Geographical Variation in Body Size and the Bergmann’s Rule in Andrew’s Toad (Bufo andrewsi)
Abstract
Simple Summary
Abstract
1. Introduction
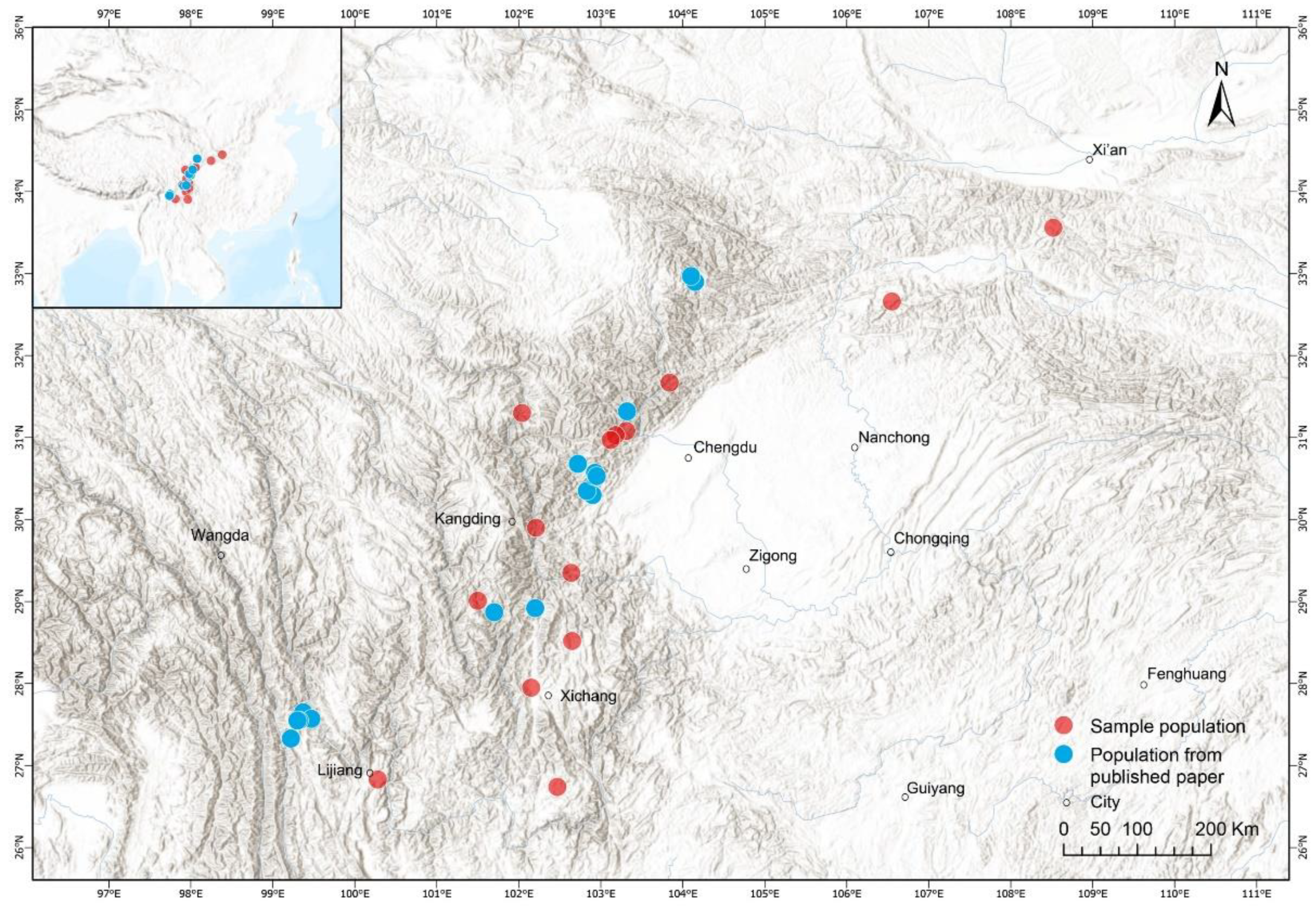
2. Materials and Methods
2.1. Data on Body Size and Age Estimation
2.2. Environmental Predictors
2.3. Statistical Analysis
3. Results
3.1. Geographical Variation in Age
3.2. Geographical Variation in Body Size
3.3. Effect of Environmental Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenzweig, C.; Karoly, D.; Vicarelli, M.; Neofotis, P.; Wu, Q.; Casassa, G.; Menzel, A.; Root, T.L.; Estrella, N.; Seguin, B.; et al. Attributing physical and biological impacts to anthropogenic climate change. Nature 2008, 453, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Souchet, J.; Gangloff, E.J.; Micheli, G.; Bossu, C.; Trochet, A.; Bertrand, R.; Clobert, J.; Calvez, O.; Martinez-Silvestre, A.; Darnet, E.; et al. High-elevation hypoxia impacts perinatal physiology and performance in a potential montane colonizer. Integr. Zool. 2020, 15, 544–557. [Google Scholar] [CrossRef] [PubMed]
- De Meester, G.; Šunje, E.; Prinsen, E.; Verbruggen, E.; Van Damme, R. Toxin variation among salamander populations: Discussing potential causes and future directions. Integr. Zool. 2021, 16, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.; Agudo, R. The future of species under climate change: Resilience or decline? Science 2013, 341, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Hu, J.; Liu, Y.; Giam, X.; Lee, T.M.; Luo, H.; Wu, J.; Liang, Q.; Zhao, J.; Long, X.; et al. Community-wide changes in intertaxonomic temporal co-occurrence resulting from phenological shifts. Glob. Chang. Biol. 2016, 22, 1746–1754. [Google Scholar] [CrossRef]
- Liang, T.; Meiri, S.; Shi, L. Sexual size dimorphism in lizards: Rensch’s rule, reproductive mode, clutch size, and line fitting method effects. Integr. Zool. 2022, 17, 787–803. [Google Scholar] [CrossRef]
- Hu, J.; Xie, F.; Li, C.; Jiang, J. Elevational patterns of species richness, range and body size for spiny frogs. PLoS ONE 2011, 6, e19817. [Google Scholar] [CrossRef][Green Version]
- Rutherford, L.; Murray, L.E. Personality and behavioral changes in Asian elephants (Elephas maximus) following the death of herd members. Integr. Zool. 2021, 16, 170–188. [Google Scholar] [CrossRef]
- Zedda, M.; Sathe, V.; Chakraborty, P.; Palombo, M.R.; Farina, V. A first comparison of bone histomorphometry in extant domestic horses (Equus caballus) and a Pleistocene Indian wild horse (Equus namadicus). Integr. Zool. 2021, 16, 448–460. [Google Scholar] [CrossRef]
- Munoz-munoz, F.; Pages, N.; Durao, A.F.; England, M.; Werner, D.; Talavera, S. Narrow versus broad: Sexual dimorphism in the wing form of western European species of the subgenus Avaritia (Culicoides, Ceratopogonidae). Integr. Zool. 2021, 16, 769–784. [Google Scholar] [CrossRef]
- Huang, C.H.; Zhong, M.J.; Liao, W.B.; Kotrschal, A. Investigating the role of body size, ecology, and behavior in Anuran eye size evolution. Evol. Ecol. 2019, 33, 585–598. [Google Scholar] [CrossRef]
- Mai, C.L.; Liao, W.B.; Kotrschal, A.; Lüpold, S. Relative brain size is predicted by the intensity of intrasexual competition in frogs. Am. Nat. 2020, 196, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mai, C.L.; Liao, W.B.; Kotrschal, A. Body mass variation is negatively associated with brain size: Evidence for the fat-brain trade-off in Anurans. Evolution 2020, 74, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Balciauskas, L.; Amshokova, A.; Balciauskiene, L.; Benedek, A.M.; Cichocki, J.; Csanady, A.; De Mendonca, P.G.; Nistreanu, V. Geographical clines in the size of the herb field mouse (Apodemus uralensis). Integr. Zool. 2020, 15, 55–68. [Google Scholar] [CrossRef]
