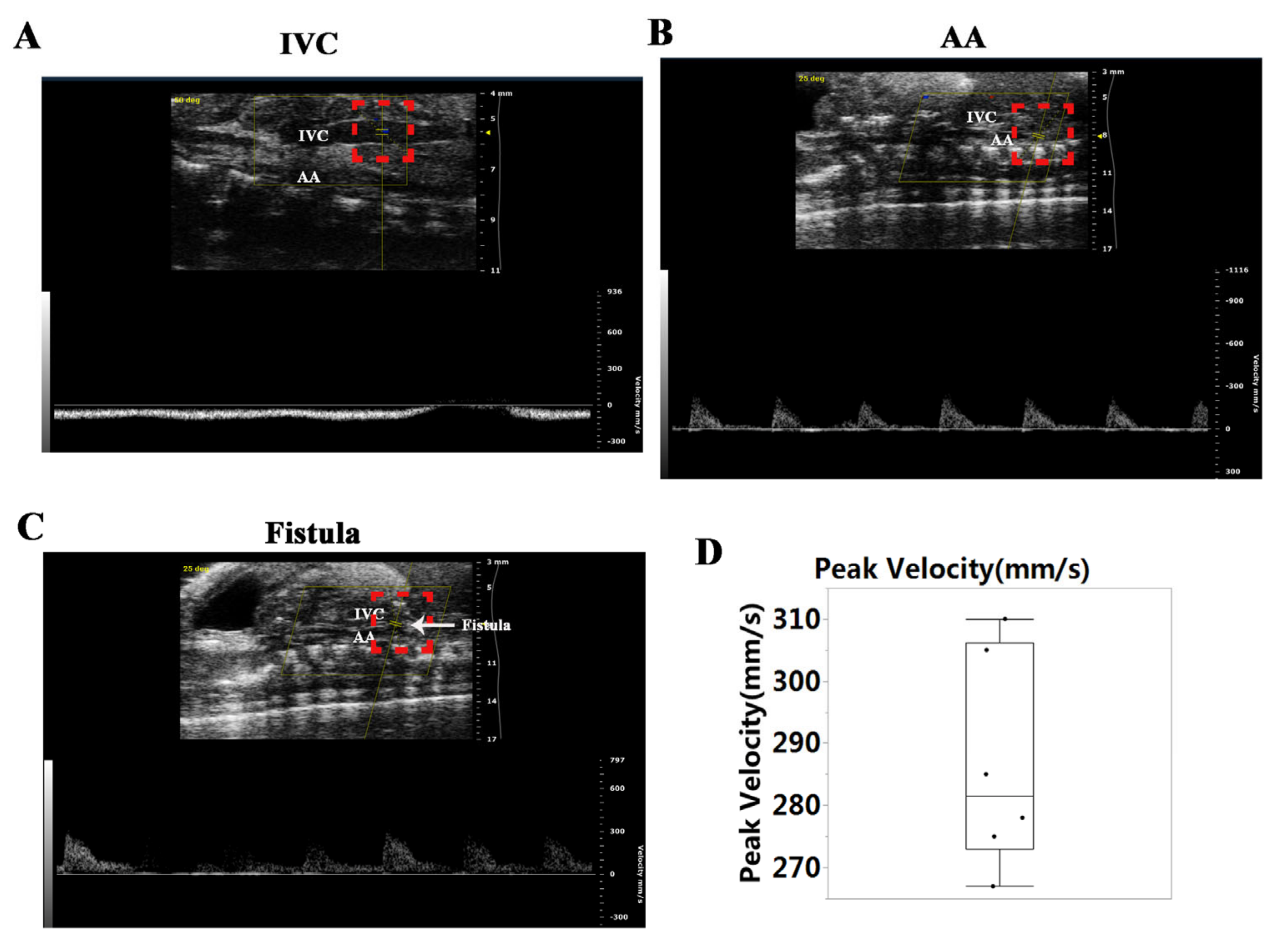
2.2. Abdominal Ultrasonography
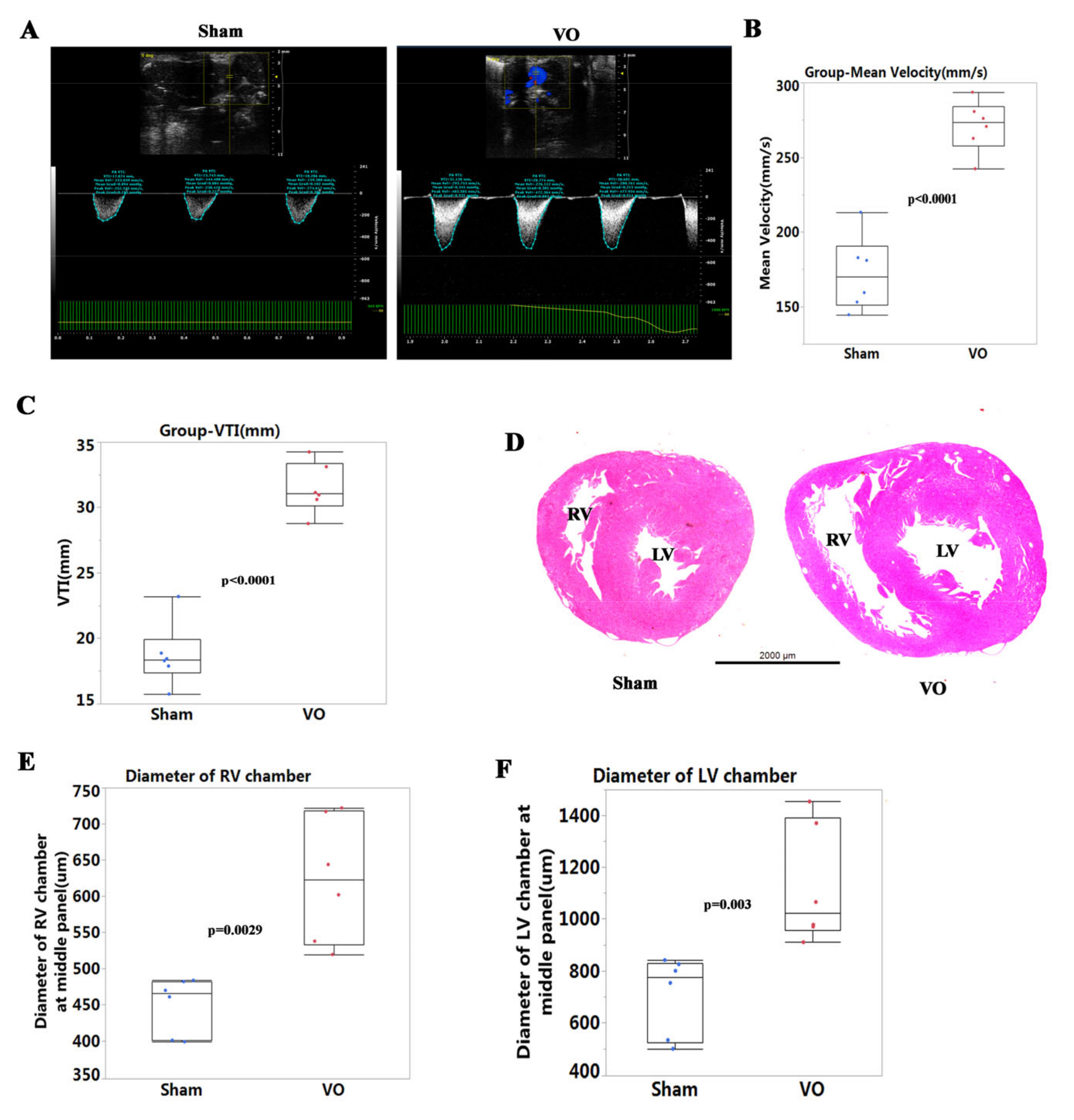
2.3. Echocardiography
2.4. Histopathological Analysis
2.5. Oil Red Stain
2.6. Western Blotting Analysis
2.7. Library Construction and Sequencing
2.8. Quality and Quantification of Gene Expression Levels
2.9. Analysis of Differentially Expressed Genes
2.10. Statistical Analysis
3. Results
3.1. Establishment of Ventricular VO via AVF Surgery
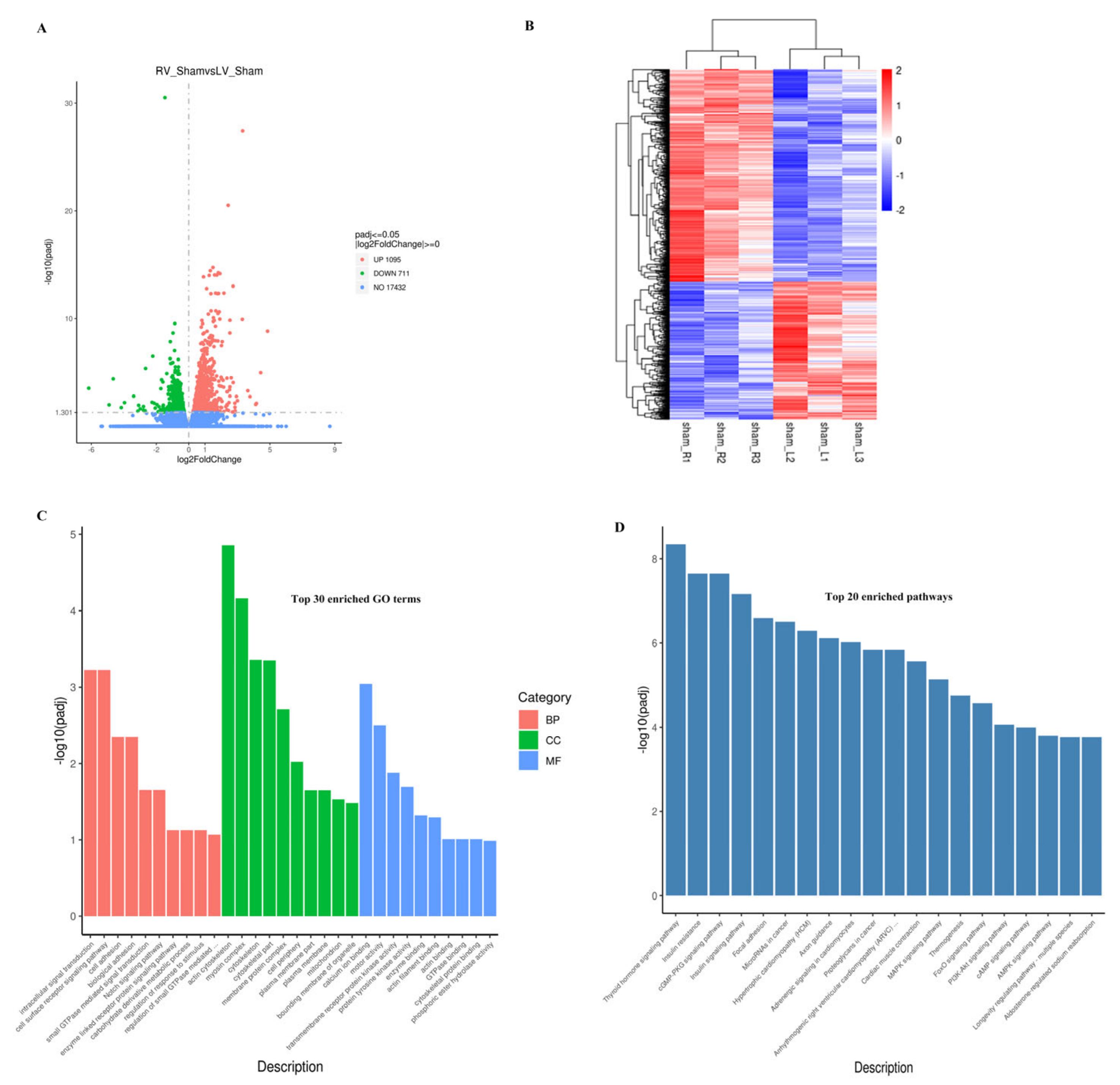
3.2. Molecular Differences between Neonatal LV and RV
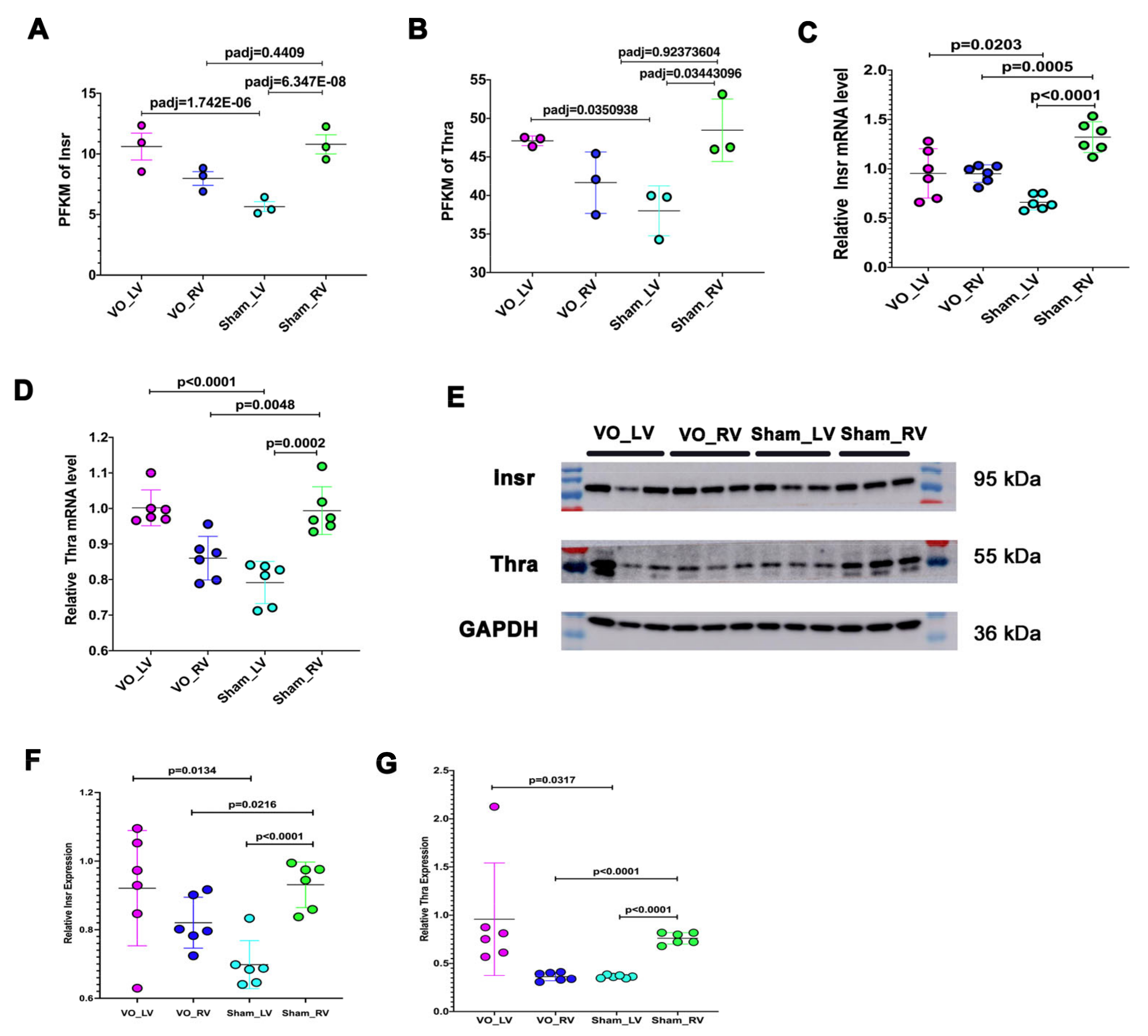
3.3. Neonatal LV Was more Transcriptionally Active at Baseline in Response to VO Than the RV
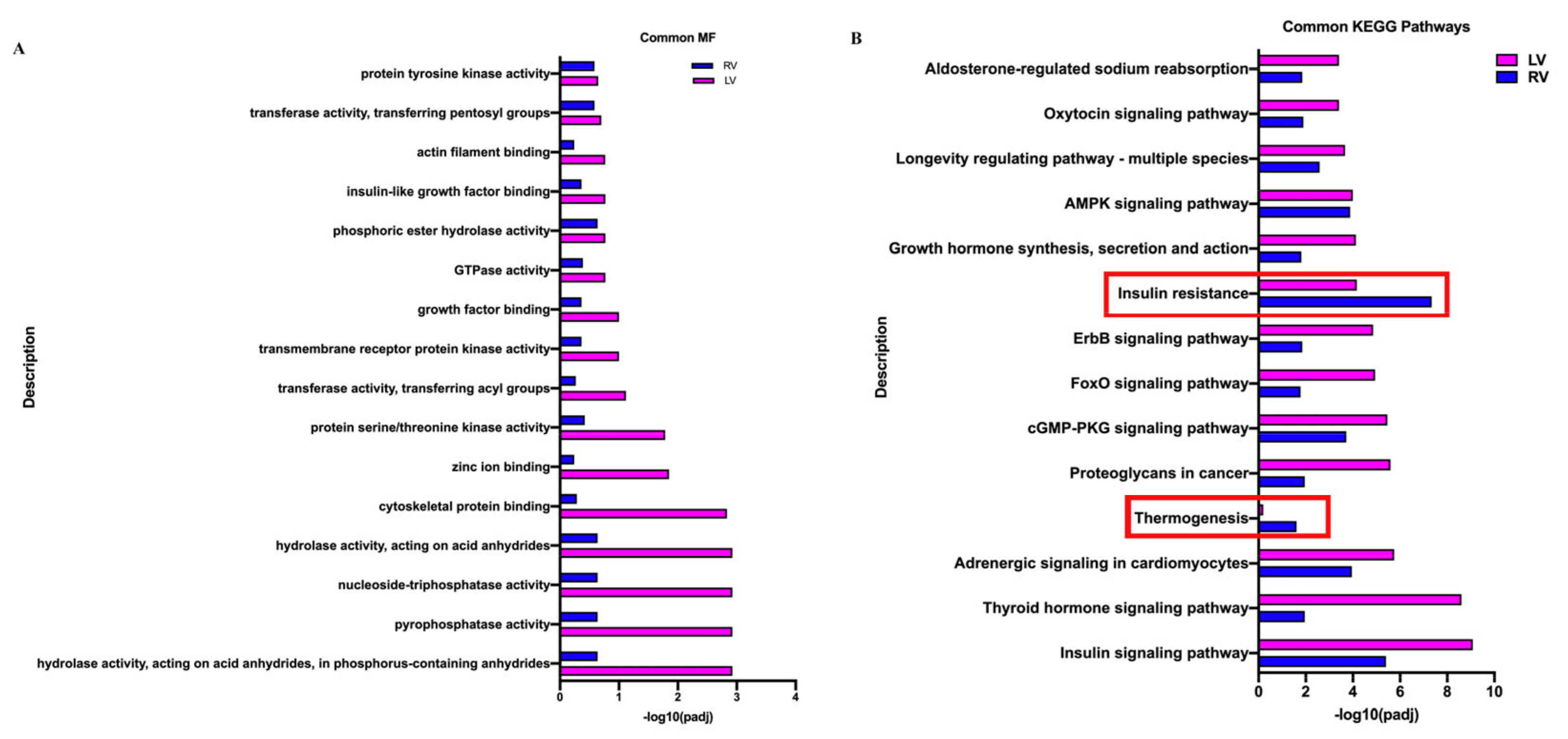
3.4. Common Responses between Neonatal LV and RV under the Influence of VO
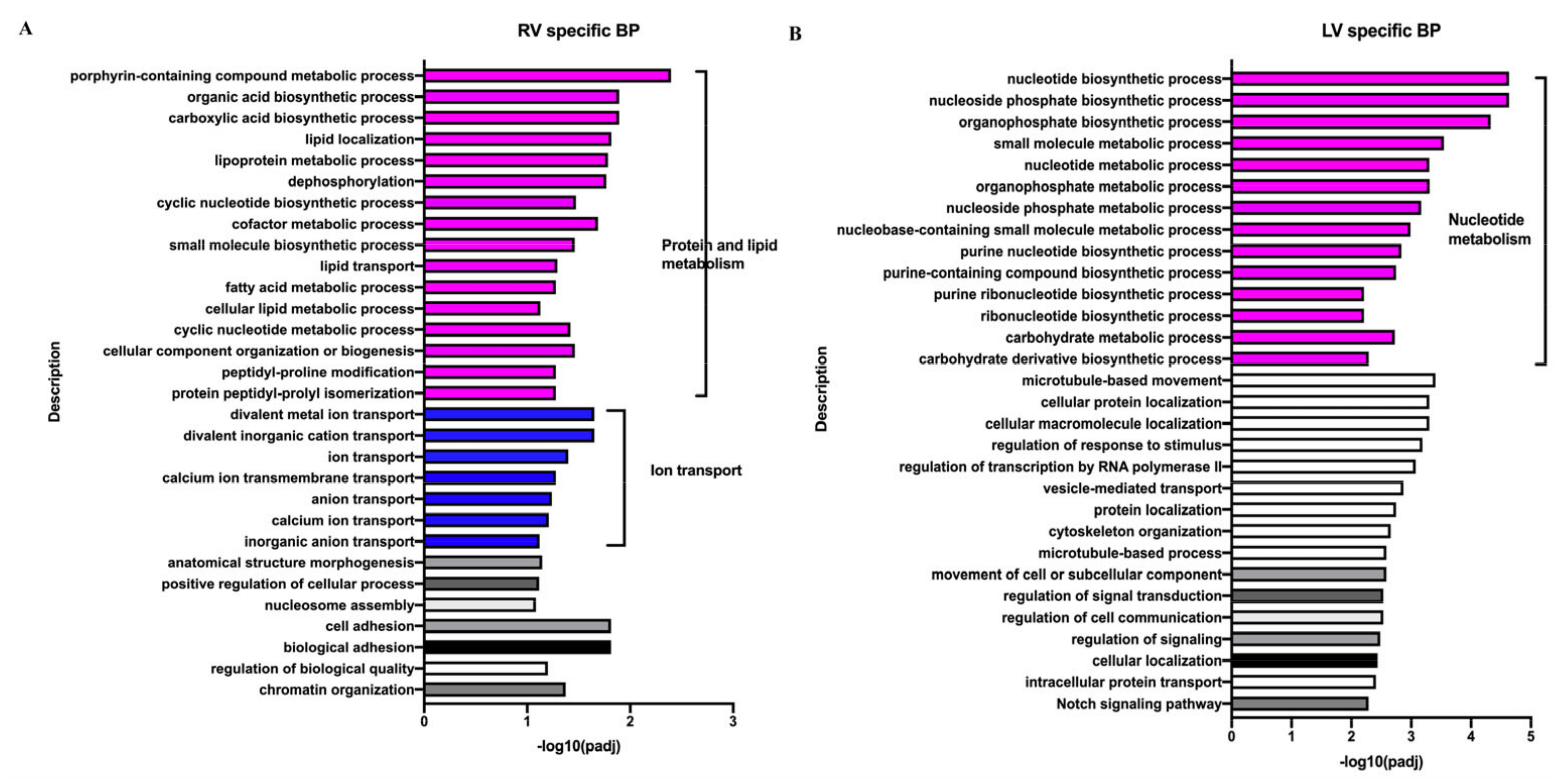
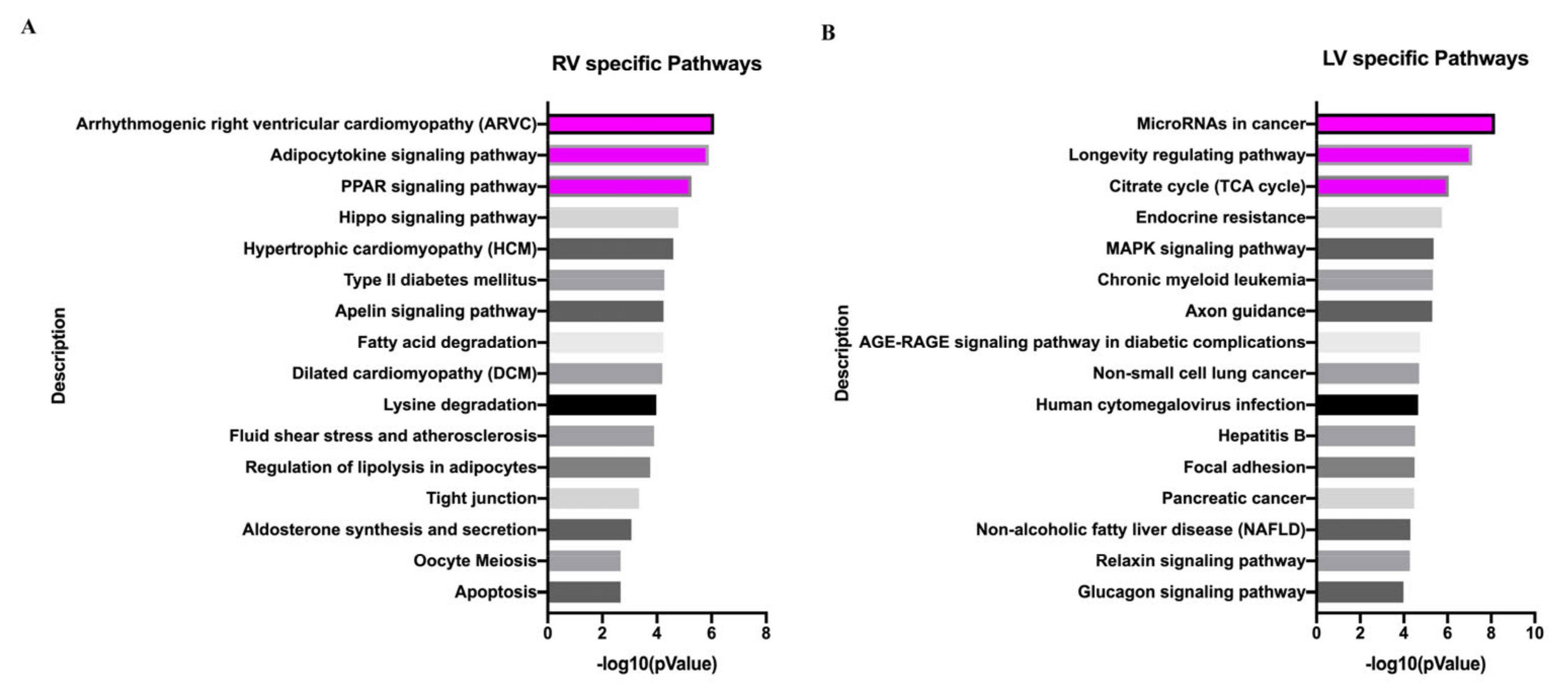
3.5. Different Responses of Neonatal LV and RV under the Influence of VO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feinstein, J.A.; Benson, D.W.; Dubin, A.M.; Cohen, M.S.; Maxey, D.M.; Mahle, W.T.; Pahl, E.; Villafañe, J.; Bhatt, A.B.; Peng, L.F.; et al. Hypoplastic left heart syndrome: Current considerations and expectations. J. Am. Coll. Cardiol. 2012, 59, S1–S42. [Google Scholar] [CrossRef] [PubMed]
- Ohye, R.G.; Schranz, D.; D’Udekem, Y. Current Therapy for Hypoplastic Left Heart Syndrome and Related Single Ventricle Lesions. Circulation 2016, 134, 1265–1279. [Google Scholar] [CrossRef] [PubMed]
