Nile Tilapia (Oreochromis niloticus Linnaeus, 1758) Invasion Caused Trophic Structure Disruptions of Fish Communities in the South China River—Pearl River
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Stable Isotope Metrics
2.4. Isotopic Diversity Indices
2.5. Statistical Analyses
3. Results
3.1. Fish Community Structure
3.2. Relationship between Nile tilapia Invasion and Trophic Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallardo, B.; Clavero, M.; Sànchez, M.I.; Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Baxter, C.V.; Fausch, K.D.; Murakami, M.; Chapman, P.L. Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology 2004, 85, 2656–2663. [Google Scholar] [CrossRef]
- Cucherousset, J.; Olden, J.D. Ecological impacts of non-native freshwater fishes. Fisheries 2011, 36, 215–230. [Google Scholar] [CrossRef]
- Firth, B.L.; Poesch, M.S.; Koops, M.A.; Drake, D.A.R.; Power, M. Diet overlap of common and at-risk riverine benthic fishes before and after Round Goby (Neogobius melanostomus) invasion. Biol. Invasions 2021, 23, 221–234. [Google Scholar] [CrossRef]
- Krakowiak, P.J.; Pennuto, C.M. Fish and macroinvertebrate communities in tributary streams of Eastern Lake Erie with and without round gobies (Neogobius melanostomus, Pallas 1814). J. Great Lakes Res. 2008, 34, 675–689. [Google Scholar] [CrossRef]
- Kessel, N.V.; Dorenbosch, M.; Kranenbarg, J.; Velde, G.V.D.; Leuven, R.S.E.W. Invasive Ponto-Caspian gobies rapidly reduce the abundance of protected native bullhead. Aquat. Invasions 2016, 11, 179–188. [Google Scholar] [CrossRef]
- Yoshioka, A.; Miyazaki, Y.; Sekizaki, Y.; Suda, S.I.; Kadoya, T.; Washitani, I. A “lost biodiversity” approach to revealing major anthropogenic threats to regional freshwater ecosystems. Ecol. Indic. 2014, 36, 348–355. [Google Scholar] [CrossRef]
- Sagouis, A.; Cucherousset, J.; Villéger, S.; Santoul, F.; Boulêtreau, S. Non-native species modify the isotopic structure of freshwater fish communities across the globe. Ecography 2015, 38, 213–227. [Google Scholar] [CrossRef] [Green Version]
- Strassburg, B.B.N.; Iribarrem, A.; Beyer, H.L.; Cordeiro, C.L.; Crouzeilles, R.; Jakovac, C.C.; Braga Junqueira, A.; Lacerda, E.; Latawiec, A.E.; Balmford, A.; et al. Global priority areas for ecosystem restoration. Nature 2020, 586, 724–729. [Google Scholar] [CrossRef]
- Wainright, C.A.; Muhlfeld, C.C.; Elser, J.J.; Bourretd, S.L.; Devlina, S.P. Species 390 invasion progressively disrupts the trophic structure of native food webs. Proc. Natl. Acad. Sci. USA 2021, 118, e2102179118. [Google Scholar] [CrossRef]
- Marr, S.M.; Marchetti, M.P.; Olden, J.D.; García-Berthou, E.; Morgan, D.L.; Arismendi, I.; Day, J.A.; Griffiths, C.L.; Skelton, P.H. Freshwater fish introductions in Mediterranean-climate regions: Are there commonalities in the conservation problem? Divers. Distrib. 2010, 16, 606–619. [Google Scholar] [CrossRef]
- Pysek, P.; Jarosik, V.; Hulme, P.E.; Kühn, I.; Wilda, J.; Arianoutsou, M.; Bacher, S.; Chiron, F.; Didziulis, V.; Essl, F.; et al. Disentangling the role of environmental and human pressures on biological invasions across Europe. Proc. Natl. Acad. Sci. USA 2010, 107, 12157–12162. [Google Scholar] [CrossRef] [Green Version]