- Donihue, C.M.; Daltry, J.C.; Challenger, S.; Herrel, A. Population increase and changes in behavior and morphology in the Critically Endangered Redonda ground lizard (Pholidoscelis atratus) following the successful removal of alien rats and goats. Integr. Zool. 2021, 16, 379–389. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, Y.; Jin, L.; Liao, W.B. No evidence for effects of ecological and behavioral factors on eye size evolution in Anurans. Front. Ecol. Evol. 2021, 9, 755818. [Google Scholar] [CrossRef]
- Liao, W.B.; Jiang, Y.; Li, D.Y.; Jin, L.; Zhong, M.J.; Qi, Y.; Lüpold, S.; Kotrschal, A. Cognition contra camouflage: How the brain mediates predator–driven crypsis evolution. Sci. Adv. 2022, 8, eabq1878. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, C.; Liao, W.B. Anuran interorbital distance variation: The role of ecological and behavioral factors. Integr. Zool. 2022, 17, 777–786. [Google Scholar] [CrossRef]
- Jiang, Y.; Luan, X.F.; Liao, W.B. Anuran brain size predicts food availability-driven population density. Sci. China Life Sci. 2022, 65, 1–3. [Google Scholar] [CrossRef]
- Hu, J.; Hu, H.; Jiang, Z. The impacts of climate change on the wintering distribution of an endangered migratory bird. Oecologia 2010, 164, 555–565. [Google Scholar] [CrossRef]
- Deme, G.G.; Hao, X.; Ma, L.; Sun, B.J.; Du, W.G. Elevational variation in reproductive strategy of a widespread lizard: High-elevation females lay fewer but larger eggs. Asian Herpetol. Res. 2022, 13, 198–204. [Google Scholar]
- Liao, W.B.; Luo, Y.; Lou, S.L.; Lu, D.; Jehle, R. Geographic variation in life-history traits: Growth season affects age structure, egg size and clutch size in Andrew’s toad (Bufo andrewsi). Front. Zool. 2016, 13, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiong, J.L.; Guo, J.P.; Huang, Y.; Zhang, B.W.; Ren, H.T.; Pan, T. Age and body size of the Shangcheng Stout Salamander Pachyhynobius shangchengensis (Caudata: Hynobiidae) from Southeastern China. Asian Herpetol. Res. 2020, 11, 219–224. [Google Scholar]
- Liao, W.B.; Liu, W.C.; Merilä, J. Andrew meets Rensch: Sexual size dimorphism and the inverse of Rensch’s rule in Andrew’s toad (Bufo andrewsi). Oecologia 2015, 177, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Li, S.R.; Hao, X.; Sun, B.J.; Bi, J.H.; Zhang, Y.P.; Du, W.G. Phenotypic consequences of maternally selected nests: A cross-fostering experiment in a desert lizard. Integr. Zool. 2021, 16, 741–754. [Google Scholar] [CrossRef]
- Kamdem, M.M.; Ngakou, A.; Yanou Njintang, N.; Otomo Voua, P. Habitat components and population density drive plant litter consumption by Eudrilus eugeniae (Oligochaeta) under tropical conditions. Integr. Zool. 2021, 16, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J.; Chown, S.L.; Evans, K.L. Ecogeographical rules: Elements of a synthesis. J. Biogeogr. 2008, 35, 483–500. [Google Scholar] [CrossRef]
- Alho, J.S.; Herczeg, G.; Laugen, A.T.; Räsänen, K.; Laurila, A.; Merilä, J. Allen’s rule revisited: Quantitative genetics of extremity length in the common frog along a latitudinal gradient. J. Evol. Biol. 2011, 24, 59–70. [Google Scholar] [CrossRef]
- Mcwhinnie, R.B.; Sckrabulis, J.P.; Raffel, T.R. Temperature and mass scaling affect cutaneous and pulmonary respiratory performance in a diving frog. Integr. Zool. 2021, 16, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, C.C. Body sizes of poikilotherm vertebrates at different latitudes. Evolution 1966, 20, 456–465. [Google Scholar]