- Saraf, A.; Book, W.M.; Nelson, T.J.; Xu, C. Hypoplastic left heart syndrome: From bedside to bench and back. J. Mol. Cell Cardiol. 2019, 135, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Brida, M.; Diller, G.P.; Gatzoulis, M.A. Systemic Right Ventricle in Adults with Congenital Heart Disease: Anatomic and Phenotypic Spectrum and Current Approach to Management. Circulation 2018, 137, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Khairy, P.; Fernandes, S.M.; Mayer, J.E., Jr.; Triedman, J.K.; Walsh, E.P.; Lock, J.E.; Landzberg, M.J. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008, 117, 85–92. [Google Scholar] [CrossRef]
- Dewan, S.; Krishnamurthy, A.; Kole, D.; Conca, G.; Kerckhoffs, R.; Puchalski, M.D.; Omens, J.H.; Sun, H.; Nigam, V.; McCulloch, A.D. Model of Human Fetal Growth in Hypoplastic Left Heart Syndrome: Reduced Ventricular Growth Due to Decreased Ventricular Filling and Altered Shape. Front. Pediatr. 2017, 5, 25. [Google Scholar] [CrossRef]
- Feit, L.R.; Copel, J.A.; Kleinman, C.S. Foramen ovale size in the normal and abnormal human fetal heart: An indicator of transatrial flow physiology. Ultrasound Obstet. Gynecol. 1991, 1, 313–319. [Google Scholar] [CrossRef] [PubMed]
- García-Otero, L.; Soveral, I.; Sepúlveda-Martínez, Á.; Rodriguez-López, M.; Torres, X.; Guirado, L.; Nogué, L.; Valenzuela-Alcaraz, B.; Martínez, J.M.; Gratacós, E.; et al. Reference ranges for fetal cardiac, ventricular and atrial relative size, sphericity, ventricular dominance, wall asymmetry and relative wall thickness from 18 to 41 gestational weeks. Ultrasound Obstet Gynecol. 