MaCts1, an Endochitinase, Is Involved in Conidial Germination, Conidial Yield, Stress Tolerances and Microcycle Conidiation in Metarhizium acridum
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Gene Deletion and Complementation
2.3. Phenotypic Analyses
2.4. Fungal Pathogenicity Assays
2.5. Microscopic Observation of the Conidiation
2.6. Data Analysis
3. Results
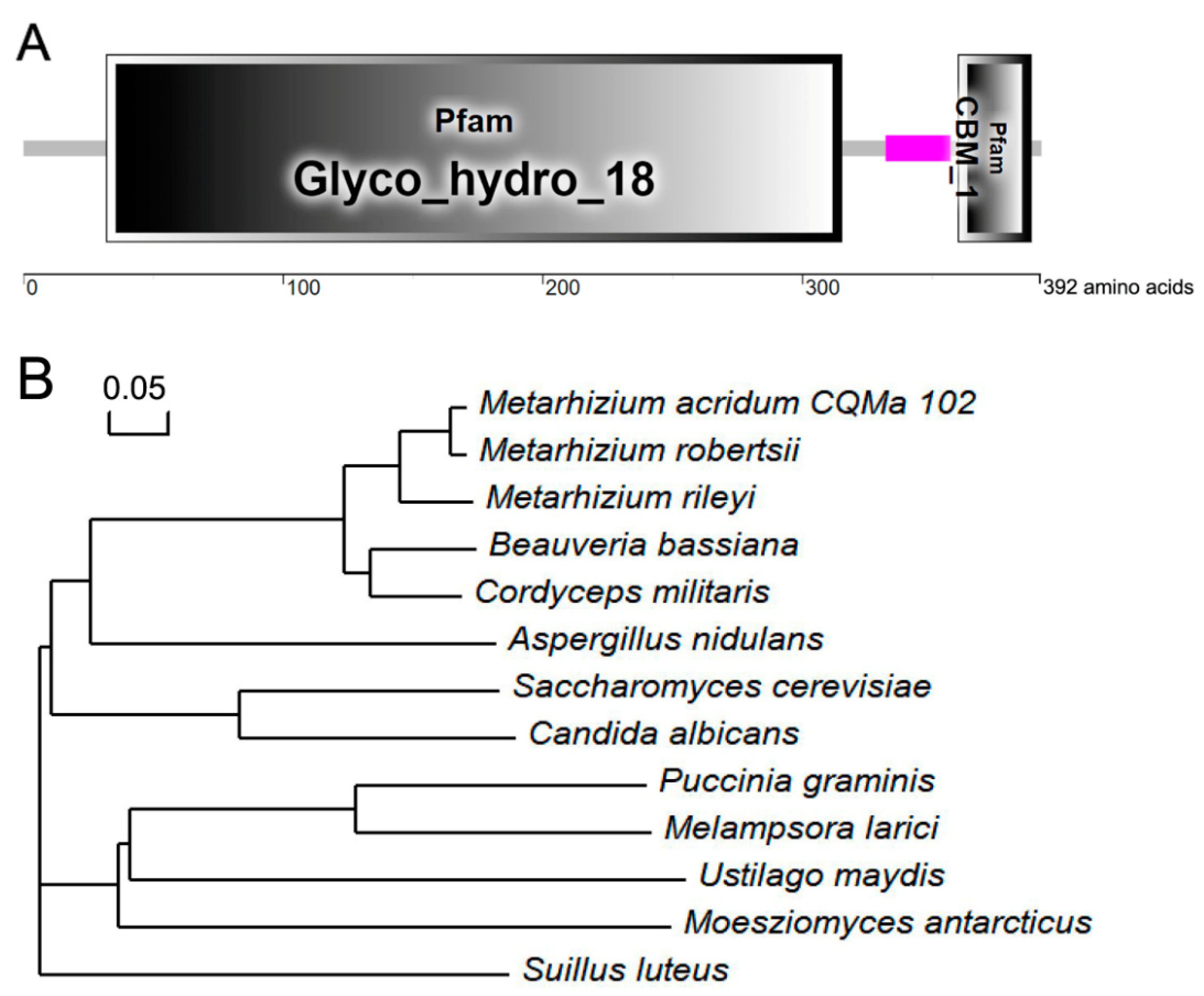
3.1. Features of MaCts1
3.2. MaCts1 Is Involved in Conidial Germination and Influences Conidial Yield but Not Virulence
3.3. Disruption of MaCts1 Impairs Fungal Resistances to UV-B Irradiation and Heat-Shock
3.4. MaCts1 Makes Contributions to the Microcycle Conidiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- St Leger, R.J.; Joshi, L.; Bidochka, M.J.; Roberts, D.W. Construction of an improved mycoinsecticide overexpressing a toxic protease. Proc. Natl. Acad. Sci. USA 1996, 93, 6349–6354. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; St Leger, R.J.; Wang, C. Advances in genomics of entomopathogenic fungi. Adv. Genet. 2016, 94, 67–105. [Google Scholar] [PubMed]
- Holder, D.J.; Keyhani, N.O. Adhesion of the entomopathogenic fungus Beauveria (cordyceps) bassiana to substrata. Appl. Environ. Microbiol. 2005, 71, 5260–5266. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.M.; Charnley, A.K. New insights into the mechanisms of fungal pathogenesis in insect. Trends Microbiol. 1996, 4, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.M.; Feng, M.G. Phenotypic and molecular insights into heat tolerance of formulated cells as active ingredients of fungal insecticides. Appl. Microbiol. Biotechnol. 2020, 104, 5711–5724. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004, 22, 189–259. [Google Scholar] [CrossRef]
- Moore, D.; Bateman, R.P.; Carey, M.; Prior, C. Long-term storage of Metarhizium flavoviride conidia in oil formulations for the control of locusts and grasshoppers. Biocontrol Sci. Technol. 1995, 5, 193–200. [Google Scholar] [CrossRef]
- Hanlin, R.T. Microcycle conidiation—A review. Mycoscience 1994, 35, 113–123. [Google Scholar] [CrossRef]
- Jung, B.; Kim, S.; Lee, J. Microcyle conidiation in filamentous fungi. Mycobiology 2014, 42, 1–5. [Google Scholar] [CrossRef]
- Anderson, J.G.; Smith, J.E. The production of conidiophores and conidia by newly germinated conidia of Aspergillus niger (microcycle conidiation). J. Gen. Microbiol. 1971, 69, 185–197. [Google Scholar] [CrossRef]