- Angilletta, M.J., Jr.; Dunham, A.E. The temperature-size rule in ectotherms: Simple evolutionary explanations may not be general. Am. Nat. 2003, 162, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tan, S.; Yao, Z.Y.; Liu, G.F.; Fu, J.Z.; Chen, J.F. Sex but not altitude, modulates phenotypic covariations between growth and physiological traits in adult Asiatic toads. Asian Herpetol. Res. 2022, 13, 34–42. [Google Scholar]
- Roff, D.A. Life History Evolution; Sunderland, M.A., Ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2002. [Google Scholar]
- Lüpold, S.; Jin, L.; Liao, W.B. Population density and structure drive differential investment in pre- and postmating sexual traits in frogs. Evolution 2017, 71, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Bao, J.H.; Lee, P.S.; Wang, J.; Wang, S.; Zhang, F. Nonlinear phenomena conveying body size information and improving attractiveness of the courtship calls in the males of Odorrana tormota. Asian Herpetol. Res. 2021, 12, 117–123. [Google Scholar]
- Bláha, M.; Patoka, J.; Japoshvili, B.; Let, M.; Buric, M.; Kouba, A.; Mpumladze, L. Genetic diversity, phylogenetic position and morphometric analysis of Astacus colchicus (Decapoda, Astacidae): A new insight into Eastern European crayfish fauna. Integr. Zool. 2021, 16, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, B.R.; Surkova, E.N.; Shenbrot, G.I.; Khokhlova, I.S. Latitudinal gradients in body size and sexual size dimorphism in fleas: Males drive Bergmann’s pattern. Integr. Zool. 2022, 1–13. [Google Scholar] [CrossRef]
- Peters, R.H. The Ecological Implications of Body Size; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Brown, J.H. Macroecology; University of Chicago Press: Chicago, IL, USA, 1995. [Google Scholar]
- Gaston, K.J.; Blackburn, T.M. Pattern and Process in Macroecology; Blackwell Science: Oxford, UK, 2000. [Google Scholar]
- Smith, F.A.; Lyons, K. On being the right size: The importance of size in life history, ecology and evolution. In Animal Body Size: Linking Pattern and Process across Space, Time, and Taxonomic Group; Smith, F.A., Lyons, K., Eds.; University of Chicago Press: Chicago, IL, USA, 2013; pp. 1–12. [Google Scholar]
- Scheun, J.; Neller, S.; Bennett, N.C.; Kemp, L.V.; Ganswindt, A. Endocrine correlates of gender and throat coloration in the southern ground-hornbill (Bucorvus leadbeateri). Integr. Zool. 2021, 16, 189–201. [Google Scholar] [CrossRef]
- Stellatelli, O.A.; Vega, L.E.; Block, C.; Rocca, C.; Bellagamba, P.; Daji, J.E.; Cruz, F.B. Latitudinal pattern of the thermal sensitivity of running speed in the endemic lizard Liolaemus multimaculatus. Integr. Zool. 2022, 17, 619–637. [Google Scholar] [CrossRef]
- Rodriguez, M.A.; Lopez-Sanudo, I.L.; Hawkins, B.A. The geographic distribution of mammal body size in Europe. Glob. Ecol. Biogeogr. 2006, 15, 173–181. [Google Scholar] [CrossRef]
- Liao, W.B.; Lu, X. Adult body size = f (initial size + growth rate × age): Explaining the proximate cause of Bergman’s cline in a toad along altitudinal gradients. Evol. Ecol. 2012, 26, 579–590. [Google Scholar] [CrossRef]
- Adams, D.C.; West, M.E.; Collyer, M.L. Location-specific sympatric morphological divergence as a possible response to species interactions in West Virginia Plethodon salamander communities. J. Anim. Ecol. 2007, 76, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kozak, K.H.; Mendyk, R.W.; Wiens, J.J. Can parallel diversification occur in sympatry? Repeated patterns of body-size evolution in coexisting clades of north American salamanders. Evolution 2010, 63, 1769–1784. [Google Scholar] [CrossRef] [PubMed]
- Clifton, I.T.; Chamberlain, J.D.; Gifford, M.E. Role of phenotypic plasticity in morphological differentiation between water snake populations. Integr. Zool. 2020, 15, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C. Ueber die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Größe. Göttinger. Studien. 1847, 3, 595–708. [Google Scholar]
