The Glacier Ice Worm, Mesenchytraeus solifugus, Elevates Mitochondrial Inorganic Polyphosphate (PolyP) Levels in Response to Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Specimens
2.3. Polymerase Chain Reaction (PCR) Species Identification
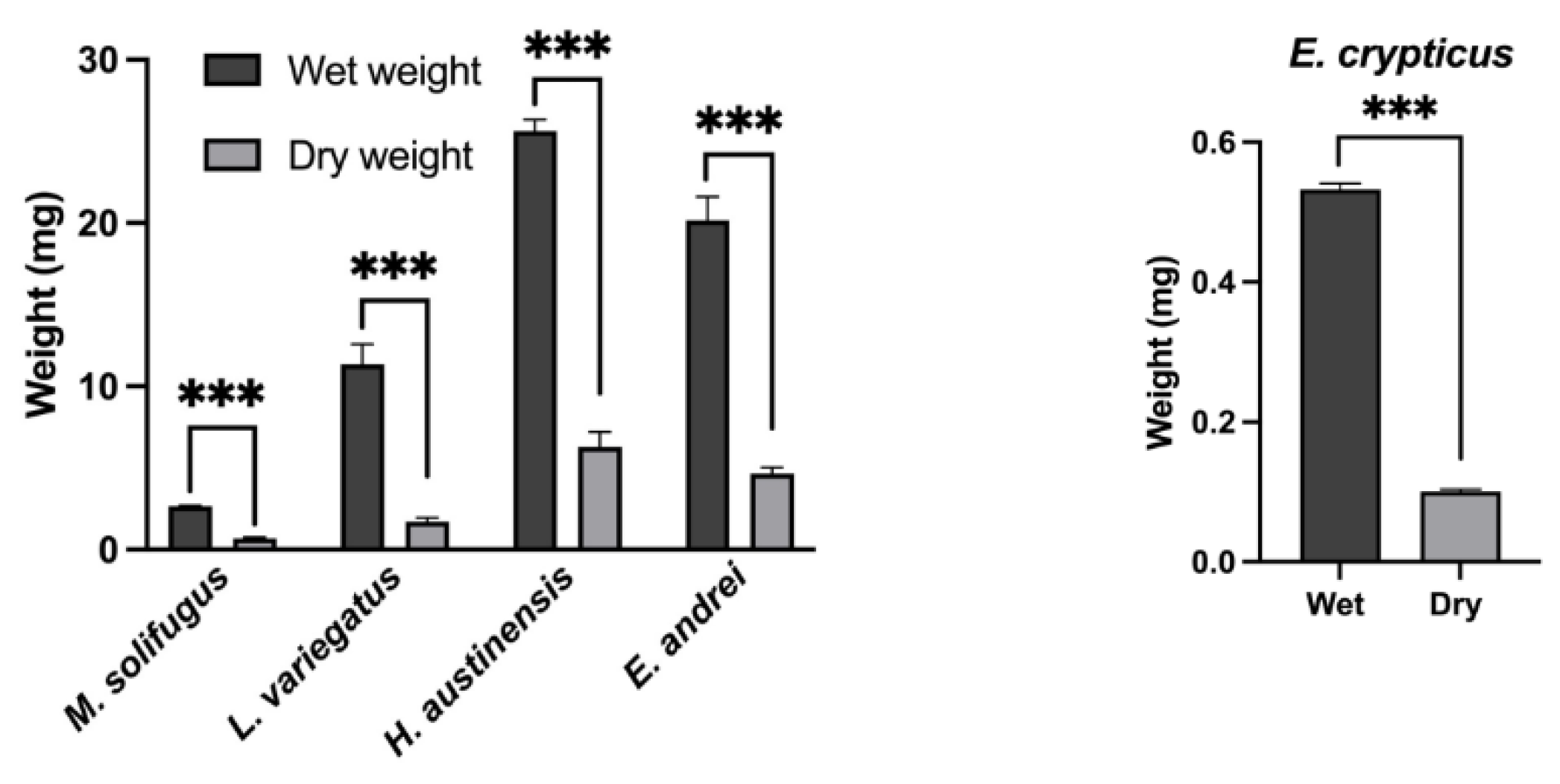
2.4. Dry Weight Quantification
2.5. Mitochondrial Isolation
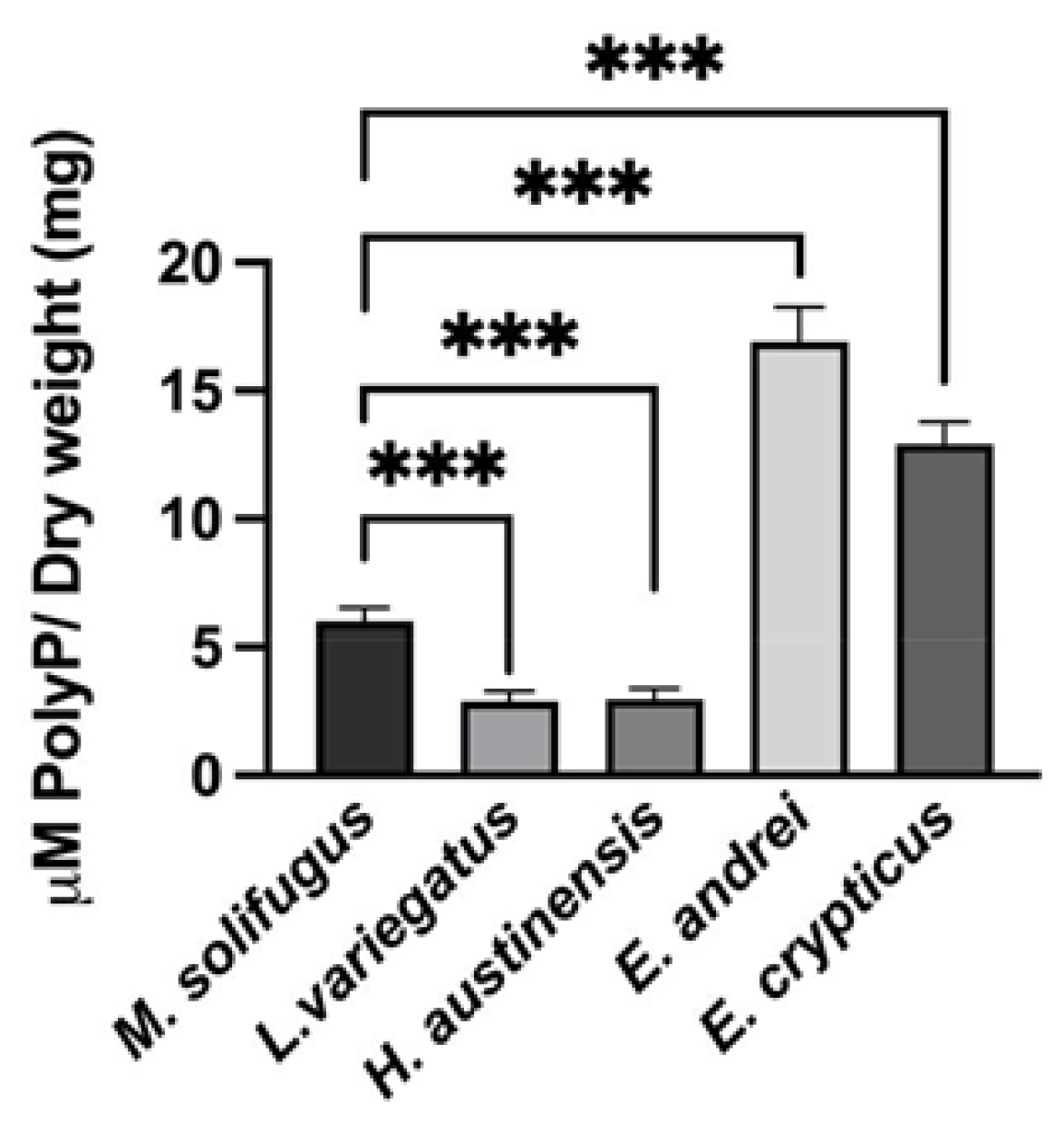
2.6. PolyP Quantification
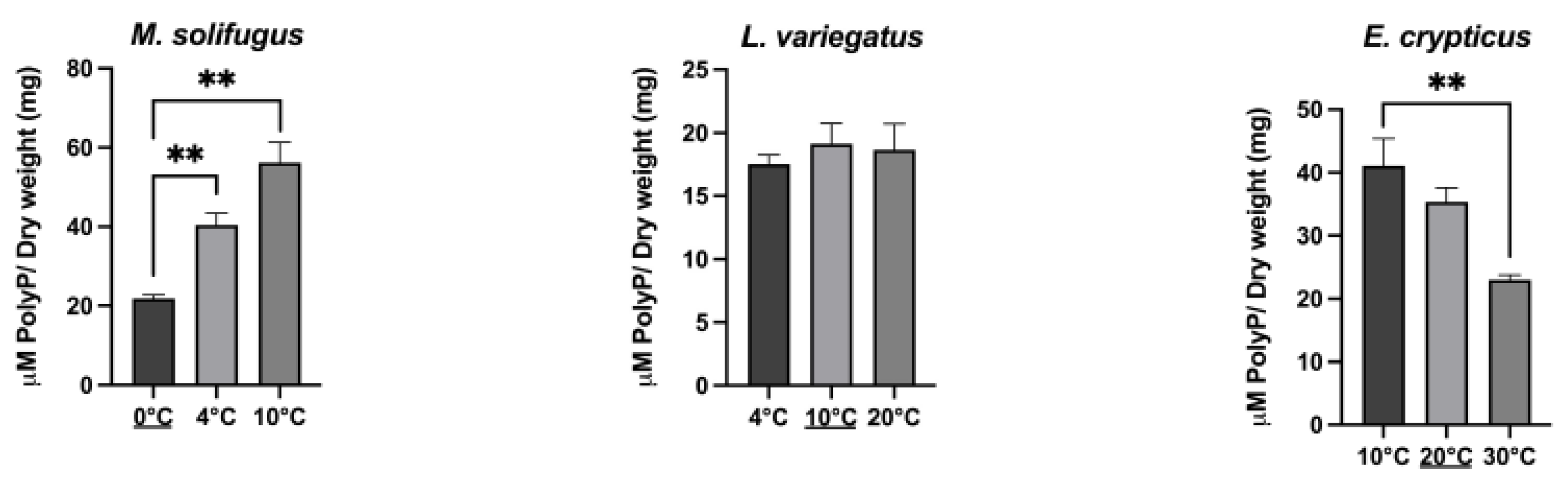
2.7. Temperature-Induced Stress
2.8. Hypoxia
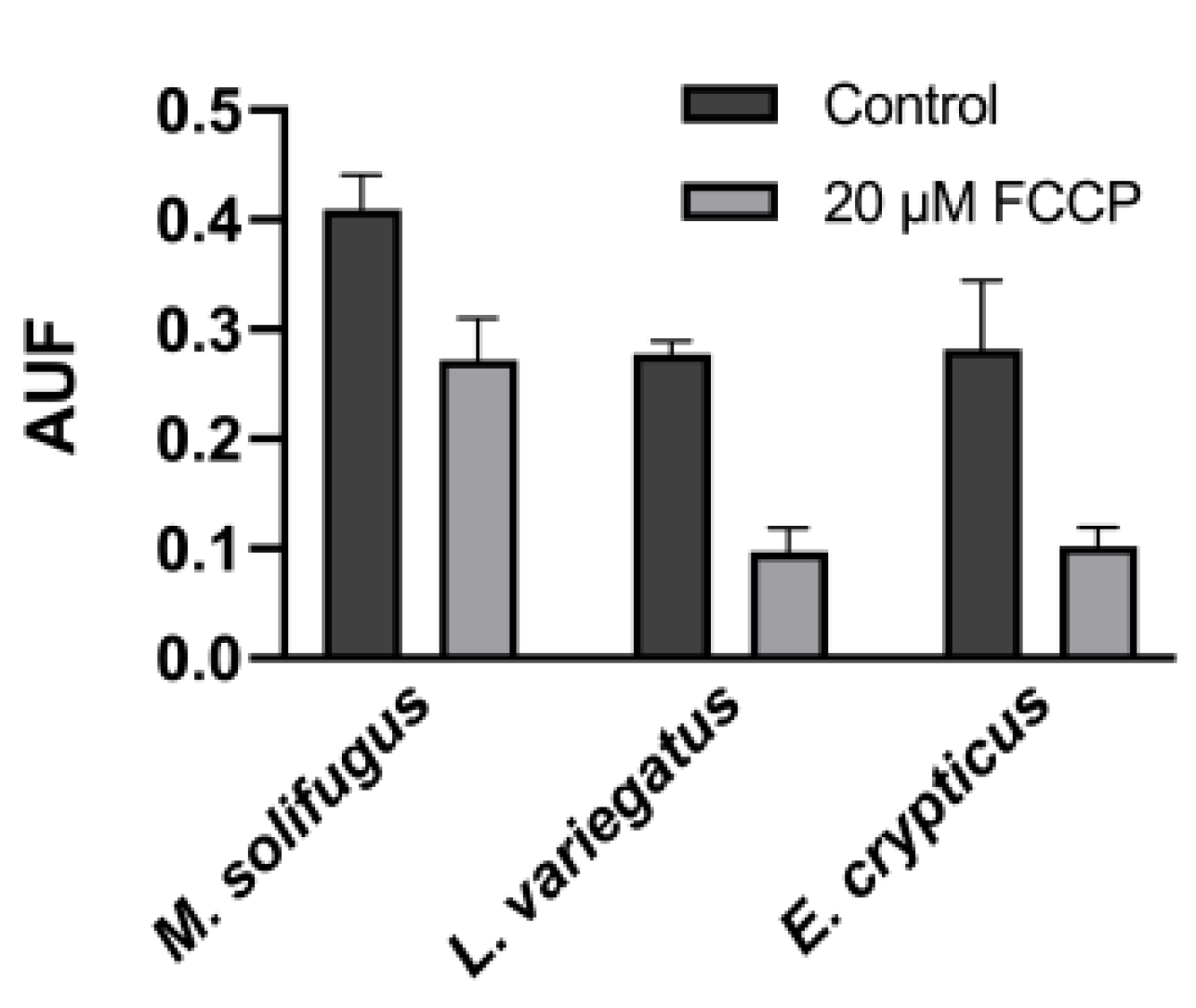
2.9. Mitochondrial Membrane Potential Assay
2.10. Statistical Analysis
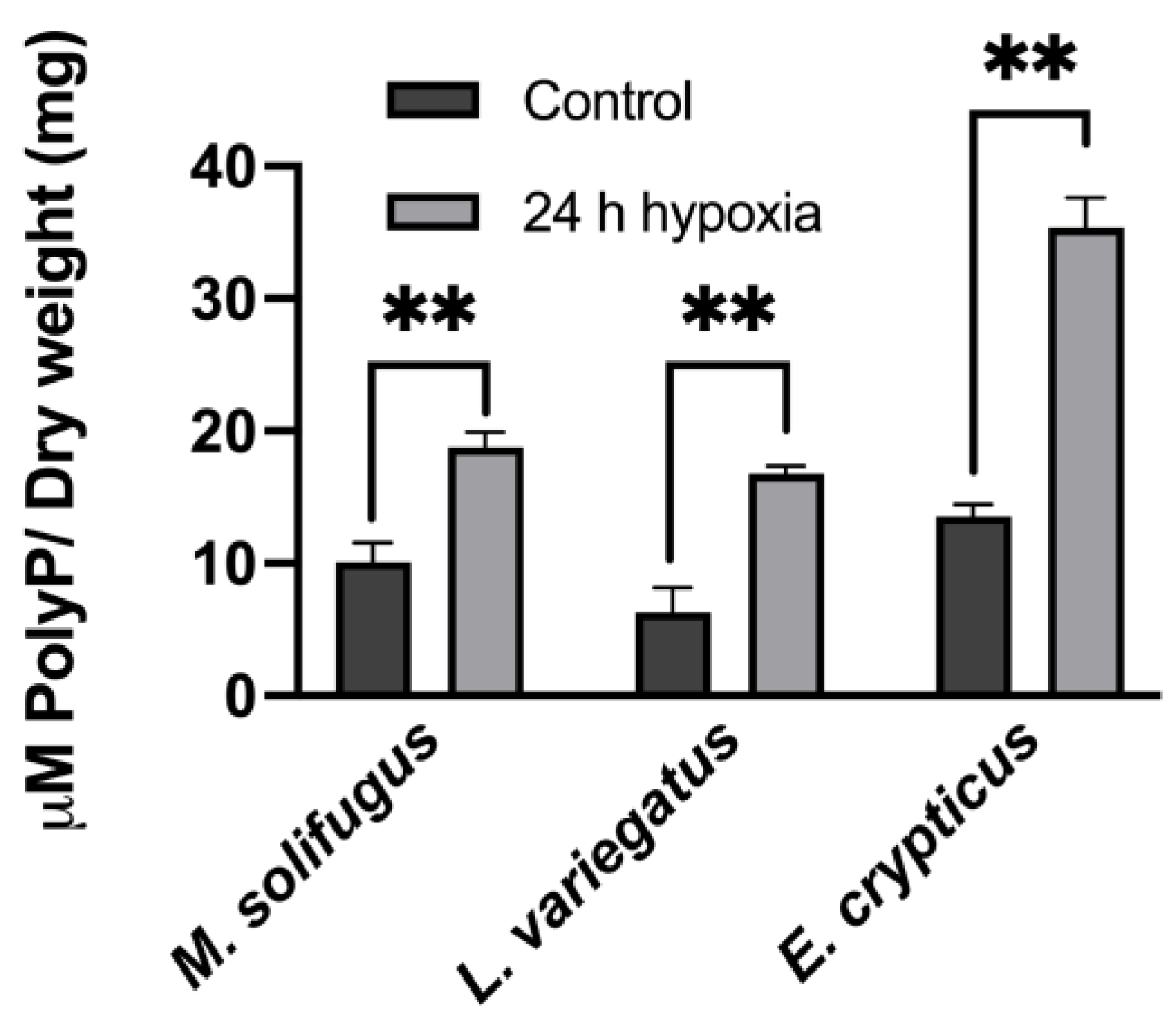
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, D.C. Colloquium paper: Bioenergetics, the origins of complexity, and the ascent of man. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. 2), 8947–8953. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.H.; Choi, S.H.; Smith, S.A. Polyphosphate: An ancient molecule that links platelets, coagulation, and inflammation. Blood 2012, 119, 5972–5979. [Google Scholar] [CrossRef] [PubMed]
- Saiardi, A. How inositol pyrophosphates control cellular phosphate homeostasis? Adv. Biol. Regul. 2012, 52, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Solesio, M.E.; Garcia Del Molino, L.C.; Elustondo, P.A.; Diao, C.; Chang, J.C.; Pavlov, E.V. Inorganic polyphosphate is required for sustained free mitochondrial calcium elevation, following calcium uptake. Cell Calcium. 2020, 86, 102127. [Google Scholar] [CrossRef]