2021, 58, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Martínez, A.; García-Otero, L.; Soveral, I.; Guirado, L.; Valenzuela-Alcaraz, B.; Torres, X.; Rodriguez-Lopez, M.; Gratacos, E.; Gómez, O.; Crispi, F. Comparison of 2D versus M-mode echocardiography for assessing fetal myocardial wall thickness. J. Matern Fetal Neonatal. Med. 2019, 32, 2319–2327. [Google Scholar] [CrossRef]
- Ito, T.; Harada, K.; Takada, G. In situ morphometric analysis of left and right ventricles in fetal rats: Changes in ventricular volume, mass, wall thickness, and valvular size. Tohoku J. Exp. Med. 2001, 193, 37–44. [Google Scholar] [CrossRef]
- Ye, L.; Wang, S.; Xiao, Y.; Jiang, C.; Huang, Y.; Chen, H.; Zhang, H.; Zhang, H.; Liu, J.; Xu, Z.; et al. Pressure Overload Greatly Promotes Neonatal Right Ventricular Cardiomyocyte Proliferation: A New Model for the Study of Heart Regeneration. J. Am. Heart Assoc. 2020, 9, e015574. [Google Scholar] [CrossRef] [PubMed]
- Galdos, F.X.; Guo, Y.; Paige, S.L.; VanDusen, N.J.; Wu, S.M.; Pu, W.T. Cardiac Regeneration: Lessons From Development. Circ. Res. 2017, 120, 941–959. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hu, Y.; Xiao, Y.; Wang, S.; Jiang, C.; Liu, J.; Zhang, H.; Hong, H.; Li, F.; Ye, L. Postnatal Right Ventricular Developmental Track Changed by Volume Overload. J. Am. Heart Assoc. 2021, 10, e020854. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Sun, S.; Hu, M.; Xiao, Y.; Yu, X.; Ye, L.; Qiu, L. Downregulated developmental processes in the postnatal right ventricle under the influence of a volume overload. Cell Death Discov. 2021, 7, 208. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Zhou, C.; Xiao, Y.; Sun, S.; Jiang, C.; Chen, L.; Liu, J.; Zhang, H.; Li, F.; et al. Molecular Changes in Prepubertal Left Ventricular Development Under Experimental Volume Overload. Front. Cardiovasc. Med. 2022, 9, 850248. [Google Scholar] [CrossRef]
