Bioaccumulation of Trace Elements from Aqueous Solutions by Selected Terrestrial Moss Species
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Devices and Reagents
2.2. Quality Control
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahapatra, B.; Dhal, N.K.; Dash, A.K.; Panda, B.P.; Panigrahi, K.C.S.; Pradhan, A. Perspective of Mitigating Atmospheric Heavy Metal Pollution: Using Mosses as Biomonitoring and Indicator Organism. Environ. Sci. Pollut. Res. 2019, 26, 29620–29638. [Google Scholar] [CrossRef] [PubMed]
- Abas, A. A Systematic Review on Biomonitoring Using Lichen as the Biological Indicator: A Decade of Practices, Progress and Challenges. Ecol. Indic. 2021, 121, 107197. [Google Scholar] [CrossRef]
- Winkler, A.; Contardo, T.; Lapenta, V.; Sgamellotti, A.; Loppi, S. Assessing the Impact of Vehicular Particulate Matter on Cultural Heritage by Magnetic Biomonitoring at Villa Farnesina in Rome, Italy. Sci. Total Environ. 2022, 823, 153729. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, M.C.; Capozzi, F.; Wuyts, K.; Joosen, S.; Mubiana, V.K.; Giordano, S.; Samson, R.; Spagnuolo, V. Mobile Biomonitoring of Atmospheric Pollution: A New Perspective for the Moss-Bag Approach. Plants 2021, 10, 2384. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, V.; Giordano, S.; Pérez-Llamazares, A.; Ares, A.; Carballeira, A.; Fernández, J.A.; Aboal, J.R. Distinguishing Metal Bioconcentration from Particulate Matter in Moss Tissue: Testing Methods of Removing Particles Attached to the Moss Surface. Sci. Total Environ. 2013, 463–464, 727–733. [Google Scholar] [CrossRef]
- Tretiach, M.; Pittao, E.; Crisafulli, P.; Adamo, P. Influence of Exposure Sites on Trace Element Enrichment in Moss-Bags and Characterization of Particles Deposited on the Biomonitor Surface. Sci. Total Environ. 2011, 409, 822–830. [Google Scholar] [CrossRef]
- Kılıç, Ö.; Belivermiş, M.; Sıkdokur, E.; Sezer, N.; Akyıl Erentürk, S.; Haciyakupoglu, S.; Madadzada, A.; Frontasyeva, M. Assessment of 210Po and 210Pb by Moss Biomonitoring Technique in Thrace Region of Turkey. J. Radioanal. Nucl. Chem. 2019, 322, 699–706. [Google Scholar] [CrossRef]
- Di Palma, A.; Capozzi, F.; Spagnuolo, V.; Giordano, S.; Adamo, P. Atmospheric Particulate Matter Intercepted by Moss-Bags: Relations to Moss Trace Element Uptake and Land Use. Chemosphere 2017, 176, 361–368. [Google Scholar] [CrossRef]
- Spagnuolo, V.; Zampella, M.; Giordano, S.; Adamo, P. Cytological Stress and Element Uptake in Moss and Lichen Exposed in Bags in Urban Area. Ecotoxicol. Environ. Saf. 2011, 74, 1434–1443. [Google Scholar] [CrossRef]
- Branquinho, C.; Catarino, F.; Brown, D.H.; Pereira, M.J.; Soares, A. Improving the Use of Lichens as Biomonitors of Atmospheric Metal Pollution. Sci. Total Environ. 1999, 232, 67–77. [Google Scholar] [CrossRef]
- Aboal, J.R.; Pérez-Llamazares, A.; Carballeira, A.; Giordano, S.; Fernández, J.A. Should Moss Samples Used as Biomonitors of Atmospheric Contamination Be Washed? Atmos. Environ. 2011, 45, 6837–6840. [Google Scholar] [CrossRef]
- Bustamante, J.; Liñero, O.; Arrizabalaga, I.; Carrero, J.A.; Arana, G.; Diego, A. De Sample Pretreatment to Differentiate between Bioconcentration and Atmospheric Deposition of Polycyclic Aromatic Hydrocarbons in Mosses. Chemosphere 2015, 122, 295–300. [Google Scholar] [CrossRef]