- Simberloff, D.; Martin, J.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; Garcia-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What's what and the way forward. Trends Ecol. Evol. 2013, 8, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Rooney, N.; McCann, K.S. Integrating food web diversity, structure and stability. Trends Ecol. Evol. 2012, 27, 40–46. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R: Bayesian isotopic niche metrics. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Goto, D.; Dunlop, E.S.; Young, J.D.; Jackson, D.A. Shifting trophic control of fishery—Ecosystem dynamics following biological invasions. Ecol. Appl. 2020, 30, e02190. [Google Scholar] [CrossRef]
- Edlund, M.B.; Jude, D.J.; Nalepa, T.F. Diets of the benthic amphipod Diporeia in southern Lake Michigan before and after the dreissenid invasion. J. Great Lakes Res. 2021, 47, 447–462. [Google Scholar] [CrossRef]
- Polis, G.A.; Winemiller, K.O. Food Webs: Integration of Patterns & Dynamics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Cucherousset, J.; Aymes, J.C.; Santoul, F.; Céréghino, R. Stable isotope evidence of trophic interactions between introduced brook trout Salvelinus fontinalis and native brown trout Salmo trutta in a mountain stream of south-west France. J. Fish Biol. 2007, 71, 210–223. [Google Scholar] [CrossRef]
- Layman, C.A.; Arrington, D.A.; Montaña, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef]
- Walsworth, T.E.; Budy, P.; Thiede, G.P. Longer food chains and crowded niche space: Effects of multiple invaders on desert stream food web structure. Ecol. Freshw. Fish 2013, 22, 439–452. [Google Scholar] [CrossRef]
- Cucherousset, J.; Villéger, S. Quantifying the multiple facets of isotopic diversity: New metrics for stable isotope ecology. Ecol. Indic. 2015, 56, 152–160. [Google Scholar] [CrossRef]
- González-Bergonzoni, I.; Silva, I.; Mello, F.T.; D'Anatro, A.; Boccardi, L.; Stebniki, S.; Brugnoli, E.; Tesitore, G.; Vidal, N.; Naya, D.E. Evaluating the role of predatory fish controlling the invasion of the Asian golden mussel Limnoperna fortunei in a subtropical river. J. Appl. Ecol. 2020, 57, 717–728. [Google Scholar] [CrossRef]
- Koel, T.M.; Tronstad, L.M.; Arnold, J.L.; Gunther, K.A.; White, P.J. Predatory fish invasion induces within and across ecosystem effects in yellowstone national park. Sci. Adv. 2019, 5, eaav1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lévèque, C.; Oberdorff, T.; Paugy, D.; Stiassny, M.L.J.; Tedesco, P.A. Global diversity of fish (Pisces) in fresh water. Hydrobiologia 2008, 595, 545–567. [Google Scholar] [CrossRef]
- Jenkins, M. Prospects for biodiversity. Science 2003, 302, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Michelan, T.S.; Thomaz, S.M.; Mormul, R.P.; Carvalho, P. Effects of an exotic invasive macrophyte (tropical signalgrass) on native plant community composition, species richness and functional diversity. Freshw. Biol. 2010, 55, 1315–1326. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Non-natives: Plusses of invasion ecology. Nature 2011, 475, 36. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.X. Pearl River Fishery Resources; Guangdong Science and Technology Press: Guanghzou, China, 1990; pp. 91–121. [Google Scholar]