- Solesio, M.E.; Demirkhanyan, L.; Zakharian, E.; Pavlov, E.V. Contribution of inorganic polyphosphate towards regulation of mitochondrial free calcium. Biochim. Biophys. Acta 2016, 1860, 1317–1325. [Google Scholar] [CrossRef]
- Seidlmayer, L.K.; Juettner, V.V.; Kettlewell, S.; Pavlov, E.V.; Blatter, L.A.; Dedkova, E.N. Distinct mPTP activation mechanisms in ischaemia-reperfusion: Contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc. Res. 2015, 106, 237–248. [Google Scholar] [CrossRef]
- Seidlmayer, L.K.; Gomez-Garcia, M.R.; Shiba, T.; Porter, G.A., Jr.; Pavlov, E.V.; Bers, D.M.; Dedkova, E.N. Dual role of inorganic polyphosphate in cardiac myocytes: The importance of polyP chain length for energy metabolism and mPTP activation. Arch. Biochem. Biophys. 2019, 662, 177–189. [Google Scholar] [CrossRef]
- Suess, P.M.; Watson, J.; Chen, W.; Gomer, R.H. Extracellular polyphosphate signals through Ras and Akt to prime Dictyostelium discoideum cells for development. J. Cell Sci. 2017, 130, 2394–2404. [Google Scholar] [CrossRef]
- Müller, W.E.; Wang, S.; Neufurth, M.; Kokkinopoulou, M.; Feng, Q.; Schröder, H.C.; Wang, X. Polyphosphate as a donor of high-energy phosphate for the synthesis of ADP and ATP. J. Cell Sci. 2017, 130, 2747–2756. [Google Scholar] [CrossRef]