- Graham, E.M.; Bradley, S.M.; Atz, A.M. Preoperative management of hypoplastic left heart syndrome. Expert Opin. Pharmacother. 2005, 6, 687–693. [Google Scholar] [CrossRef]
- Stieh, J.; Fischer, G.; Scheewe, J.; Uebing, A.; Dütschke, P.; Jung, O.; Grabitz, R.; Trampisch, H.J.; Kramer, H.H. Impact of preoperative treatment strategies on the early perioperative outcome in neonates with hypoplastic left heart syndrome. J. Thorac. Cardiovasc. Surg. 2006, 131, 1122–1129.e2. [Google Scholar] [CrossRef]
- Mazurak, M.; Kusa, J. A milestone in congenital cardiac surgery: Four decades of the Norwood procedure. J. Card. Surg. 2021, 36, 2919–2923. [Google Scholar] [CrossRef]
- Kelleher, D.K.; Laussen, P.; Teixeira-Pinto, A.; Duggan, C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition 2006, 22, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Mahle, W.T.; Hu, C.; Trachtenberg, F.; Menteer, J.; Kindel, S.J.; Dipchand, A.I.; Richmond, M.E.; Daly, K.P.; Henderson, H.T.; Lin, K.Y.; et al. Heart failure after the Norwood procedure: An analysis of the Single Ventricle Reconstruction Trial. J. Heart Lung Transplant. 2018, 37, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, M.K.; Rychik, J. Outcomes in Hypoplastic Left Heart Syndrome. Pediatr. Clin. North Am. 2020, 67, 945–962. