RETRACTED: Cross-Generational Impact of Epigenetic Male Influence on Physical Activity in Rat
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Determination of Inheritance of the Level of Motor Activity
2.3. Determination of Inheritance of the Level of Anxiety
2.4. Determination of the Inheritance of the Learnability
2.5. Statistics
2.6. Animal Welfare
2.7. Groups of Rats
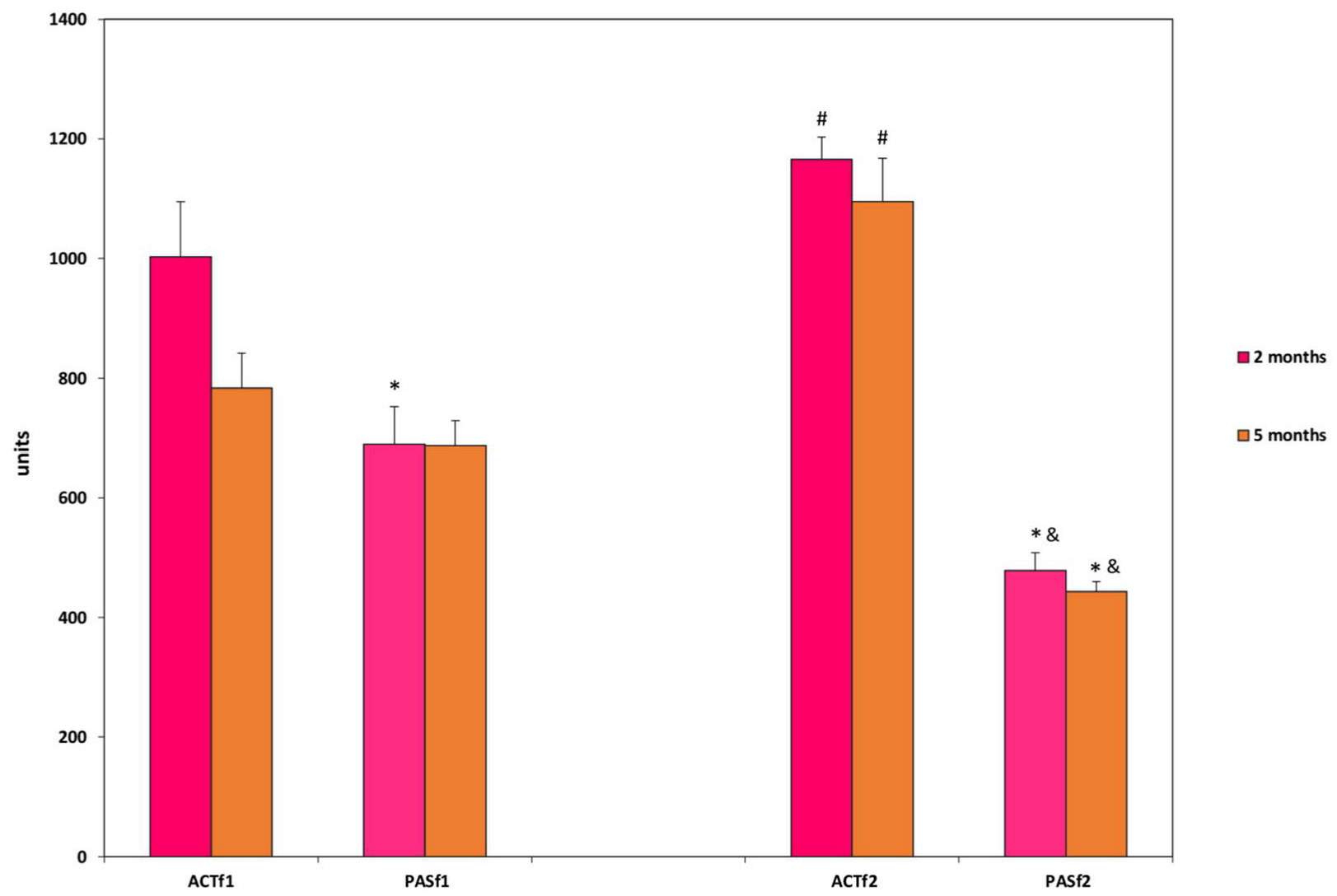
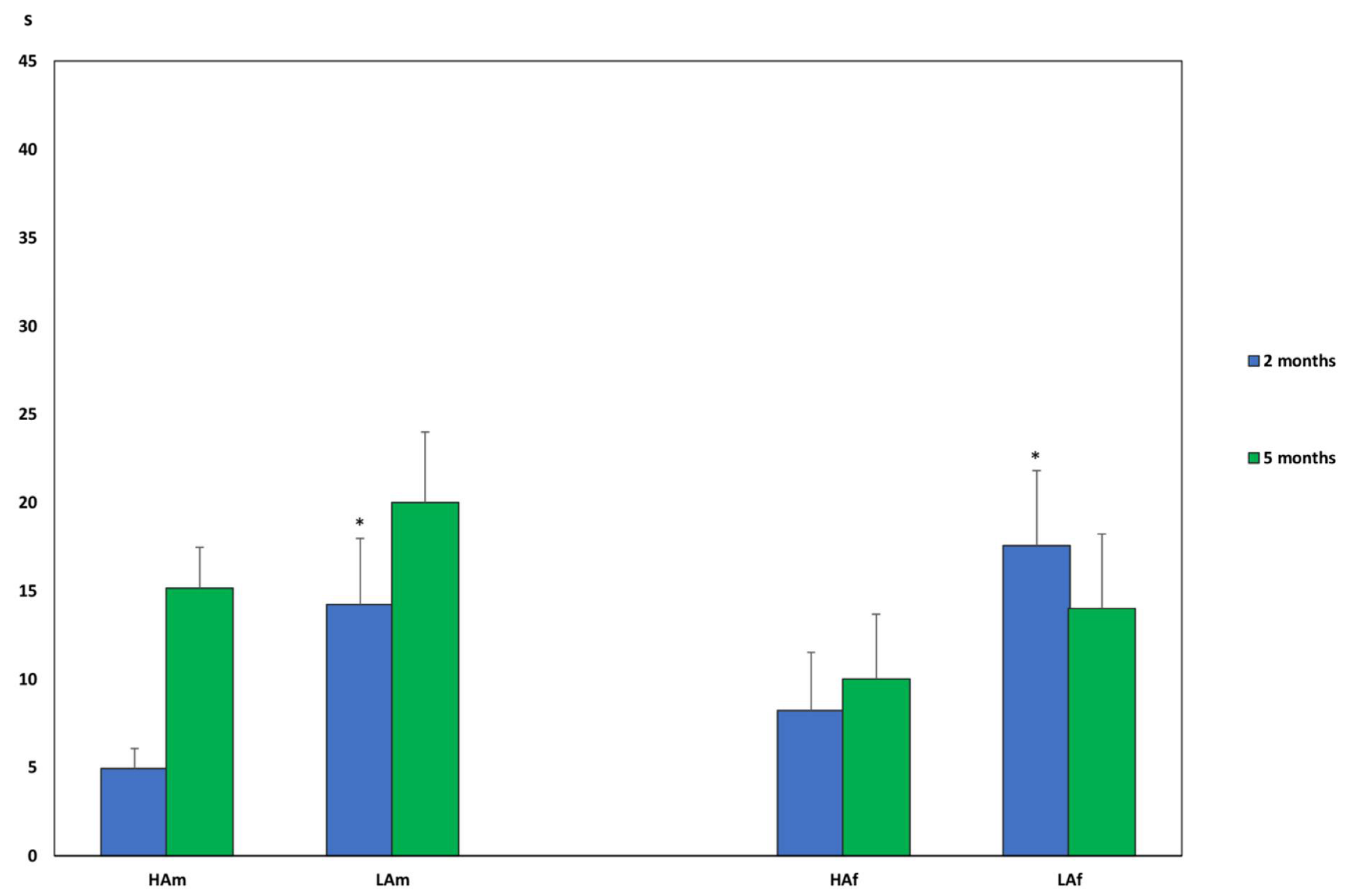
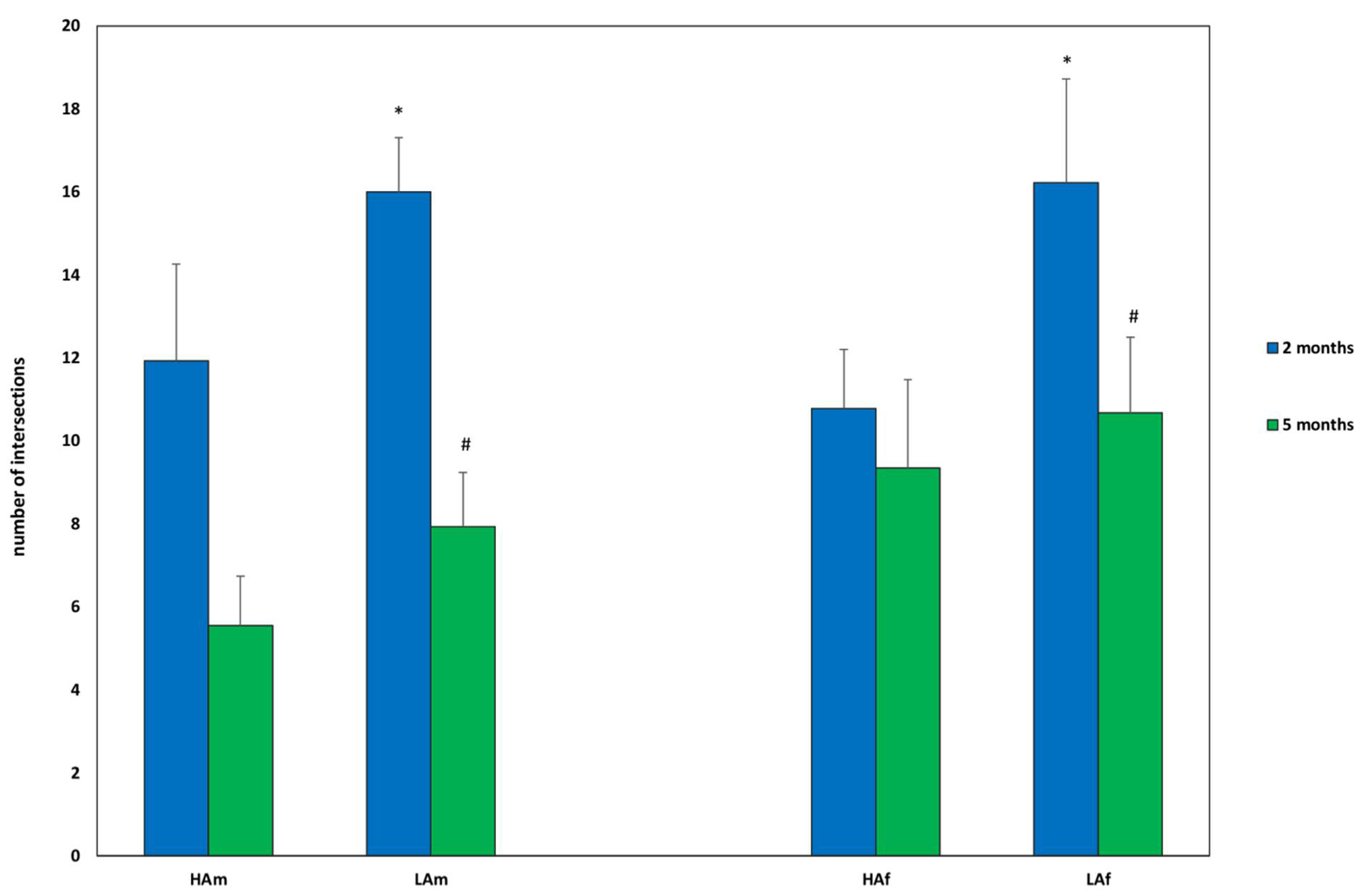
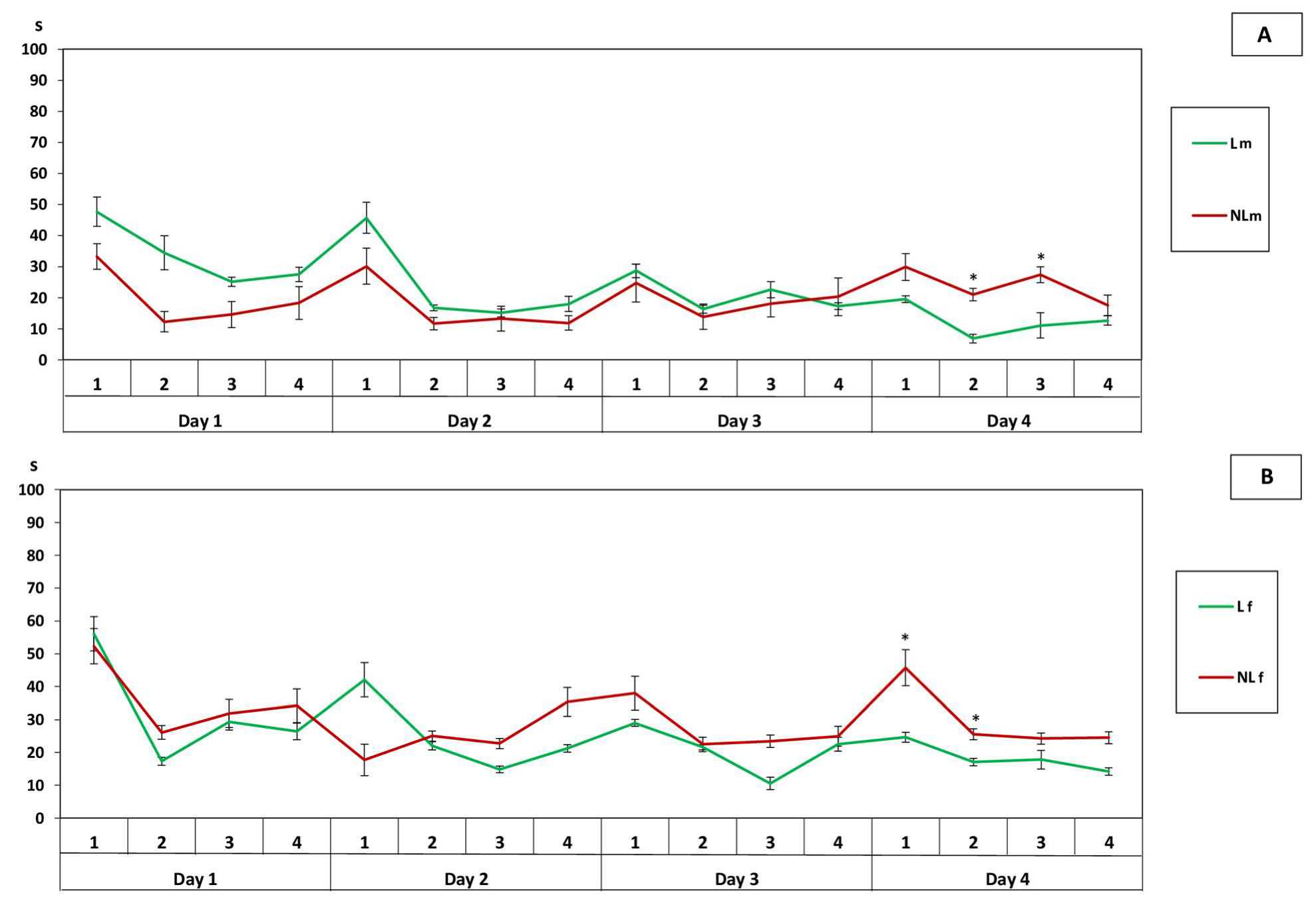
3. Results
3.1. Determination of Inheritance for the Level of Motor Activity
3.2. Determination of Inheritance for the Level of Anxiety
3.3. Determination of Inheritance of Learnability
4. Discussion
5. Conclusions
- 1 .
- At the moment of conception, exertion of epigenetic influences, which have a significant impact on the behavior of future offspring, is possible.
- 2 .
- The activity levels of the offspring depend on the activity levels of the bystander males.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birch, J. Natural selection and the maximization of fitness. Biol. Rev. 2015, 91, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Garland, T.; Kelly, S.A. Phenotypic plasticity and experimental evolution. J. Exp. Biol. 2006, 209, 2344–2361. [Google Scholar] [CrossRef] [PubMed]