- Cao, L.; Naylor, R.; Henriksson, P.; Leadbitter, D.; Metian, M.; Troell, M.; Zhang, W. China’s aquaculture and the world’s wild fisheries. Science 2015, 347, 133–135. [Google Scholar] [CrossRef]
- Grammer, G.L.; Slack, W.T.; Peterson, M.S.; Dugo, M.A. Nile tilapia Oreochromis niloticus (Linnaeus, 1758) establishment in temperate Mississippi, USA: Multi-year survival confirmed by otolith ages. Aquat. Invasions 2012, 7, 367–376. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020—Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Deines, A.M.; Wittmann, M.E.; Deines, J.M.; Lodge, D.M. Tradeoffs among ecosystem services associated with global tilapia introductions. Rev. Fish. Sci. Aquac. 2016, 2, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Zengeya, T.A.; Robertson, M.P.; Booth, A.J.; Chimimba, C.T. Ecological niche modeling of the invasive potential of Nile tilapia Oreochromis niloticus in African river systems: Concerns and implications for the conservation of indigenous congenerics. Biol. Invasions 2013, 15, 1507–1521. [Google Scholar] [CrossRef]
- Shuai, F.; Li, X.; Chen, F.; Li, Y.; Lek, S. Spatial patterns of fish assemblages in the Pearl River, China: Environmental correlates. Fund. Appl. Limnol. 2017, 189, 329–340. [Google Scholar] [CrossRef]
- Russell, D.J.; Thuesen, P.A.; Thomson, F.E. A review of the biology, ecology, distribution and control of Mozambique tilapia, Oreochromis mossambicus (Peters 1852) (Pisces: Cichlidae) with particular emphasis on invasive Australian populations. Rev. Fish Biol. Fisher. 2012, 22, 533–554. [Google Scholar] [CrossRef]
- Gu, D.E.; Ma, G.M.; Zhu, Y.J.; Xu, M.; Luo, D.; Li, Y.Y.; Wei, H.; Mu, X.D.; Luo, J.R.; Hu, Y.C. The impacts of invasive Nile tilapia (Oreochromis niloticus) on the fisheries in the main rivers of Guangdong Province, China. Biochem. Syst. Ecol. 2015, 59, 1–7. [Google Scholar] [CrossRef]
- Stauffer, J.R.; Chirwa, E.R.; Jere, W.; Konings, A.F.; Tweddle, D.; Weyl, O. Nile tilapia, oreochromis niloticus (teleostei: Cichlidae): A threat to native fishes of lake malawi? Biol. Invasions 2022, 24, 1585–1597. [Google Scholar] [CrossRef]
- Zambrano, L.; Martínez-Meyer, E.; Menezes, N.; Peterson, A.T. Invasive potential of common carp (Cyprinus carpio) and nile tilapia (Oreochromis niloticus) in American freshwater systems. Can. J. Fish. Aquat. Sci. 2006, 63, 1903–1910. [Google Scholar] [CrossRef] [Green Version]
- Henson, M.N.; Aday, D.D.; Rice, J.A.; Layman, C.A. Assessing the influence of tilapia on sport fish species in north Carolina reservoirs. Trans. Am. Fish. Soc. 2018, 147, 350–362. [Google Scholar] [CrossRef] [Green Version]
- Rader, J.A.; Newsome, S.D.; Sabat, P.; Chesser, R.T.; Dillon, M.E.; Martínez del Rio, C. Isotopic niches support the resource breadth hypothesis. J. Anim. Ecol. 2017, 86, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Rennie, M.D.; Sprules, W.G.; Johnson, T.B. Resource switching in fish following a major food web disruption. Oecologia 2009, 159, 789–802. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 4.0; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R–project.org/ (accessed on 1 May 2022).