- Müller, W.E.; Wang, S.; Ackermann, M.; Neufurth, M.; Steffen, R.; Mecja, E.; Muñoz-Espí, R.; Feng, Q.; Schröder, H.C.; Wang, X. Rebalancing beta-Amyloid-Induced Decrease of ATP Level by Amorphous Nano/Micro Polyphosphate: Suppression of the Neurotoxic Effect of Amyloid beta-Protein Fragment 25–35. Int. J. Mol. Sci. 2017, 18, 2154. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Hurlimann, H.C.; Jakob, C.A.; Werner, T.P.; Amrhein, N. Systematic screening of polyphosphate (poly P) levels in yeast mutant cells reveals strong interdependence with primary metabolism. Genome Biol. 2006, 7, R109. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Fraley, C.; Diao, C.T.; Winkfein, R.; Colicos, M.A.; Duchen, M.R.; French, R.J.; Pavlov, E. Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proc. Natl. Acad. Sci. USA 2007, 104, 18091–18096. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, S.R. Adenosine triphosphate synthesis from polyphosphate by an enzyme from Escherichia coli. Biochim. Biophys. Acta 1957, 26, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Solesio, M.E.; Elustondo, P.A.; Zakharian, E.; Pavlov, E.V. Inorganic polyphosphate (polyP) as an activator and structural component of the mitochondrial permeability transition pore. Biochem. Soc. Trans. 2016, 44, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Solesio, M.E.; Xie, L.; McIntyre, B.; Ellenberger, M.; Mitaishvili, E.; Bhadra-Lobo, S.; Bettcher, L.F.; Bazil, J.N.; Raftery, D.; Jakob, U.; et al. Depletion of mitochondrial inorganic polyphosphate (polyP) in mammalian cells causes metabolic shift from oxidative phosphorylation to glycolysis. Biochem. J. 2021, 478, 1631–1646. [Google Scholar] [CrossRef]