- Mayr, E. Geographical character gradients and climatic adaptation. Evolution 1956, 10, 105–108. [Google Scholar]
- Atkinson, D.; Sibly, R.M. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol. Evol. 1997, 12, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.; Shine, R.; Porter, W.P. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc. Natl. Acad. Sci. USA 2009, 106, 3835–3840. [Google Scholar] [CrossRef]
- Adams, D.C.; Church, J.O. Amphibians do not follow Bergmann’s rule. Evolution 2008, 62, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Ashton, K.G. Sensitivity of intraspecific latitudinal clines of body size for tetrapods to sampling, latitude and body size. Integr. Comp. Biol. 2004, 44, 403–412. [Google Scholar] [CrossRef]
- Laugen, A.T.; Jönsson, I.; Laurila, A.; Söderman, F.; Merilä, J. Do common frogs (Rana temporaria) follow Bermann’s rule? Evol. Ecol. Res. 2005, 7, 717–731. [Google Scholar]
- Ashton, K.G.; Feldman, C.R. Bergmann’s rule in nonavian reptiles: Turtles follow it, lizards and snakes reverse it. Evolution 2003, 57, 1151–1163. [Google Scholar] [PubMed]
- Ma, X.Y.; Lu, X.; Merilä, J. Altitudinal decline of body size in a Tibetan frog Nanorana parkeri. J. Zool. 2009, 279, 364–371. [Google Scholar] [CrossRef]
- Servino, L.M.; Verdade, V.K.; Sawaya, R.J. For neither heat nor water conservation: Body size variation in Atlantic Forest frogs does not follow a general mechanism. J. Biogeogr. 2022, 49, 460–468. [Google Scholar] [CrossRef]
- Nevo, E. Adaptive variation in size of cricket frogs. Ecology 1973, 54, 1271–1278. [Google Scholar] [CrossRef]
- Valenzuela-Sánchez, A.; Cunningham, A.A.; Soto-Azat, C. Geographic body size variation in ectotherms: Effects of seasonality on an Anuran from the southern temperate forest. Front. Zool. 2015, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Olalla-Tárraga, M.A.; Rodríguez, M.A. Energy and interspecific body size patterns of amphibian faunas in Europe and North America: Anurans follow Bergmann’s rule, urodeles its converse. Glob. Ecol. Biogeogr. 2007, 16, 606–617. [Google Scholar] [CrossRef]
- Kozłowski, J.; Czarnołeski, M.; Dańko, M. Can optimal resource allocation models explain why ectotherms grow larger in cold? Integr. Comp. Biol. 2004, 44, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.W.; Zhang, L.X.; Lu, X. Global gaps in age data based on skeletochronology for amphibians. Integr. Zool. 2022, 17, 752–763. [Google Scholar] [CrossRef]
- Guo, C.; Gao, S.; Krzton, A.; Zhang, L. Geographic body size variation of a tropical Anuran: Effects of water deficit and precipitation seasonality on Asian common toad from southern Asia. BMC Evol. Biol. 2019, 19, 208. [Google Scholar] [CrossRef]
- Lu, X.; Li, B.; Liang, J.J. Comparative demography of a temperate Anuran, Rana chensinensis, along a relatively fine altitudinal gradient. Can. J. Zool. 2006, 84, 1789–1795. [Google Scholar] [CrossRef]
- Martin, A.K.; Sheridan, J.A. Body size responses to the combined effects of climate and land use changes within an urban framework. Glob. Chang. Biol. 2022, 28, 5385–5398. [Google Scholar] [CrossRef] [PubMed]
- Olarte, O.; Sanchez-Montes, G.; Martinez-Solano, I. Integrative demographic study of the Iberian painted frog (Discoglossus galganoi): Inter-annual variation in the effective to census population size ratio, with insights on mating system and breeding success. Integr. Zool. 2020, 15, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, A.R.; Kiesecker, J.M. Complexity in conservation: Lessons from the global decline of amphibian populations. Ecol. Lett. 2002, 5, 597–608. [Google Scholar] [CrossRef]