- Smyers, M.E.; Bachir, K.Z.; Britton, S.L.; Koch, L.G.; Novak, C.M. Physically active rats lose more weight during calorie restriction. Physiol. Behav. 2014, 139, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Correia, E.C.; Izídio, G.S.; Brüske, G.R. Genetic Selection of Two New Rat Lines Displaying Different Levels of Anxiety-Related Behaviors. Behav. Genet. 2003, 33, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Ohl, F. Animal Models of Anxiety. Handb. Exp. Pharmacol. 2005, 169, 35–69. [Google Scholar] [CrossRef]
- Landgraf, R.; Keßler, M.S.; Bunck, M.; Murgatroyd, C.; Spengler, D.; Zimbelmann, M.; Nußbaumer, M.; Czibere, L.; Turck, C.W.; Singewald, N.; et al. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: Focus on vasopressin and glyoxalase-I. Neurosci. Biobehav. Rev. 2007, 31, 89–102. [Google Scholar] [CrossRef]
- Giorgi, O.; Piras, G.; Corda, M.G. The psychogenetically selected Roman high- and low-avoidance rat lines: A model to study the individual vulnerability to drug addiction. Neurosci. Biobehav. Rev. 2007, 31, 148–163. [Google Scholar] [CrossRef]
- Ohta, R.; Kojima, K. Hatano rats selectively bred for high- and low-avoidance learning: An overview. Exp. Anim. 2019, 68, 127–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Gao, Y.; Zhang, C. Effects of fetal microwave radiation exposure on offspring behavior in mice. J. Radiat. Res. 2014, 56, 261–268. [Google Scholar] [CrossRef]
- Azimzadeh, M.; Jelodar, G. Prenatal and early postnatal exposure to radiofrequency waves (900 MHz) adversely affects passive avoidance learning and memory. Toxicol. Ind. Health 2020, 36, 1024–1030. [Google Scholar] [CrossRef]
- Rouzer, S.K.; Cole, J.M.; Johnson, J.M.; Varlinskaya, E.I.; Diaz, M.R. Moderate Maternal Alcohol Exposure on Gestational Day 12 Impacts Anxiety-Like Behavior in Offspring. Front. Behav. Neurosci. 2017, 11, 183. [Google Scholar] [CrossRef]
- Dong, T.; Guan, Q.; Hu, W.; Zhang, M.; Zhang, Y.; Chen, M.; Wang, X.; Xia, Y. Prenatal exposure to glufosinate ammonium disturbs gut microbiome and induces behavioral abnormalities in mice. J. Hazard. Mater. 2020, 389, 122152. [Google Scholar] [CrossRef]
- Babenko, O.; Kovalchuk, I.; Metz, G.A.S. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci. Biobehav. Rev. 2015, 48, 70–91. [Google Scholar] [CrossRef]