- Park, H.; Yu, J. Genetic control of asexual sporulation in filamentous fungi. Curr. Opin. Microbiol. 2012, 15, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.; Yantorno, O. Microcycle conidiation in the entomopathogenic fungus Beauveria bassiana bals (Vuill). Process Biochem. 1999, 34, 707–716. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, G.; Xia, Y. Microcycle conidiation and the conidial properties in the entomopathogenic fungus Metarhizium acridum on agar medium. Biocontrol Sci. Techn. 2010, 20, 809–819. [Google Scholar] [CrossRef]
- Adams, D.J. Fungal cell wall chitinases and glucanases. Microbiology 2004, 150, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.; Chelius, C.; Riekhof, W.; Marten, M.R.; Harris, S.D. Micafungin induced cell wall damage stimulates morphlogical changes consistent with microcycle conidiation in Aspergillus nidulans. J. Fungi 2021, 7, 525. [Google Scholar] [CrossRef]
- 16. Cabib, E.; Arroyo, J. How carbohydrates sculpt cells: Chemical control of morphogenesis in the yeast cell wall. Nat. Rev. Microbiol. 2013, 11, 648–655. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Du, Y.; Keyhani, N.O.; Xia, Y.; Jin, K. Members of chitin synthase family in Metarhizium acridum differentially affect fungal growth, stress tolerances, cell wall integrity and virulence. PLoS Pathog. 2019, 15, e1007964. [Google Scholar] [CrossRef]
- Gokul, B.; Lee, J.H.; Song, K.B.; Rhee, S.K.; Kim, C.H.; Panda, T. Characterization and applications of chitinases from Trichoderma harzianum—A review. Bioprocess Eng. 2000, 23, 691–694. [Google Scholar] [CrossRef]
- Roncero, C.; Vázquez de Aldana, C.R. Glucanases and chitinases. Curr. Top. Microbiol. Immunol. 2020, 425, 131–166. [Google Scholar]
- Courtade, G.; Aachmann, F.L. Chitin-Active Lytic Polysaccharide Monooxygenases. Adv. Exp. Med. Biol. 2019, 1142, 253–272. [Google Scholar]
- Reindl, M.; Stock, J.; Hussnaetter, K.P.; Genc, A.; Brachmann, A.; Schipper, K. A novel factor essential for unconventional secretion of chitinase Cts1. Front. Microbiol. 2020, 11, 1529. [Google Scholar] [CrossRef] [PubMed]
- Kuranda, M.J.; Robbins, P.W. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J. Biol. Chem. 1991, 266, 19758–19767. [Google Scholar] [CrossRef] [PubMed]
- King, L.; Butler, G. Ace2p, a regulator of CTS1 (chitinase) expression, affects pseudohyphal production in Saccharomyces cerevisiae. Curr. Genet. 1998, 34, 183–191. [Google Scholar] [CrossRef]
- Takaya, N.; Yamazaki, D.; Horiuchi, H.; Ohta, A.; Takagi, M. Cloning and characterization of a chitinase-encoding gene (chiA) from Aspergillus nidulans, disruption of which decreases germination frequency and hyphal growth. Biosci. Biotechnol. Biochem. 1998, 62, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Dünkler, A.; Walther, A.; Specht, C.A.; Wendland, J. Candida albicans CHT3 encodes the functional homolog of the Cts1 chitinase of Saccharomyces cerevisiae. Fungal Genet. Biol. 2005, 42, 935–947. [Google Scholar] [CrossRef]