- Byun, M.Y.; Kim, D.; Youn, U.J.; Lee, S.; Lee, H. Improvement of Moss Photosynthesis by Humic Acids from Antarctic Tundra Soil. Plant Physiol. Biochem. 2021, 159, 37–42. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Aprile, G.; Conte, B.; Castaldo Cobianchi, R. Comparison of the Heavy Metal Bioaccumulation Capacity of an Epiphytic Moss and an Epiphytic Lichen. Environ. Pollut. 2008, 151, 401–407. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhang, P. Micro-Morphology, Ultrastructure and Chemical Composition Changes of Bryum Argenteum from a Desert Biological Soil Crust Following One-Year Desiccation. Bryologist 2014, 117, 232–240. [Google Scholar] [CrossRef]
- Di Palma, A.; González, A.G.; Adamo, P.; Giordano, S.; Reski, R.; Pokrovsky, O.S. Biosurface Properties and Lead Adsorption in a Clone of Sphagnum Palustre (Mosses): Towards a Unified Protocol of Biomonitoring of Airborne Heavy Metal Pollution. Chemosphere 2019, 236, 124375. [Google Scholar] [CrossRef]
- Fortuna, L.; González, A.G.; Tretiach, M.; Pokrovsky, O.S. Influence of Secondary Metabolites on Surface Chemistry and Metal Adsorption of a Devitalized Lichen Biomonitor. Environ. Pollut. 2021, 273, 116500. [Google Scholar] [CrossRef]
- Capozzi, F.; Sorrentino, M.C.; Di Palma, A.; Mele, F.; Arena, C.; Adamo, P.; Spagnuolo, V.; Giordano, S. Implication of Vitality, Seasonality and Specific Leaf Area on PAH Uptake in Moss and Lichen Transplanted in Bags. Ecol. Indic. 2020, 108, 105727. [Google Scholar] [CrossRef]
- Chen, Y.E.; Cui, J.M.; Yang, J.C.; Zhang, Z.W.; Yuan, M.; Song, C.; Yang, H.; Liu, H.M.; Wang, C.Q.; Zhang, H.Y.; et al. Biomonitoring Heavy Metal Contaminations by Moss Visible Parameters. J. Hazard. Mater. 2015, 296, 201–209. [Google Scholar] [CrossRef]
- Świsłowski, P.; Nowak, A.; Rajfur, M. Is Your Moss Alive during Active Biomonitoring Study? Plants 2021, 10, 2389. [Google Scholar] [CrossRef]
- Boquete, M.T.; Aboal, J.R.; Carballeira, A.; Fernández, J.A. Do Mosses Exist Outside of Europe? A Biomonitoring Reflection. Sci. Total Environ. 2017, 593–594, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Boquete, M.T.; Bermúdez-Crespo, J.; Aboal, J.R.; Carballeira, A.; Fernández, J.Á. Assessing the Effects of Heavy Metal Contamination on the Proteome of the Moss Pseudoscleropodium Purum Cross-Transplanted between Different Areas. Environ. Sci. Pollut. Res. 2014, 21, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Elvira, N.J.; Medina, N.G.; Leo, M.; Cala, V.; Estébanez, B. Copper Content and Resistance Mechanisms in the Terrestrial Moss Ptychostomum Capillare: A Case Study in an Abandoned Copper Mine in Central Spain. Arch. Environ. Contam. Toxicol. 2020, 79, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bellini, E.; Betti, C.; Toppi, L.S. Di Responses to Cadmium in Early-Diverging Streptophytes (Charophytes and Bryophytes): Current Views and Potential Applications. Plants 2021, 10, 770. [Google Scholar] [CrossRef]
- ICP Vegetation. Heavy Metals, Nitrogen and POPs in European Mosses: 2020 Survey; 2020. Available online: https://icpvegetation.ceh.ac.uk/sites/default/files/ICP%20Vegetation%20moss%20monitoring%20manual%202020.pdf (accessed on 22 October 2022).