- Zheng, C.Y. Ichthyology of the Pearl River; Science Press: Beijing, China, 1989. [Google Scholar]
- Zhou, J.; Zhang, C.G. Freshwater Fishes of Guangxi, China; Guangxi People’s Publishing House: Nanning, China, 2005. [Google Scholar]
- Cucherousset, J.; Bouletreau, S.; Martino, A.; Roussel, J.M.; Santoul, F. Using stable isotope analyses to determine the ecological effects of non-native fishes. Fish. Manag. Ecol. 2012, 19, 111–119. [Google Scholar] [CrossRef]
- Holt, D.; Lawton, J.H.G.; Polis, A.; Martinez, N.D. Trophic rank and the species-area relationship. Ecology 1999, 80, 1495–1504. [Google Scholar] [CrossRef] [Green Version]
- Hoeinghaus, D.J.; Zeug, S.C. Can stable isotope ratios provide for community-wide measures of trophic structure?Comment. Ecology 2008, 89, 2353–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuai, F.; Li, X.; Liu, Q.; Zhu, S.; Wu, Z.; Zhang, Y. Nile tilapia (Oreochromis niloticus) invasions disrupt the functional patterns of fish community in a large subtropical river in China. Fish. Manag. Ecol. 2019, 26, 578–589. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Vadeboncoeur, Y.; Mccann, K.S.; Zanden, M.J.V.; Rasmussen, J.B. Effects of multi-chain omnivory on the strength of trophic control in lakes. Ecosystems 2005, 8, 682–693. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.; Dominguez-Garcia, V.; Donetti, L.; Muñoz, M.A. Trophic coherence determines food-web stability. Proc. Natl. Acad. Sci. USA 2014, 111, 17923–17928. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K. State of aquatic invasive species in tropical India: An overview. Aquat. Ecosyst. Health 2021, 2, 13–23. [Google Scholar] [CrossRef]
- Zambrano, L.; Valiente, E.; Vander Zanden, M.J. Food web overlap among native axolotl (Ambystoma mexicanum) and two exotic fishes: Carp (Cyprinus carpio) and tilapia (Oreochromis niloticus) in Xochimilco, Mexico City. Biol. Invasions 2010, 12, 3061–3069. [Google Scholar] [CrossRef]
- Jackson, M.C.; Donohue, I.; Jackson, A.L.; Britton, J.R.; Harper, D.M.; Grey, J. Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology. PLoS ONE 2012, 7, e31757. [Google Scholar] [CrossRef]
- Manlick, P.J.; Pauli, J.N. Human disturbance increases trophic niche overlap in terrestrial carnivore communities. Proc. Natl. Acad. Sci. USA 2020, 117, 26842–26848. [Google Scholar] [CrossRef]
- McCue, M.D.; Javal, M.; Clusella-Trullas, S.; Le Roux, J.J.; Jackson, M.C.; Ellis, A.G.; Richardson, D.M.; Valentine, A.J.; Terblanche, J.S. Using stable isotope analysis to answer fundamental questions in invasion ecology: Progress and prospects. Methods Ecol. Evol. 2020, 11, 196–214. [Google Scholar] [CrossRef]