- Xu, F.; Li, J.; Yang, W.K. Invasive American bullfrogs age, body size, and sexual size dimorphism geographical variation in Northwestern China. Diversity 2022, 14, 953. [Google Scholar] [CrossRef]
- Ashton, K.G. Patterns of within-species body size variation of birds: Strong evidence for Bergmann’s rule. Glob. Ecol. Biogeogr. 2002, 11, 505–523. [Google Scholar] [CrossRef]
- Schäuble, C.S. Variation in body size and sexual dimorphism across geographical and environmental space in the frogs Limnodynastes tasmaniensis and L. peronii. Biol. J. Linn. Soc. 2004, 82, 39–56. [Google Scholar] [CrossRef]
- Adams, D.C.; Church, J.O. The evolution of large-scale body size clines in Plethodon salamanders: Evidence of heat-balance or species-specific artifact? Ecography 2011, 34, 1067–1075. [Google Scholar] [CrossRef]
- Marangoni, F.; Tejedo, M. Variation in body size and metamorphic traits of Iberian spadefoot toads over a short geographic distance. J. Zool. 2008, 275, 97–105. [Google Scholar] [CrossRef]
- Marangoni, F.; Tejedo, M.; Gomez-Mestre, I. Extreme reduction in body size and reproductive output associated with sandy substrates in two Anuran species. Amphib. Reptil. 2008, 29, 541–553. [Google Scholar] [CrossRef]
- Bidau, C.J.; Martí, D.A.; Baldo, D. Inter-and intraspecific geographic variation of body size in South American redbelly toads of the genus Melanophryniscus Gallardo, 1961 (Anura: Bufonidae). J. Herpetol. 2011, 45, 66–74. [Google Scholar] [CrossRef]
- Gouveia, S.F.; Dobrovolski, R.; Lemes, P.; Cassemiro, F.A.; Diniz-Filho, J.A.F. Environmental steepness, tolerance gradient, and ecogeographical rules in glassfrogs (Anura: Centrolenidae). Biol. J. Linn. Soc. 2013, 108, 773–783. [Google Scholar] [CrossRef]
- Boaratti, A.Z.; Da Silva, F.R. Relationships between environmental gradients and geographic variation in the intraspecific body size of three species of frogs (Anura). Austral Ecol. 2015, 40, 869–876. [Google Scholar] [CrossRef]
- Eaton, B.R.; Paszkowski, C.A.; Kristensen, K.; Hiltz, M. Life-history variation among populations of Canadian toads in Alberta, Canada. Can. J. Zool. 2005, 83, 1421–1430. [Google Scholar] [CrossRef]
- Ma, X.Y.; Tong, L.N.; Lu, X. Variation of body size, age structure and growth of a temperate frog, Rana chensinensis, over an altitudinal gradient in northern China. Amphib. Reptil. 2009, 30, 111–117. [Google Scholar] [CrossRef]
- Matthews, R.K.; Miaud, C. A skeletochronological study of the age structure, growth, and longevity of the mountain yellow-legged frog, Rana muscosa, in the sierra Nevada, California. Copeia 2007, 4, 986–993. [Google Scholar] [CrossRef]
- Cvetković, D.; Tomašević, N.; Ficetola, G.F.; Crnobrnja-Isailović, J.; Miaud, C. Bergmann’s rule in amphibians: Combining demographic and ecological parameters to explain body size variation among populations in the common toad Bufo bufo. J. Zool. Syst. Evol. Res. 2009, 47, 171–180. [Google Scholar] [CrossRef]
- Sinsch, U.; Marangoni, F.; Oromi, N.; Leskovar, C.; Sanuy, D.; Tejedo, M. Proximate mechanisms determining size variability in natterjack toads. Zoology 2010, 281, 272–281. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Maiorano, L. Contrasting effects of temperature and precipitation change on amphibian phenology, abundance and performance. Oecologia 2016, 181, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, X.; Yang, S.; Li, C.; Hu, J. Morphological variation and its environmental correlates in the Taihangshan swelled-vented frog across the Qinling mountains. Animals 2022, 12, 2328. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Hakkinen, J.; Pasanen, S.; Kukkonen, J.V.K. The effects of solar UV-B radiation on embryonic mortality and development in three boreal Anurans (Rana temporaria, Rana arvalis and Bufo bufo). Chemosphere 2001, 44, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Verschooren, E.; Brown, R.K.; Vercammen, F.; Pereboom, J. Ultraviolet B radiation (UV-B) and the growth and skeletal development of the Amazonian milk frog (Trachycephalus resinifictrix) from metamorphsis. J. Physiol. Pathophysiol. 2011, 2, 34–42. [Google Scholar]