- Guitart-Mampel, M.; Urquiza, P.; Carnevale Neto, F.; Anderson, J.R.; Hambardikar, V.; Scoma, E.R.; Merrihew, G.E.; Wang, L.; MacCoss, M.J.; Raftery, D.; et al. Mitochondrial Inorganic Polyphosphate (polyP) Is a Potent Regulator of Mammalian Bioenergetics in SH-SY5Y Cells: A Proteomics and Metabolomics Study. Front. Cell Dev. Biol. 2022, 10, 833127. [Google Scholar] [CrossRef]
- Hambardikar, V.; Guitart-Mampel, M.; Scoma, E.R.; Urquiza, P.; Nagana, G.G.A.; Raftery, D.; Collins, J.A.; Solesio, M.E. Enzymatic Depletion of Mitochondrial Inorganic Polyphosphate (polyP) Increases the Generation of Reactive Oxygen Species (ROS) and the Activity of the Pentose Phosphate Pathway (PPP) in Mammalian Cells. Antioxidants 2022, 11, 685. [Google Scholar] [CrossRef]
- McCormack, J.G.; Halestrap, A.P.; Denton, R.M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 1990, 70, 391–425. [Google Scholar] [CrossRef]
- Borden, E.A.; Furey, M.; Gattone, N.J.; Hambardikar, V.D.; Liang, X.H.; Scoma, E.R.; Abou Samra, A.; LR, D.G.; Dennis, D.J.; Fricker, D.; et al. Is there a link between inorganic polyphosphate (polyP), mitochondria, and neurodegeneration? Pharmacol. Res. 2021, 163, 105211. [Google Scholar] [CrossRef]