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.E., Jr.; Chang, A.C.; Murdison, K.A.; Baffa, J.M.; Norwood, W.I.; Murphy, J.D. Outcome and assessment after the modified Fontan procedure for hypoplastic left heart syndrome. Circulation 1992, 85, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Khoo, N.S.; Smallhorn, J.F.; Kaneko, S.; Myers, K.; Kutty, S.; Tham, E.B. Novel insights into RV adaptation and function in hypoplastic left heart syndrome between the first 2 stages of surgical palliation. JACC Cardiovasc. Imaging 2011, 4, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef]
- Cui, Q.; Sun, S.; Zhu, H.; Xiao, Y.; Jiang, C.; Zhang, H.; Liu, J.; Ye, L.; Shen, J. Volume Overload Initiates an Immune Response in the Right Ventricle at the Neonatal Stage. Front. Cardiovasc. Med. 2021, 8, 772336. [Google Scholar] [CrossRef]
- Catterall, W.; Epstein, P.N. Ion channels. Diabetologia 1992, 35, S23–S33. [Google Scholar] [CrossRef]
- Hilgemann, D.W. Cytoplasmic ATP-dependent regulation of ion transporters and channels: Mechanisms and messengers. Annu. Rev. Physiol. 1997, 59, 193–220. [Google Scholar] [CrossRef]
- Te Riele, A.S.; Hauer, R.N. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: Clinical challenges in a changing disease spectrum. Trends Cardiovasc. Med. 2015, 25, 191–198. [Google Scholar] [CrossRef]
- Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005, 87, 99–109. [Google Scholar] [CrossRef]
- Feige, J.N.; Gelman, L.; Michalik, L.; Desvergne, B.; Wahli, W. From molecular action to physiological outputs: Peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog. Lipid Res. 2006, 45, 120–159. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009, 4, 199–227. [Google Scholar] [CrossRef]