- Maerz, S.; Seiler, S. Tales of RAM and MOR: NDR kinase signaling in fungal morphogenesis. Curr. Opin. Microbiol. 2010, 13, 663–671. [Google Scholar] [CrossRef]
- Jorgensen, P.; Nelson, B.; Robinson, M.D.; Chen, Y.; Andrews, B.; Tyers, M.; Boone, C. High-resolution genetic mapping with ordered arrays of Saccharomyces cerevisiae deletion mutants. Genetics 2002, 162, 1091–1099. [Google Scholar] [CrossRef]
- Nelson, B.; Kurischko, C.; Horecka, J.; Mody, M.; Nair, P.; Pratt, L.; Zougman, A.; Mc Broom, L.D.B.; Hughes, T.R.; Boone, C.; et al. RAM: A conserved signaling network that regulates Ace2p transcriptional activity and polarized morphogenesis. Mol. Biol. Cell 2003, 14, 3782–3803. [Google Scholar] [CrossRef]
- Saputo, S.; Chabrier-Rosello, Y.; Luca, F.C.; Kumar, A.; Krysan, D.J. The RAM network in pathogenic fungi. Eukaryot. Cell 2012, 11, 708–717. [Google Scholar] [CrossRef]
- Racki, W.J.; Bécam, A.; Nasr, F.; Herbert, C.J. Cbk1p, a protein similar to the human myotonic dystrophy kinase, is essential for normal morphogenesis in Saccharomyces cerevisiae. EMBO J. 2000, 19, 4524–4532. [Google Scholar] [CrossRef]
- Butler, G.; Thiele, D.J. ACE2, an activator of yeast metallothionein expression which is homologous to SWI5. Mol. Cell. Biol. 1991, 11, 476–485. [Google Scholar] [PubMed]
- Jin, K.; Ming, Y.; Xia, Y.X. MaHog1, a Hog1-type mitogen-activated protein kinase gene, contributes to stress tolerance and virulence of the entomopathogenic fungus Metarhizium acridum. Microbiology 2012, 158, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.R.; Jin, K.; Xia, Y.X. Involvement of masom1, a downstream transcriptional factor of cAMP/PKA pathway, in conidial yield, stress tolerances, and virulence in Metarhizium acridum. Appl. Microbiol. Biotechnol. 2018, 102, 5611–5623. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Q.; Tian, H.T.; Xia, Y.; Jin, K. MaPmt1, a protein O-mannosyltransferase, contributes to virulence through governing the appressorium turgor pressure in Metarhizium acridum. Fungal Genet. Biol. 2020, 145, 103480. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Q.; Xia, Y.X.; Jin, K. MaSln1, a conserved histidine protein kinase, contributes to conidiation pattern shift independent of the MAPK pathway in Metarhizium acridum. Microbiol. Spectr. 2022, 10, e0205121. [Google Scholar] [CrossRef]
- Li, C.; Xia, Y.; Jin, K. N-terminal zinc fingers of MaNCP1 contribute to growth, stress tolerance, and virulence in Metarhizium acridum. Int. J. Biol. Macromol. 2022, 216, 426–436. [Google Scholar] [CrossRef]