- Fei, L.; Ye, C.Y. The Colour Handbook of Amphibians of Sichuan; China Forestry Publishing House: Beijing, China, 2001. [Google Scholar]
- Liao, W.B.; Lu, X. Male mate choice in the Andrew’s toad Bufo andrewsi: A preference for larger females. J. Ethol. 2009, 27, 413–417. [Google Scholar] [CrossRef]
- Liao, W.B.; Lu, X. Sex recognition by male Andrew’s toad Bufo andrewsi in a subtropical montane region. Behav. Proc. 2009, 82, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.B.; Lu, X. Proximate mechanisms leading to large male-mating advantage in the Andrew’s toad Bufo andrewsi. Behaviour 2011, 148, 1087–1102. [Google Scholar]
- Guo, B.C.; Lu, D.; Liao, W.B.; Merilä, J. Genome-wide scan for adaptive differentiation along altitudinal gradient in the Andrew’s toad Bufo andrewsi. Mol. Ecol. 2016, 25, 3884–3900. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, L.; Luan, X.F.; Liao, W.B. Testis size variation and its environmental correlates in Andrew’s toad (Bufo andrewsi). Animals 2022, 12, 3011. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, C.; Jiang, Y.; Zhao, L.; Jin, L. Geographical variation of organ size in Andrew’s toad (Bufo andrewsi). Front. Ecol. Evol. 2022, 10, 972942. [Google Scholar] [CrossRef]
- Zhao, L.; Mai, C.L.; Liu, G.H.; Liao, W.B. Altitudinal implications in organ size in the Andrew’s toad (Bufo andrewsi). Anim. Biol. 2019, 69, 365–376. [Google Scholar] [CrossRef]
- Norris, J.; Tingley, R.; Meiri, S.; Chapple, D.G. Environmental correlates of morphological diversity in Australian geckos. Glob. Ecol. Biogeogr. 2021, 30, 1086–1100. [Google Scholar] [CrossRef]
- Buckley, L.B.; Jetz, W. Environmental and historical constraints on global patterns of amphibian richness. Proc. R. Soc. B 2007, 274, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, M.; Vaclavik, T.; Manceur, A.M.; Sprtova, L.; von Wehrden, H.; Welk, E.; Cord, A.F. glUV: A global UV-B radiation data set for macroecological studies. Methods Ecol. Evol. 2014, 5, 372–383. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Environmental Systems Research Institute (ESRI). ArcGIS Desktop 10.8. Environmental Systems; Environmental Systems Research Institute: Redlands, CA, USA, 2020. [Google Scholar]
- Hu, J.H.; Huang, Y.; Clifton, J.P.; Guisan, A. Genetic diversity in frogs linked to past and future climate changes on the roof of the world. J. Anim. Ecol. 2019, 88, 953–963. [Google Scholar] [CrossRef] [PubMed]
- R Project for Statistical Computing. Available online: https://www.R–project.org/ (accessed on 1 November 2022).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Adolph, S.C.; Porter, W.P. Growth, seasonality and lizard life histories: Age and size at maturity. Oikos 1996, 77, 267–278. [Google Scholar] [CrossRef]
- Angilletta, M.J.; Niewiarowski, H.P.; Dunham, A.E.; Leaché, A.D.; Porter, W.P. Bergmann’s clines in ectotherms: Illustrating a life-history perspective with sceloporine lizards. Am. Nat. 2004, 164, 168–183. [Google Scholar] [CrossRef]
- Gvoždík, V.; Moravec, J.; Kratochvíl, L. Geographic morphological variation in parapatric Western Palearctic tree frogs, Hyla arborea and Hyla savignyi: Are related species similarly affected by climatic conditions? Biol. J. Linn. Soc. 2008, 95, 539–556. [Google Scholar] [CrossRef]