- Kalayinia, S.; Arjmand, F.; Maleki, M.; Malakootian, M.; Singh, C.P. MicroRNAs: Roles in cardiovascular development and disease. Cardiovasc. Pathol. 2021, 50, 107296. [Google Scholar] [CrossRef] [PubMed]
- Iliadis, F.; Kadoglou, N.; Didangelos, T. Insulin and the heart. Diabetes Res. Clin. Pract. 2011, 93, S86–S91. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.K.; Struthers, A.D.; Choy, A.M.; Lang, C.C. Insulin sensitization therapy and the heart: Focus on metformin and thiazolidinediones. Heart Fail Clin. 2012, 8, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Benes, J.; Skaroupkova, P.; Sedmera, D.; Strnad, H.; Kolar, M.; Vlcek, C.; Petrak, J.; Benes, J., Jr.; Papousek, F.; et al. Metabolic characterization of volume overload heart failure due to aorto-caval fistula in rats. Mol. Cell Biochem. 2011, 354, 83–96. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Q.; Su, Z.; Xing, J.; Wu, J.; Xiang, L.; Huang, Y.; Pan, H.; Wu, X.; Zhang, X.; et al. Suppression of Myocardial Hypoxia-Inducible Factor-1α Compromises Metabolic Adaptation and Impairs Cardiac Function in Patients With Cyanotic Congenital Heart Disease During Puberty. Circulation 2021, 143, 2254–2272. [Google Scholar] [CrossRef]
- Klein, I.; Ojamaa, K. Thyroid hormone and the cardiovascular system. N. Engl. J. Med. 2001, 344, 501–509. [Google Scholar] [CrossRef]
- Jabbar, A.; Pingitore, A.; Pearce, S.H.; Zaman, A.; Iervasi, G.; Razvi, S. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar] [CrossRef]