- Kumble, K.D.; Kornberg, A. Inorganic polyphosphate in mammalian cells and tissues. J. Biol. Chem. 1995, 270, 5818–5822. [Google Scholar] [CrossRef]
- Pavlov, E.; Aschar-Sobbi, R.; Campanella, M.; Turner, R.J.; Gomez-Garcia, M.R.; Abramov, A.Y. Inorganic polyphosphate and energy metabolism in mammalian cells. J. Biol. Chem. 2010, 285, 9420–9428. [Google Scholar] [CrossRef] [PubMed]
- Bayev, A.Y.; Angelova, P.R.; Abramov, A.Y. Inorganic polyphosphate is produced and hydrolysed in F0F1-ATP synthase of mammalian mitochondria. Biochem. J. 2020, 477, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.J.; Nagele, R.G.; Shain, D.H. The ice worm, Mesenchytraeus solifugus, elevates adenylate levels at low physiological temperature. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 137, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.J.; Shain, D.H. Quantitating adenylate nucleotides in diverse organisms. J. Biochem. Biophys. Methods 2005, 63, 69–77. [Google Scholar] [CrossRef]
- Lang, S.A.; Shain, D.H. Atypical Evolution of the F1Fo Adenosine Triphosphate Synthase Regulatory ATP6 subunit in Glacier Ice Worms (Annelida: Clitellata: Mesenchytraeus). Evol. Bioinform. Online 2018, 14, 1176934318788076. [Google Scholar] [CrossRef]
- Hotaling, S.; Shain, D.H.; Lang, S.A.; Bagley, R.K.; Tronstad, L.M.; Weisrock, D.W.; Kelley, J.L. Long-distance dispersal, ice sheet dynamics and mountaintop isolation underlie the genetic structure of glacier ice worms. Proc. Biol. Sci. 2019, 286, 20190983. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Solesio, M.E.; Peixoto, P.M.; Debure, L.; Madamba, S.M.; de Leon, M.J.; Wisniewski, T.; Pavlov, E.V.; Fossati, S. Carbonic anhydrase inhibition selectively prevents amyloid beta neurovascular mitochondrial toxicity. Aging Cell 2018, 17, e12787. [Google Scholar] [CrossRef]
- Baltanas, A.; Solesio, M.E.; Zalba, G.; Galindo, M.F.; Fortuno, A.; Jordan, J. The senescence-accelerated mouse prone-8 (SAM-P8) oxidative stress is associated with upregulation of renal NADPH oxidase system. J. Physiol. Biochem. 2013, 69, 927–935. [Google Scholar] [CrossRef]
- Aschar-Sobbi, R.; Abramov, A.Y.; Diao, C.; Kargacin, M.E.; Kargacin, G.J.; French, R.J.; Pavlov, E. High sensitivity, quantitative measurements of polyphosphate using a new DAPI-based approach. J. Fluoresc. 2008, 18, 859–866. [Google Scholar] [CrossRef]
- Wang, W.; Nema, S.; Teagarden, D. Protein aggregation--pathways and influencing factors. Int. J. Pharm. 2010, 390, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Rao, N.N.; Fraley, C.D.; Kornberg, A. Inorganic polyphosphate is essential for long-term survival and virulence factors in Shigella and Salmonella spp. Proc. Natl. Acad. Sci. USA 2002, 99, 7675–7680. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Kornberg, A. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2000, 97, 4885–4890. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; McEwen, B.S. Psychological Stress and Mitochondria: A Conceptual Framework. Psychosom. Med. 2018, 80, 126–140. [Google Scholar] [CrossRef]