- Wen, Z.Q.; Fan, Y.; Xia, Y.; Jin, K. MaOpy2, a transmembrane protein, is involved in stress tolerances and pathogenicity and negatively regulates conidial yield by shifting the conidiation pattern in Metarhizium acridum. J. Fungi 2022, 8, 587. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Peng, G.X.; Xie, J.Q.; Guo, R.; Keyhani, N.O.; Zeng, D.Y.; Yang, P.Y.; Xia, Y.X. Long-term field evaluation and large-scale application of a Metarhizium anisopliae strain for controlling major rice pests. J. Pest. Sci. 2021, 94, 969–980. [Google Scholar] [CrossRef]
- Selvaggini, S.; Munro, C.A.; Paschoud, S.; Sanglard, D.; Gow, N.A. Independent regulation of chitin synthase and chitinase activity in Candida albicans and Saccharomyces cerevisiae. Microbiology 2004, 150, 921–928. [Google Scholar] [CrossRef]
- Werner, S.; Sugui, J.A.; Steinberg, G.; Deising, H.B. A chitin synthase with a myosin-like motor domain is essential for hyphal growth, appressorium differentiation, and pathogenicity of the maize anthracnose fungus Colletotrichum graminicola. Mol. Plant Microbe Interact. 2007, 20, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R.; Latgé, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Keyhani, N.O. Lipid biology in fungal stress and virulence: Entomopathogenic fungi. Fungal Biol. 2018, 122, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Millet, N.; Latgé, J.; Mouyna, I. Members of glycosyl-hydrolase family 17 of A. fumigatus differentially affect morphogenesis. J. Fungi 2018, 4, 18. [Google Scholar] [CrossRef]
- Harman, G.E.; Hayes, C.K.; Lorito, M.; Broadway, R.M.; Di Pietro, A.; Peterbauer, C.; Tronsmo, A. Chitinolytic enzymes of Trichoderma harzianum: Purification of chitobiosidase and endochitinase. Phytopathology 1993, 83, 313–318. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Liu, K.; Lu, J. Purification and partial characterization of a chitinase from the mycoparasitic fungus Trichothecium roseum. J. Gen. Appl. Microbiol. 2004, 50, 35–39. [Google Scholar] [CrossRef][Green Version]
- Duo-Chuan, L.I.; Shu, C.; Jing, L.U. Purification and partial characterization of two chitinases from the mycoparasitic fungus Talaromyces flavus. Mycopathologia 2005, 159, 223–229. [Google Scholar] [CrossRef]
- Mathivanan, N.; Kabilan, V.; Murugesan, K. Purification, characterization, and antifungal activity of chitinase from Fusarium chlamydosporum, a mycoparasite to groundnut rust, Puccinia arachidis. Can. J. Microbiol. 1998, 44, 646–651. [Google Scholar] [CrossRef]
- Kozome, D.; Uechi, K.; Taira, T.; Fukada, H.; Kubota, T.; Ishikawa, K. Structural analysis and construction of a thermostable antifungal chitinase. Appl. Environ. Microbiol. 2022, 88, e0065222. [Google Scholar] [CrossRef]