- Świsłowski, P.; Kosior, G.; Rajfur, M. The Influence of Preparation Methodology on the Concentrations of Heavy Metals in Pleurozium Schreberi Moss Samples Prior to Use in Active Biomonitoring Studies. Environ. Sci. Pollut. Res. 2021, 28, 10068–10076. [Google Scholar] [CrossRef]
- Sari, A.; Mendil, D.; Tuzen, M.; Soylak, M. Biosorption of Cd(II) and Cr(III) from Aqueous Solution by Moss (Hylocomium Splendens) Biomass: Equilibrium, Kinetic and Thermodynamic Studies. Chem. Eng. J. 2008, 144, 1–9. [Google Scholar] [CrossRef]
- Henriques, F.S. Leaf Chlorophyll Fluorescence: Background and Fundamentals for Plant Biologists. Bot. Rev. 2009, 75, 249–270. [Google Scholar] [CrossRef]
- Baker, N.R.; Harbinson, J.; Kramer, D.M. Determining the Limitations and Regulation of Photosynthetic Energy Transduction in Leaves. Plant Cell Environ. 2007, 30, 1107–1125. [Google Scholar] [CrossRef]
- Šraj Kržič, N.; Gaberščik, A. Photochemical Efficiency of Amphibious Plants in an Intermittent Lake. Aquat. Bot. 2005, 83, 281–288. [Google Scholar] [CrossRef]
- Laisk, A.; Oja, V.; Eichelmann, H.; Dall’Osto, L. Action Spectra of Photosystems II and i and Quantum Yield of Photosynthesis in Leaves in State 1. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 315–325. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific Inc. iCE 3000 Series AA Spectrometers Operator’s Manual; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2011; Available online: http://photos.labwrench.com/equipmentManuals/9291-6306.pdf (accessed on 22 October 2022).
- Tien, D.P.T.; Khiem, L.H.; Trinh, T.T.T.; Frontasyeva, M.V.; Sang, N.T.M.; Nguyen, S.A. Comparing Atmospheric Trace Element Accumulation of Three Moss Species. Sci. Technol. Dev. J. 2020, 23, 752–757. [Google Scholar] [CrossRef]