- Bock, J.; Wainstock, T.; Braun, K.; Segal, M. Stress In Utero: Prenatal Programming of Brain Plasticity and Cognition. Biol. Psychiatry 2015, 78, 315–326. [Google Scholar] [CrossRef]
- Stasinopoulou, M.; Fragopoulou, A.; Stamatakis, A.; Mantziaras, G.; Skouroliakou, K.; Papassideri, I.; Stylianopoulou, F.; Lai, H.; Kostomitsopoulos, N.; Margaritis, L. Effects of pre- and postnatal exposure to 1880–1900 MHz DECT base radiation on development in the rat. Reprod. Toxicol. 2016, 65, 248–262. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zhang, K.-Y.; Guo, L.; Chen, Q.-L.; Gao, P.; Wang, T.; Li, J.; Guo, G.-Z.; Ding, G.-R. Effects of 1.8 GHz Radiofrequency Fields on the Emotional Behavior and Spatial Memory of Adolescent Mice. Int. J. Environ. Res. Public Health 2017, 14, 1344. [Google Scholar] [CrossRef]
- Spear, L.P. Effects of adolescent alcohol consumption on the brain and behaviour. Nat. Rev. Neurosci. 2018, 19, 197–214. [Google Scholar] [CrossRef]
- Laviolette, S.R. Molecular and neuronal mechanisms underlying the effects of adolescent nicotine exposure on anxiety and mood disorders. Neuropharmacology 2020, 184, 108411. [Google Scholar] [CrossRef]
- Branchi, I. The mouse communal nest: Investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci. Biobehav. Rev. 2009, 33, 551–559. [Google Scholar] [CrossRef]