- Sandfoss, M.R.; Mccue, M.D.; Lillywhite, H.B. Trophic niche and home range of an insular pit viper following loss of food resources. J. Zool. 2021, 314, 296–310. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, W.; Li, B.; Wen, L.; Lei, G. Habitat alteration facilitates the dominance of invasive species through disrupting niche partitioning in floodplain wetlands. Divers. Distrib. 2021, 27, 1861–1871. [Google Scholar] [CrossRef]
- Figueredo, C.C.; Giani, A. Ecological interactions between Nile tilapia (Oreochromis niloticus, L.) and the phytoplanktonic community of the Furnas Reservoir (Brazil). Freshw. Biol. 2005, 50, 1391–1403. [Google Scholar] [CrossRef]
- Starling, F.; Lazzaro, X.; Cavalcanti, C.; Moreira, R. Contribution of omnivorous tilapia to eutrophication of a shallow tropical reservoir: Evidence from a fish kill. Freshw. Biol. 2002, 47, 2443–2452. [Google Scholar] [CrossRef] [Green Version]
- Esselman, P.C.; Schmitter-Soto, J.J.; Allan, J.D. Spatiotemporal dynamics of the spread of African tilapias (Pisces: Oreochromis spp.) into rivers of northeastern Mesoamerica. Biol. Invasions 2013, 15, 1471–1491. [Google Scholar] [CrossRef]
- Yao, G.C.; Ye, W. New Technology of Ecological and Efficient Culture of Tilapia; China Ocean Press: Beijing, China, 2014. [Google Scholar]
- Fisheries and Fishery Administration Bureau of Ministry of Agriculture 2021. China Fisheries Statistical Yearbook; China Agricultural Publishing House: Beijing, China, 2021. [Google Scholar]
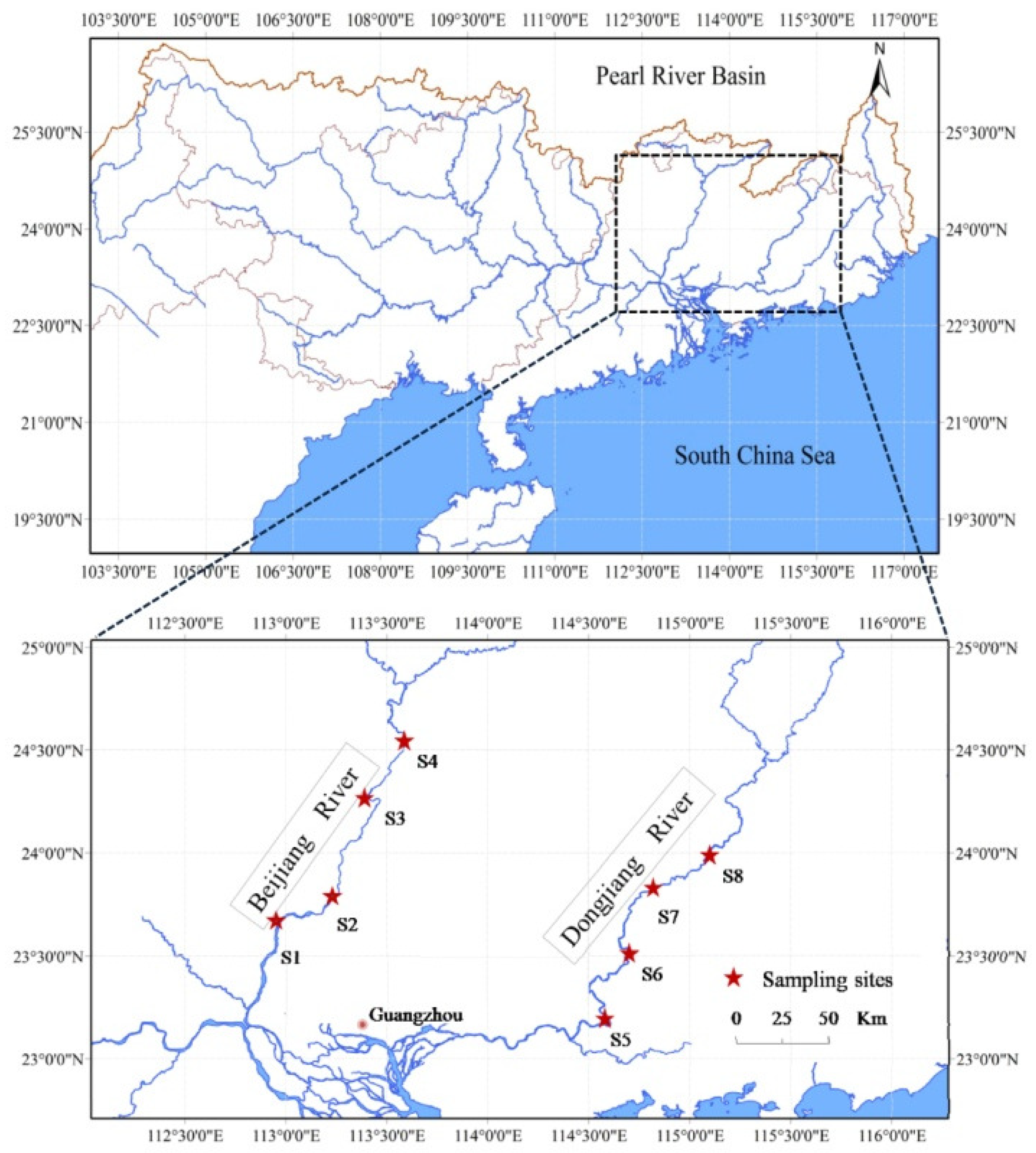




| Sites | Name | Coordinates | River Width (m) | Species Richness | Subordinate River |
|---|---|---|---|---|---|
| S1 | Lubao | 112°53′23″ E, 23°20′53″ N | 791 | 35 | Beijiang |
| S2 | Shijiao | 112°57′59″ E, 23°33′41″ N | 882 | 53 | |
| S3 | Qingyuan | 113°3′49″ E, 23°41′50″ N | 935 | 46 | |
| S4 | Lianjiang | 113°18′16″ E, 24°1′29″ N | 635 | 32 | |
| S5 | Hengli | 114°36′55″ E, 23°10′26″ N | 770 | 42 | Dongjiang |
| S6 | Guzhu | 114°41′26″ E, 23°30′25″ N | 462 | 50 | |
| S7 | Heyuan | 114°42′45″ E, 23°44′18″ N | 714 | 44 | |
| S8 | Huangtian | 114°59′36″ E, 23°53′17″ N | 341 | 21 |
| Feeding Habit | Species | English Name | Percentage (%) | Type | Category | |
|---|---|---|---|---|---|---|
| Beijiang | Dongjiang | |||||
| Piscivore | Culter recurviceps | Culter hainan | 3.62 | 0.72 | E | SE |
| Culter dabryi | Dashi culter | 1.04 | − | N | SE | |
| Culter alburnus | Topmouth culter | 0.07 | 0.59 | N | SE | |