- Kłos, A.; Rajfur, M.; Šrámek, I.; Wacławek, M. Use of Lichen and Moss in Assessment of Forest Contamination with Heavy Metals in Praded and Glacensis Euroregions (Poland and Czech Republic). Water. Air. Soil Pollut. 2011, 222, 367–376. [Google Scholar] [CrossRef]
- BROWN, D.H.; BATES, J.W. Bryophytes and Nutrient Cycling. Bot. J. Linn. Soc. 1990, 104, 129–147. [Google Scholar] [CrossRef]
- Kłos, A.; Gordzielik, E.; Jówiak, M.A.; Rajfur, M. Sorption of Cadmium and Zinc in Selected Species of Epigeic Mosses. Bull. Environ. Contam. Toxicol. 2014, 92, 323–328. [Google Scholar] [CrossRef]
- Kłos, A.; Rajfur, M. Influence of Hydrogen Cations on Kinetics and Equilibria of Heavy-Metal Sorption by Algae-Sorption of Copper Cations by the Alga Palmaria Palmata (Linnaeus) Weber & Mohr (Rhodophyta). J. Appl. Phycol. 2013, 25, 1387–1394. [Google Scholar] [CrossRef]
- Stanković, J.D.; Sabovljević, A.D.; Sabovljević, M.S. Bryophytes and Heavy Metals: A Review. Acta Bot. Croat. 2018, 77, 109–118. [Google Scholar] [CrossRef]
- Ćosić, M.; Vujičić, M.M.; Sabovljević, M.S.; Sabovljević, A.D. What Do We Know about Salt Stress in Bryophytes? Plant Biosyst. 2019, 153, 478–489. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.; Bernardes da Silva, A.; Branquinho, C.; Marques da Silva, J. Influence of Dehydration Rate on Cell Sucrose and Water Relations Parameters in an Inducible Desiccation Tolerant Aquatic Bryophyte. Environ. Exp. Bot. 2015, 120, 18–22. [Google Scholar] [CrossRef]
- Koster, K.L.; Balsamo, R.A.; Espinoza, C.; Oliver, M.J. Desiccation Sensitivity and Tolerance in the Moss Physcomitrella Patens: Assessing Limits and Damage. Plant Growth Regul. 2010, 62, 293–302. [Google Scholar] [CrossRef]