- Monica, Z.; Maura, B.; Alessandro, N.; Klaus, P.; Luca, M.; Piero, M.; Alberto, N.; Benedettoet, R. Identification of the Arabidopsis RAM/MOR signaling network: Adding new regulatory players in plant stem cell maintenance and cell polarization. Ann. Bot. 2015, 1, 69. [Google Scholar]
- Sartorel, E.; Perez-Martin, J. The distinct interaction between cell cycle regulation and the widely conserved morphogenesis-related (MOR) pathway in the fungus Ustilago maydis determines morphology. J. Cell Sci. 2012, 125, 4597–4608. [Google Scholar] [PubMed]
- O’Conalláin, C.; Doolin, M.; Taggart, C.; Thornton, F.; Butler, G. Regulated nuclear localisation of the yeast transcription factor Ace2p controls expression of chitinase (CTS1) in Saccharomyces cerevisiae. Mol. Gen. Genet. 1999, 262, 275–282. [Google Scholar] [CrossRef]
- Voth, W.P.; Olsen, A.E.; Sbia, M.; Freedman, K.H.; Stillman, D.J. ACE2, CBK1, and BUD4 in budding and cell separation. Eukaryot. Cell 2005, 4, 1018–1028. [Google Scholar] [CrossRef]
- Lopez-Mirabal, H.R.; Winther, J.R.; Thorsen, M.; Kielland-Brandt, M.C. Mutations in the RAM network confer resistance to the thiol oxidant 4,4’-dipyridyl disulfide. Mol. Genet. Genom. 2008, 279, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Bidlingmaier, S.; Weiss, E.L.; Seidel, C.; Drubin, D.G.; Snyder, M. The cbk1p pathway is important for polarized cell growth and cell separation in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001, 21, 2449–2462. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Li, C.; Wang, S.; Xia, Y.; Jin, K. MaCts1, an Endochitinase, Is Involved in Conidial Germination, Conidial Yield, Stress Tolerances and Microcycle Conidiation in Metarhizium acridum. Biology 2022, 11, 1730. https://doi.org/10.3390/biology11121730
Zou Y, Li C, Wang S, Xia Y, Jin K. MaCts1, an Endochitinase, Is Involved in Conidial Germination, Conidial Yield, Stress Tolerances and Microcycle Conidiation in Metarhizium acridum. Biology. 2022; 11(12):1730. https://doi.org/10.3390/biology11121730
Chicago/Turabian StyleZou, Yuneng, Chan Li, Shuqin Wang, Yuxian Xia, and Kai Jin. 2022. "MaCts1, an Endochitinase, Is Involved in Conidial Germination, Conidial Yield, Stress Tolerances and Microcycle Conidiation in Metarhizium acridum" Biology 11, no. 12: 1730. https://doi.org/10.3390/biology11121730
APA StyleZou, Y., Li, C., Wang, S., Xia, Y., & Jin, K. (2022). MaCts1, an Endochitinase, Is Involved in Conidial Germination, Conidial Yield, Stress Tolerances and Microcycle Conidiation in Metarhizium acridum. Biology, 11(12), 1730. https://doi.org/10.3390/biology11121730