| Erythroculter hypselonotus | Bigeyse culterfish | 0.07 | + | N | SE | |
| Elopichthys bambusa | Yellow cheek carp | 0.03 | − | N | RL | |
| Pelteobagrus fulvidraco | Yellow catfish | 1.45 | 0.71 | N | SE | |
| Pelteobagrus vachelli | Darkbarbel catfish | 1.32 | 1.49 | N | SE | |
| Leiocassis crassilabris | Ussuri catfish | 1.05 | 0.01 | N | SE | |
| Mystus guttatus | Spotted longbarbel catfish | 0.47 | 0.25 | N | SE | |
| Silurus asotus | Catfish | 0.31 | 0.22 | N | SE | |
| Clarias fuscus | Oriental catfish | 0.08 | 0.66 | N | SE | |
| Mystus macropterus | Largefin longbarbel catfish | 0.01 | − | N | SE | |
| Siniperca kneri | Bigeye mandarinfish | 0.36 | 0.07 | N | SE | |
| Siniperca scherzeri | Spotted mandarinfish | 0.15 | − | N | SE | |
| Channa asiatica | Chinese snakehead | 0.03 | 0.29 | N | SE | |
| Channa maculata | Taiwan snakehead | 0.01 | 0.17 | N | SE | |
| Channa argus | Snakehead | 0.01 | − | N | SE | |
| Anguilla japonica | Japanese eel | + | 0.02 | N | RS | |
| Invertivore | Squalidus wolterstorffi | Dot chub | 3.14 | 0.05 | N | RL |
| Saurogobio dabryi | Longnose gudgeon | 1.52 | 4.63 | N | RL | |
| Hemibarbus labeo | 1.22 | 0.77 | N | SE | ||
| Hemibarbus maculatus | 1.20 | 1.13 | N | SE | ||
| Opsariichthys bidens Günther | Chinese hooksnout carp | 1.00 | 0.90 | N | SE | |
| Coilia grayii | Gray’s grsnadier anchovy | 3.79 | 4.31 | N | SE | |
| Lateolabrax japonicus | Spotted sea bass | 0.67 | + | N | RS | |
| Eleotris oxycephala | Sharphead sleeper | 0.49 | 0.20 | N | SE | |
| Mastacembelus armatus | Tire track eel | 0.37 | 0.61 | N | SE | |
| Rhinogobius giurinus | Amur goby | 0.27 | 1.69 | N | SE | |
| Glossogobius giuris | Tongue goby | − | 2.79 | N | SE | |
| Hypseleotris hainanensis | − | 0.01 | N | SE | ||
| Leiocassis argentivittatus | Longitudinal catfish | 0.24 | 0.33 | N | SE | |
| Ietalurus punetaus | Channel catfish | − | 0.07 | Non | SE | |
| Leiocassis virgatus | Striped catfish | − | 0.39 | N | SE | |
| Glyptothorax fukiensis | − | 0.09 | N | SE | ||
| Monopterus albus | Finless eel | 0.07 | 0.05 | N | RS | |
| Takifugu ocellatus | Ocellated puffer | + | − | N | RS | |
| Omnivore | Squalidus argentatus | Chub | 18.17 | 7.25 | N | RL |
| Hemiculter leucisculus | Common sawbelly | 15.32 | 17.22 | N | SE | |
| Pseudohemiculter dispar | 3.54 | 0.36 | N | SE | ||
| Squaliobarbus curriculus | Barbel chub | 2.92 | 1.50 | N | RL | |
| Abbottina rivularis | Amur false gudgeon | 2.60 | 0.02 | N | SE | |
| Cyprinus carpio | Carp | 1.94 | 1.52 | N | SE | |