- Debén, S.; Fernández, J.A.; Carballeira, A.; Aboal, J.R. Using Devitalized Moss for Active Biomonitoring of Water Pollution. Environ. Pollut. 2016, 210, 315–322. [Google Scholar] [CrossRef]
- Printarakul, N.; Meeinkuirt, W. The Bryophyte Community as Bioindicator of Heavy Metals in a Waterfall Outflow. Sci. Rep. 2022, 12, 6942. [Google Scholar] [CrossRef] [PubMed]
- Kłos, A.; Rajfur, M.; Wacławek, M.; Wacławek, W. Ion Equilibrium in Lichen Surrounding. Bioelectrochemistry 2005, 66, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Fasani, E.; Li, M.; Varotto, C.; Furini, A.; Dalcorso, G. Metal Detoxification in Land Plants: From Bryophytes to Vascular Plants. STATE of the Art and Opportunities. Plants 2022, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.P.; Lewis, A.T.; Lewis, P.D. Prediction of Glycoprotein Secondary Structure Using ATR-FTIR. Vib. Spectrosc. 2013, 69, 21–29. [Google Scholar] [CrossRef]
- Maksimova, V.; Klavina, L.; Bikovens, O.; Zicmanis, A.; Purmalis, O. Structural Characterization and Chemical Classification of Some Bryophytes Found in Latvia. Chem. Biodivers. 2013, 10, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Jin, W.-Y.; Cheng, C.-G. Classification of Five Kinds of Moss Plants with the Use of Fourier Transform Infrared Spectroscopy and Chemometrics. Spectroscopy 2011, 25, 271–285. [Google Scholar] [CrossRef]
- Silvestri, D.; Wacławek, S.; Sobel, B.; Torres–Mendieta, R.; Pawlyta, M.; Padil, V.V.T.; Filip, J.; Černík, M. Modification of NZVI with a Bio-Conjugate Containing Amine and Carbonyl Functional Groups for Catalytic Activation of Persulfate. Sep. Purif. Technol. 2021, 257, 117880. [Google Scholar] [CrossRef]