- Yu, X.; Zhong, M.J.; Li, D.Y.; Jin, L.; Liao, W.B.; Kotrschal, A. Large-brained frogs mature later and live longer. Evolution 2018, 72, 1174–1183. [Google Scholar] [CrossRef]
- Lessard, J.P.; Sackett, T.E.; Reynolds, W.N.; Fowler, D.A.; Sanders, N.J. Determinants of the detrital arthropod community structure: The effects of temperature, resources, and environmental gradients. Oikos 2010, 120, 333–343. [Google Scholar] [CrossRef]
- Beck, E.; Kottke, I.; Bendix, J.; Makeschin, F.; Mosandl, R. Gradients in a tropical mountain ecosystem—A synthesis. In Gradients in A Tropical Mountain Ecosystem of Ecuador; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 451–463. [Google Scholar]
- Gliwicz, Z.M.; Guisande, C. Family-planning in Daphnia: Resistance to starvation in offspring born to mothers grown at different food levels. Oecologia 1992, 91, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, N.; Altunısık, A.; Ergül, T.; Gül, S.; Tosunoğlu, M.; Cadeddu, G.; Giacoma, C. Variation in body size and age structure among three Turkish populations of the treefrog Hyla arborea. Amphib. Reptil. 2012, 33, 25–35. [Google Scholar] [CrossRef]
- Reniers, J.; Brendonck, L.; Roberts, J.D.; Verlinden, W.; Vanschoenwinkel, B. Environmental harshness shapes life-history variation in an Australian temporary pool breeding frog: A skeletochronological approach. Oecologia 2015, 178, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Belden, L.K.; Wildy, E.L.; Blaustein, A.R. Growth, survival and behaviour of larval long-toed salamanders (Ambystoma macrodactylum) exposed to ambient levels of UV-B radiation. J. Zool. 2000, 251, 473–479. [Google Scholar] [CrossRef]
- Laugen, A.T.; Laurila, A.; Räsänen, K.; Merilä, J. Latitudinal countergradient variation in the common frog (Rana temporaria) developmental rates–evidence for local adaptation. J. Evol. Biol. 2003, 16, 996–1005. [Google Scholar] [CrossRef]
- Lindgren, B.; Laurila, A. Proximate causes of adaptive growth rates: Growth efficiency variation among latitudinal populations of Rana temporaria. J. Evol. Biol. 2005, 18, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.P.; Biek, R.; Thomas, R.; Mable, B.K. Local adaptation with high gene flow: Temperature parameters drive adaptation to altitude in the common frog (Rana temporaria). Mol. Ecol. 2014, 23, 561–574. [Google Scholar] [CrossRef]
| Source | Random | Fixed | |||||
|---|---|---|---|---|---|---|---|
| VAR | SD | p | Estimate | df | F | p | |
| Age at sexual maturity | |||||||
| Population | 0.010 | 0.099 | 0.203 | ||||
| Residual | 0.018 | 0.134 | |||||
| Altitude | 0.192 | 11.025 | 0.182 | 0.678 | |||
| Latitude | 1.752 | 11.149 | 2.009 | 0.184 | |||
| Sex | 0.196 | 12.994 | 14.965 | 0.002 | |||
| Longevity | |||||||
| Population | 0.005 | 0.073 | 0.044 | ||||
| Residual | 0.005 | 0.069 | |||||
| Altitude | −0.018 | 11.003 | 0.004 | 0.952 | |||
| Latitude | 1.643 | 11.196 | 4.297 | 0.062 | |||
| Sex | 0.018 | 12.954 | 0.476 | 0.503 | |||
| Mean age | |||||||
| Population | 0.007 | 0.082 | 0.029 | ||||
| Residual | 0.005 | 0.072 | |||||
| Altitude | 0.134 | 11.046 | 0.178 | 0.682 | |||
| Latitude | 1.320 | 11.260 | 2.284 | 0.158 | |||
| Sex | 0.068 | 12.991 | 6.227 | 0.027 | |||
| Body size | |||||||
| Population | 0.001 | 0.033 | |||||
| Residual | 0.001 | 0.025 | |||||
| Sex | 0.085 | 13.000 | 79.056 | <0.001 | |||
| Body size | |||||||
| Mean age: Population | <0.001 | 0.018 | 1.000 | ||||
| Population | 0.001 | 0.034 | 0.015 | ||||
| Residual | <0.001 | 0.018 | |||||
| Sex | 0.059 | 13.598 | 1.455 | 0.248 | |||
| Mean age | −0.093 | 18.793 | 0.164 | 0.690 | |||
| Mean age: Sex | 0.061 | 14.565 | 0.275 | 0.608 | |||
| Body size | |||||||
| Population | 0.001 | 0.035 | 0.009 | ||||
| Residual | 0.001 | 0.025 | |||||