- Gray, M.J.; Wholey, W.Y.; Wagner, N.O.; Cremers, C.M.; Mueller-Schickert, A.; Hock, N.T.; Krieger, A.G.; Smith, E.M.; Bender, R.A.; Bardwell, J.C.; et al. Polyphosphate is a primordial chaperone. Mol. Cell 2014, 53, 689–699. [Google Scholar] [CrossRef]
- Kampinga, H.H. Chaperoned by prebiotic inorganic polyphosphate molecules: An ancient transcription-independent mechanism to restore protein homeostasis. Mol. Cell 2014, 53, 685–687. [Google Scholar] [CrossRef]
- Cremers, C.M.; Knoefler, D.; Gates, S.; Martin, N.; Dahl, J.U.; Lempart, J.; Xie, L.; Chapman, M.R.; Galvan, V.; Southworth, D.R.; et al. Polyphosphate: A Conserved Modifier of Amyloidogenic Processes. Mol. Cell 2016, 63, 768–780. [Google Scholar] [CrossRef]
- Beissinger, M.; Buchner, J. How chaperones fold proteins. Biol. Chem. 1998, 379, 245–259. [Google Scholar]
- McIntyre, B.; Solesio, M.E. Mitochondrial inorganic polyphosphate (polyP): The missing link of mammalian bioenergetics. Neural Regen. Res. 2021, 16, 2227–2228. [Google Scholar] [CrossRef]
- Ellington, W.R. Evolution and physiological roles of phosphagen systems. Annu. Rev. Physiol. 2001, 63, 289–325. [Google Scholar] [CrossRef]