- Silvestri, D.; Wacławek, S.; Venkateshaiah, A.; Krawczyk, K.; Sobel, B.; Padil, V.V.T.; Černík, M.; Varma, R.S. Synthesis of Ag Nanoparticles by a Chitosan-Poly(3-Hydroxybutyrate) Polymer Conjugate and Their Superb Catalytic Activity. Carbohydr. Polym. 2020, 232, 115806it. [Google Scholar] [CrossRef]
- Merck KGaA. IR Spectrum Table; Merck KGaA: Darmstadt, Germany. Available online: https://www.sigmaaldrich.com/CZ/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 22 October 2022).
- Hou, Y.; Kondoh, H.; Shimojo, M.; Sako, E.O.; Ozaki, N.; Kogure, T.; Ohta, T. Inorganic Nanocrystal Self-Assembly via the Inclusion Interaction of β-Cyclodextrins: Toward 3D Spherical Magnetite. J. Phys. Chem. B 2005, 109, 4845–4852. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B. Bioremediation of Industrial Toxic Metals with Gum Kondagogu (Cochlospermum Gossypium): A Natural Carbohydrate Biopolymer. Indian J. Biotechnol. 2011, 10, 113–120. [Google Scholar]
- Vinod, V.T.P.; Sashidhar, R.B.B.; Sreedhar, B. Biosorption of Nickel and Total Chromium from Aqueous Solution by Gum Kondagogu (Cochlospermum Gossypium): A Carbohydrate Biopolymer. J. Hazard. Mater. 2010, 178, 851–860. [Google Scholar] [CrossRef]
- González, A.G.; Pokrovsky, O.S. Metal Adsorption on Mosses: Toward a Universal Adsorption Model. J. Colloid Interface Sci. 2014, 415, 169–178. [Google Scholar] [CrossRef]
- Nag, S.; Biswas, S. Cellulose-Based Adsorbents for Heavy Metal Removal; Springer: Cham, Switzerland, 2021; pp. 113–142. [Google Scholar] [CrossRef]
- Maruyama, T.; Terashima, Y.; Takeda, S.; Okazaki, F.; Goto, M. Selective Adsorption and Recovery of Precious Metal Ions Using Protein-Rich Biomass as Efficient Adsorbents. Process Biochem. 2014, 49, 850–857. [Google Scholar] [CrossRef]