- Hirose, K.; Payumo, A.Y.; Cutie, S.; Hoang, A.; Zhang, H.; Guyot, R.; Lunn, D.; Bigley, R.B.; Yu, H.; Wang, J.; et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 2019, 364, 184–188. [Google Scholar] [CrossRef]
- You, J.; Wu, J.; Zhang, Q.; Ye, Y.; Wang, S.; Huang, J.; Liu, H.; Wang, X.; Zhang, W.; Bu, L.; et al. Differential cardiac hypertrophy and signaling pathways in pressure versus volume overload. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H552–H562. [Google Scholar] [CrossRef]
- Bossers, G.P.L.; Hagdorn, Q.A.J.; Ploegstra, M.J.; Borgdorff, M.A.J.; Silljé, H.H.W.; Berger, R.M.F.; Bartelds, B. Volume load-induced right ventricular dysfunction in animal models: Insights in a translational gap in congenital heart disease. Eur. J. Heart Fail. 2018, 20, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Modesti, P.A.; Vanni, S.; Bertolozzi, I.; Cecioni, I.; Lumachi, C.; Perna, A.M.; Boddi, M.; Gensini, G.F. Different growth factor activation in the right and left ventricles in experimental volume overload. Hypertension 2004, 43, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Toischer, K.; Zhu, W.; Hünlich, M.; Mohamed, B.A.; Khadjeh, S.; Reuter, S.P.; Schäfer, K.; Ramanujam, D.; Engelhardt, S.; Field, L.J.; et al. Cardiomyocyte proliferation prevents failure in pressure overload but not volume overload. J. Clin. Investig. 2017, 127, 4285–4296. [Google Scholar] [CrossRef] [PubMed]