- Dahl, J.U.; Gray, M.J.; Jakob, U. Protein quality control under oxidative stress conditions. J. Mol. Biol. 2015, 427, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Jakob, U. Inorganic polyphosphate, a multifunctional polyanionic protein scaffold. J. Biol. Chem. 2019, 294, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio, T.; Scoma, E.R.; Shain, D.H.; Melissaratos, D.S.; Riggs, L.M.; Hambardikar, V.; Solesio, M.E. The Glacier Ice Worm, Mesenchytraeus solifugus, Elevates Mitochondrial Inorganic Polyphosphate (PolyP) Levels in Response to Stress. Biology 2022, 11, 1771. https://doi.org/10.3390/biology11121771
Osorio T, Scoma ER, Shain DH, Melissaratos DS, Riggs LM, Hambardikar V, Solesio ME. The Glacier Ice Worm, Mesenchytraeus solifugus, Elevates Mitochondrial Inorganic Polyphosphate (PolyP) Levels in Response to Stress. Biology. 2022; 11(12):1771. https://doi.org/10.3390/biology11121771
Chicago/Turabian StyleOsorio, Teresa, Ernest R. Scoma, Daniel H. Shain, Diana S. Melissaratos, Lindsey M. Riggs, Vedangi Hambardikar, and Maria E. Solesio. 2022. "The Glacier Ice Worm, Mesenchytraeus solifugus, Elevates Mitochondrial Inorganic Polyphosphate (PolyP) Levels in Response to Stress" Biology 11, no. 12: 1771. https://doi.org/10.3390/biology11121771
APA StyleOsorio, T., Scoma, E. R., Shain, D. H., Melissaratos, D. S., Riggs, L. M., Hambardikar, V., & Solesio, M. E. (2022). The Glacier Ice Worm, Mesenchytraeus solifugus, Elevates Mitochondrial Inorganic Polyphosphate (PolyP) Levels in Response to Stress. Biology, 11(12), 1771. https://doi.org/10.3390/biology11121771