- Balabanova, B.; Lazarova, M.; Boev, B.; Barbu-Tudoran, L.; Suciu, M. Proposing Chemometric Tool for Efficacy Surface Dust Deposition Tracking in Moss Tissue Cross Bioindication Process of Metals in Environment. In Contaminant Levels and Ecological Effects; Springer: Cham, Switzerland, 2021; pp. 131–169. [Google Scholar]
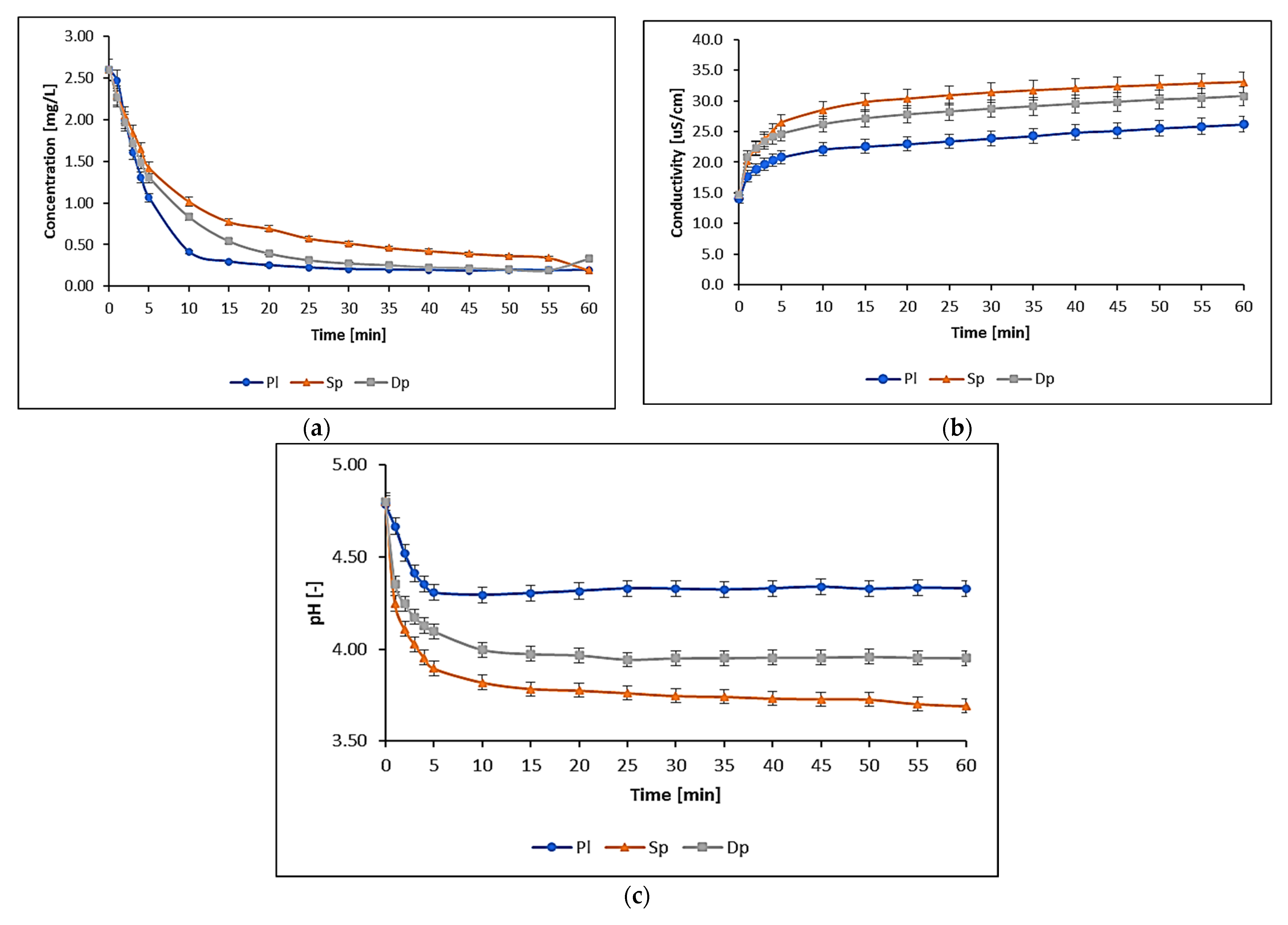
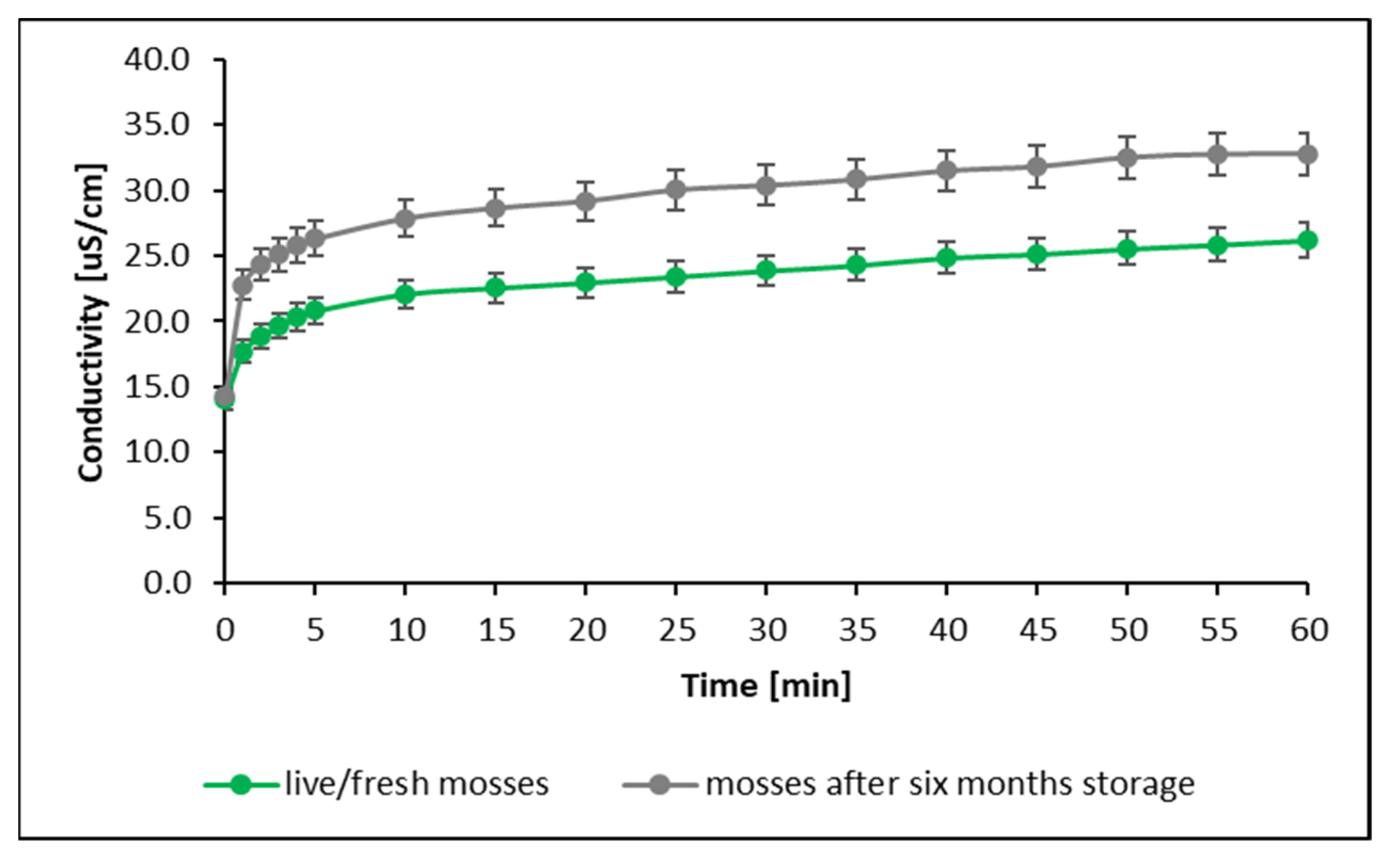
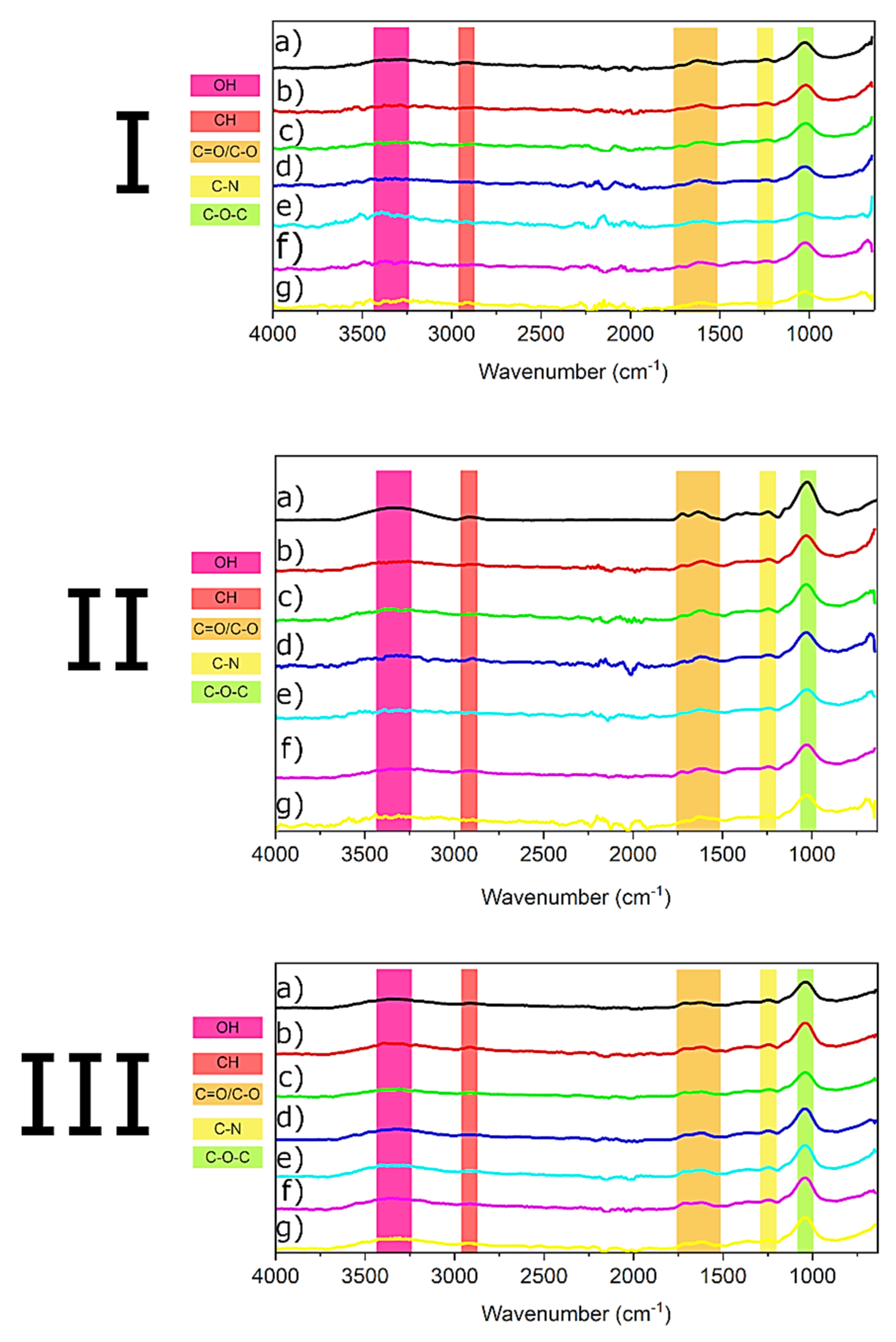
| Metal | IDL | IQL |
|---|---|---|
| Ni | 0.0043 | 0.050 |
| Cu | 0.0045 | 0.033 |
| Zn | 0.0033 | 0.010 |
| Cd | 0.0028 | 0.013 |
| Pb | 0.0130 | 0.070 |
| BCR-482 lichen (Certified) | AAS (Measured) | Dev.** | |||
|---|---|---|---|---|---|
| Metal | Concentration | ±Uncertainty | Mean | ±SD * | |
| [mg/kg dry weight] | [%] | ||||
| Ni | 2.47 | 0.07 | 2.16 | 0.32 | −13 |
| Cu | 7.03 | 0.19 | 6.63 | 0.17 | −5.7 |
| Zn | 100.6 | 2.2 | 95.1 | 2.3 | −5.5 |
| Cd | 0.56 | 0.02 | 0.53 | 0.03 | −5.3 |
| Pb | 40.9 | 1.4 | 38.2 | 1.0 | −6.6 |
| Pleurozium schreberi | Dicranum polysetum | Sphagnum fallax | ||||||
|---|---|---|---|---|---|---|---|---|
| cs,Ni(0) | cs,Ni(1) | cM,1 | cs,Ni(0) | cs,Ni(1) | cM,1 | cs,Ni(0) | cs,Ni(1) | cM,1 |
| 0.07 | <0.05 | >0.02 | 0.07 | <0.05 | >0.02 | 0.07 | <0.05 | >0.02 |
| 0.30 | <0.05 | >0.25 | 0.30 | <0.05 | >0.25 | 0.30 | <0.05 | >0.25 |
| 2.67 | 0.52 | 2.15 | 2.67 | 0.69 | 1.98 | 2.67 | 0.49 | 2.18 |
| cs,Cu(0) | cs,Cu(1) | cM,1 | cs,Cu(0) | cs,Cu(1) | cM,1 | cs,Cu(0) | cs,Cu(1) | cM,1 |
| 0.05 | <0.03 | >0.02 | 0.05 | <0.03 | >0.02 | 0.05 | <0.03 | >0.02 |
| 0.30 | <0.03 | >0.27 | 0.30 | <0.03 | >0.27 | 0.30 | <0.03 | >0.27 |
| 2.60 | 0.20 | 2.40 | 2.60 | 0.19 | 2.40 | 2.60 | 0.33 | 2.30 |
| cs,Zn(0) | cs,Zn(1) | cM,1 | cs,Zn(0) | cs, Zn (1) | cM,1 | cs, Zn (0) | cs, Zn (1) | cM,1 |
| 0.03 | <0.01 | >0.20 | 0.03 | <0.01 | >0.02 | 0.03 | <0.01 | >0.02 |