| Carassius auratus | Crucian | 1.80 | 2.52 | N | SE | |
| Cirrhinus mrigala | Mrigal carp | 1.32 | 1.10 | Non | SE | |
| Sarcocheilichthys parvus | 0.90 | − | N | SE | ||
| Rhodeus sinensis | Light’s bitterling | 0.57 | − | N | SE | |
| Xenocypris davidi | Yellow tailed xenocypris | 0.36 | 2.29 | N | RL | |
| Hemiculterella wui | 0.22 | − | E | SE | ||
| Puntius semifasciolatus | Chinese barb | 0.24 | − | N | SE | |
| Mylopharyngodon piceus | Black carp | 0.01 | 0.01 | N | RL | |
| Osteochilus salsburyi | 0.11 | 1.01 | N | SE | ||
| Xenocypris argentea | Silver xenocypris | 0.12 | 0.05 | N | RL | |
| Distoechodon tumirostris | Round mouth | 0.05 | 0.02 | N | RL | |
| Acheilognathus tonkinensis | Vietnamese bitterling | 0.03 | 0.61 | N | SE | |
| Acheilognathus macropterus | Largefin bitterling | 0.02 | − | N | SE | |
| Cyprinus carpio var.specularis | Germany mirror carp | 0.01 | − | N | SE | |
| Acheilognathus chankaensis | Khanka spiny bitterling | − | 0.26 | N | ||
| Sarcocheilichthys nigripinnis | − | 0.15 | N | |||
| Pseudorasbora parva | Stone moroko | − | 0.02 | N | SE | |
| Spinibarbus denticulatus | − | 0.02 | N | RL | ||
| Rhodeus spinalis Oshima | − | 0.01 | N | SE | ||
| Zacco platypus | Pale chub | 3.40 | 0.09 | N | SE | |
| Tinca tinca | Tench | − | 0.01 | Non | SE | |
| Oreochromis niloticus | Nile tilapia | 3.65 | 13.21 | Non | SE | |
| Tilapia zillii | Zillii tilapia | 0.27 | 0.70 | Non | SE | |
| Anabas testudineus | Climbing perch | − | 0.01 | Non | SE | |
| Prochilodus scyofa | 0.17 | 0.01 | Non | SE | ||
| Clarias gariepinus | Fuscous catfish | 0.01 | 0.18 | Non | SE | |
| Detritivore | Misgurnus anguillicaudatus | Oriental weather fish | 4.03 | 3.50 | N | SE |
| Micronoemacheilus pulcher | 0.21 | 0.08 | E | SE | ||
| Cobitis sinensis | Siberian spiny loach | 0.01 | 0.38 | N | SE | |
| Labeo rohita | Roho labeo | 0.10 | − | Non | SE | |
| Vanmanenia hainanensis | − | 0.01 | E | SE | ||
| Hypostomus plecostomus | Suckermouth catfish | 0.05 | 0.05 | Non | SE | |
| Herbivore | Cirrhinus molitorella | Mud carp | 5.67 | 12.55 | N | RL |
| Megalobrama terminalis | Black amur bream | 2.72 | 6.74 | N | RL | |
| Ctenopharyngodon idellus | Grass carp | 0.80 | 0.81 | N | RL | |
| Sinibrama wui | Bigeyes bream | 0.40 | 0.09 | E | RL | |
| Onychostoma gerlachi | Largescale shoveljaw fish | 0.31 | − | N | SE | |
| Acrossocheilus beijiangensis | 0.13 | − | N | SE | ||
| Parabramis pekinensis | White bream | 0.10 | 0.06 | N | RL | |
| Megalobrama amblycephala | Wuchang fish | 0.05 | 0.02 | Non | RL | |