- Malek Mohammadi, M.; Abouissa, A.; Heineke, J. A surgical mouse model of neonatal pressure overload by transverse aortic constriction. Nat. Protoc. 2021, 16, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Malek Mohammadi, M.; Abouissa, A.; Azizah, I.; Xie, Y.; Cordero, J.; Shirvani, A.; Gigina, A.; Engelhardt, M.; Trogisch, F.A.; Geffers, R.; et al. Induction of cardiomyocyte proliferation and angiogenesis protects neonatal mice from pressure overload-associated maladaptation. JCI Insight 2019, 23, e128336. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Li, D.; Cui, Q.; Sun, Q.; Hu, Y.; Xiao, Y.; Jiang, C.; Qiu, L.; Zhang, H.; Ye, L.; et al. Ability of the Right Ventricle to Serve as a Systemic Ventricle in Response to the Volume Overload at the Neonatal Stage. Biology 2022, 11, 1831. https://doi.org/10.3390/biology11121831
Zhou C, Li D, Cui Q, Sun Q, Hu Y, Xiao Y, Jiang C, Qiu L, Zhang H, Ye L, et al. Ability of the Right Ventricle to Serve as a Systemic Ventricle in Response to the Volume Overload at the Neonatal Stage. Biology. 2022; 11(12):1831. https://doi.org/10.3390/biology11121831
Chicago/Turabian StyleZhou, Chunxia, Debao Li, Qing Cui, Qi Sun, Yuqing Hu, Yingying Xiao, Chuan Jiang, Lisheng Qiu, Haibo Zhang, Lincai Ye, and et al. 2022. "Ability of the Right Ventricle to Serve as a Systemic Ventricle in Response to the Volume Overload at the Neonatal Stage" Biology 11, no. 12: 1831. https://doi.org/10.3390/biology11121831
APA StyleZhou, C., Li, D., Cui, Q., Sun, Q., Hu, Y., Xiao, Y., Jiang, C., Qiu, L., Zhang, H., Ye, L., & Sun, Y. (2022). Ability of the Right Ventricle to Serve as a Systemic Ventricle in Response to the Volume Overload at the Neonatal Stage. Biology, 11(12), 1831. https://doi.org/10.3390/biology11121831