| 0.30 | 0.02 | 0.28 | 0.30 | 0.02 | 0.28 | 0.30 | 0.01 | 0.29 |
| 2.81 | 0.40 | 2.41 | 2.81 | 0.64 | 2.17 | 2.81 | 0.49 | 2.32 |
| cs,Cd(0) | cs, Cd (1) | cM,1 | cs, Cd (0) | cs, Cd (1) | cM,1 | cs, Cd (0) | cs, Cd (1) | cM,1 |
| 0.03 | <0.01 | >0.02 | 0.03 | <0.01 | >0.02 | 0.03 | <0.01 | >0.02 |
| 0.30 | <0.01 | >0.29 | 0.30 | <0.01 | >0.29 | 0.30 | 0.07 | 0.23 |
| 2.67 | 0.09 | 2.58 | 2.67 | 0.27 | 2.40 | 2.67 | 0.23 | 2.44 |
| cs,Pb(0) | cs, Pb (1) | cM,1 | cs, Pb (0) | cs, Pb (1) | cM,1 | cs, Pb (0) | cs, Pb (1) | cM, 1 |
| 0.09 | <0.07 | >0.02 | 0.09 | <0.07 | >0.02 | 0.09 | <0.07 | >0.02 |
| 0.24 | <0.07 | >0.17 | 0.24 | <0.07 | >0.17 | 0.24 | <0.07 | >0.17 |
| 2.70 | 0.08 | 2.62 | 2.70 | 0.10 | 2.60 | 2.70 | 0.13 | 2.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świsłowski, P.; Nowak, A.; Wacławek, S.; Silvestri, D.; Rajfur, M. Bioaccumulation of Trace Elements from Aqueous Solutions by Selected Terrestrial Moss Species. Biology 2022, 11, 1692. https://doi.org/10.3390/biology11121692
Świsłowski P, Nowak A, Wacławek S, Silvestri D, Rajfur M. Bioaccumulation of Trace Elements from Aqueous Solutions by Selected Terrestrial Moss Species. Biology. 2022; 11(12):1692. https://doi.org/10.3390/biology11121692
Chicago/Turabian StyleŚwisłowski, Paweł, Arkadiusz Nowak, Stanisław Wacławek, Daniele Silvestri, and Małgorzata Rajfur. 2022. "Bioaccumulation of Trace Elements from Aqueous Solutions by Selected Terrestrial Moss Species" Biology 11, no. 12: 1692. https://doi.org/10.3390/biology11121692
APA StyleŚwisłowski, P., Nowak, A., Wacławek, S., Silvestri, D., & Rajfur, M. (2022). Bioaccumulation of Trace Elements from Aqueous Solutions by Selected Terrestrial Moss Species. Biology, 11(12), 1692. https://doi.org/10.3390/biology11121692