| Acrossocheilus labiatus | 0.03 | − | N | SE | ||
| Acrossocheilus stenotaeniatus | 0.02 | − | N | SE | ||
| Acrossocheilus parallens | 0.02 | − | N | SE | ||
| Sinibrama melroseib | Hainan bream | 0.02 | 0.06 | N | SE | |
| Rectoris posehensis | 0.01 | N | SE | |||
| Garra orientalis | Oriental sucking barb | − | 0.04 | N | SE | |
| Parasinilabeo assimilis | − | 0.01 | N | SE | ||
| Planktivore | Hypophthalmichthys molitrix | Silver carp | 1.16 | 1.52 | N | RL |
| Aristichthys nobilis | Bighead carp | 0.77 | 0.39 | N | RL | |
| Pseudolaubuca sinensis | − | 0.03 | N | SE | ||
| Clupanodon thrissa | Chinese gizzard shad | 0.19 | − | N | RS | |
| Konosirus punctatus | Dotted gizzard shad | 0.05 | − | N | RS | |
| River | Variable | Intercept | No. of Native PL. | No. of Native H | No. of Native D | No. of Native O | No. of Native I | No. of Native P | No. of Nile Tilapia |
|---|---|---|---|---|---|---|---|---|---|
| Dongjiang | δ13C range | 1.45 (0.039) | 1.06 (0.068) | −0.02 (0.594) | 0.04 (0.261) | 0.10 (0.075) | 0.05 (0.714) | 0.18 (0.019) | 0.22 (0.030) |
| δ15N range | 1.52 (0.026) | 1.55 (0.031) | 0.03 (0.298) | 0.05 (0.158) | 0.19 (0.064) | 0.02 (0.853) | 0.25 (0.008) | −0.19 (0.025) | |
| Beijiang | δ13C range | 1.25 (0.018) | 0.14 (0.332) | −0.01 (0.381) | 0.23 (0.042) | −0.01 (0.491) | 0.19 (0.168) | 0.22 (0.037) | 0.04 (0.453>) |
| δ15N range | 1.32 (0.025) | 0.11 (0.216) | 0.05 (0.631) | 0.19 (0.712) | 0.07 (0.329) | 0.12 (0.078) | 0.24 (0.036) | 0.08 (0.371) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, F.; Li, J. Nile Tilapia (Oreochromis niloticus Linnaeus, 1758) Invasion Caused Trophic Structure Disruptions of Fish Communities in the South China River—Pearl River. Biology 2022, 11, 1665. https://doi.org/10.3390/biology11111665
Shuai F, Li J. Nile Tilapia (Oreochromis niloticus Linnaeus, 1758) Invasion Caused Trophic Structure Disruptions of Fish Communities in the South China River—Pearl River. Biology. 2022; 11(11):1665. https://doi.org/10.3390/biology11111665
Chicago/Turabian StyleShuai, Fangmin, and Jie Li. 2022. "Nile Tilapia (Oreochromis niloticus Linnaeus, 1758) Invasion Caused Trophic Structure Disruptions of Fish Communities in the South China River—Pearl River" Biology 11, no. 11: 1665. https://doi.org/10.3390/biology11111665
APA StyleShuai, F., & Li, J. (2022). Nile Tilapia (Oreochromis niloticus Linnaeus, 1758) Invasion Caused Trophic Structure Disruptions of Fish Communities in the South China River—Pearl River. Biology, 11(11), 1665. https://doi.org/10.3390/biology11111665