| Altitude | 0.091 | 11.000 | 0.499 | 0.495 | |||
| Latitude | 0.074 | 11.286 | 0.044 | 0.838 | |||
| Sex | 0.085 | 12.928 | 78.151 | <0.001 | |||
| Variable | β | SE | t | p | |
|---|---|---|---|---|---|
| Age at sexual maturity | Annual mean temperature | −0.236 | 0.089 | −2.644 | 0.011 |
| Temperature seasonality | 0.707 | 0.354 | 1.998 | 0.050 | |
| Sex | 0.225 | 0.041 | 5.458 | <0.001 | |
| Age at sexual maturity | Annual precipitation | −0.909 | 0.651 | −1.397 | 0.168 |
| Precipitation of the driest month | −0.078 | 0.175 | −0.445 | 0.658 | |
| Precipitation seasonality | −0.327 | 0.555 | −0.589 | 0.558 | |
| Sex | 0.225 | 0.042 | 5.387 | <0.001 | |
| Age at sexual maturity | UV-B seasonality | 0.823 | 0.297 | 2.769 | 0.008 |
| Mean UV-B of the lowest month | −0.643 | 0.288 | −2.232 | 0.029 | |
| Sex | 0.225 | 0.043 | 5.285 | <0.001 | |
| Longevity | Annual mean temperature | −0.240 | 0.094 | −2.548 | 0.014 |
| Temperature seasonality | 0.575 | 0.373 | 1.542 | 0.128 | |
| Sex | 0.043 | 0.043 | 0.991 | 0.326 | |
| Longevity | Annual precipitation | −0.592 | 0.559 | −1.060 | 0.294 |
| Precipitation of the driest month | −0.018 | 0.150 | −0.118 | 0.906 | |
| Precipitation seasonality | −1.618 | 0.476 | −3.396 | 0.001 | |
| Sex | 0.043 | 0.036 | 1.202 | 0.234 | |
| Longevity | UV-B seasonality | 0.928 | 0.301 | 3.081 | 0.003 |
| Mean UV-B of the lowest month | −0.558 | 0.292 | −1.910 | 0.061 | |
| Sex | 0.043 | 0.043 | 0.998 | 0.322 | |
| Mean age | Annual mean temperature | −0.247 | 0.083 | −2.983 | 0.004 |
| Temperature seasonality | 0.429 | 0.329 | 1.306 | 0.197 | |
| Sex | 0.100 | 0.038 | 2.599 | 0.012 | |
| Mean age | Annual precipitation | −0.136 | 0.517 | −0.263 | 0.794 |
| Precipitation of the driest month | −0.140 | 0.139 | −1.012 | 0.316 | |
| Precipitation seasonality | −1.658 | 0.440 | −3.766 | <0.001 | |
| Sex | 0.100 | 0.033 | 3.006 | 0.004 | |
| Mean age | UV-B seasonality | 0.786 | 0.271 | 2.902 | 0.005 |
| Mean UV-B of the lowest month | −0.452 | 0.263 | −1.721 | 0.091 | |
| Sex | 0.100 | 0.039 | 2.567 | 0.013 | |
| Body size | Annual mean temperature | 0.015 | 0.025 | 0.607 | 0.546 |
| Temperature seasonality | 0.029 | 0.094 | 0.306 | 0.761 | |
| Sex | 0.085 | 0.011 | 7.477 | <0.001 | |
| Mean age | 0.053 | 0.037 | 1.450 | 0.152 | |
| Body size | Annual precipitation | −0.259 | 0.146 | −1.778 | 0.081 |
| Precipitation of the driest month | 0.092 | 0.039 | 2.339 | 0.023 | |
| Precipitation seasonality | −0.094 | 0.139 | −0.680 | 0.500 | |
| Sex | 0.092 | 0.010 | 9.149 | <0.001 | |
| Mean age | −0.019 | 0.037 | −0.499 | 0.620 | |
| Body size | UV-B seasonality | 0.191 | 0.076 | 2.506 | 0.015 |
| Mean UV-B of the lowest month | −0.126 | 0.071 | −1.775 | 0.081 | |
| Sex | 0.089 | 0.011 | 8.238 | <0.001 | |
| Mean age | 0.014 | 0.035 | 0.404 | 0.688 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Zhao, L.; Luan, X.; Liao, W. Geographical Variation in Body Size and the Bergmann’s Rule in Andrew’s Toad (Bufo andrewsi). Biology 2022, 11, 1766. https://doi.org/10.3390/biology11121766
Jiang Y, Zhao L, Luan X, Liao W. Geographical Variation in Body Size and the Bergmann’s Rule in Andrew’s Toad (Bufo andrewsi). Biology. 2022; 11(12):1766. https://doi.org/10.3390/biology11121766
Chicago/Turabian StyleJiang, Ying, Li Zhao, Xiaofeng Luan, and Wenbo Liao. 2022. "Geographical Variation in Body Size and the Bergmann’s Rule in Andrew’s Toad (Bufo andrewsi)" Biology 11, no. 12: 1766. https://doi.org/10.3390/biology11121766
APA StyleJiang, Y., Zhao, L., Luan, X., & Liao, W. (2022). Geographical Variation in Body Size and the Bergmann’s Rule in Andrew’s Toad (Bufo andrewsi). Biology, 11(12), 1766. https://doi.org/10.3390/biology11121766