- Kappeler, L.; Meaney, M.J. Epigenetics and parental effects. BioEssays 2010, 32, 818–827. [Google Scholar] [CrossRef]
- Sparling, J.E.; Baker, S.L.; Bielajew, C. Effects of combined pre- and post-natal enrichment on anxiety-like, social, and cognitive behaviours in juvenile and adult rat offspring. Behav. Brain Res. 2018, 353, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Hollis, F.; Isgor, C.; Kabbaj, M. The consequences of adolescent chronic unpredictable stress exposure on brain and behavior. Neuroscience 2013, 249, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Sudakov, S.; Alekseeva, E.; Nazarova, G.; Bashkatova, V. Age-Related Individual Behavioural Characteristics of Adult Wistar Rats. Animals 2021, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.E.; Krauter, E.E.; Campbell, B.A. Motor and Reflexive Behavior in the Aging Rat. J. Gerontol. 1980, 35, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Altun, M.; Bergman, E.; Edström, E.; Johnson, H.; Ulfhake, B. Behavioral impairments of the aging rat. Physiol. Behav. 2007, 92, 911–923. [Google Scholar] [CrossRef]
- Sudakov, S.K.; Nazarova, G.A.; Alekseeva, E.; Bashkatova, V.G. Estimation of the Level of Anxiety in Rats: Differences in Results of Open-Field Test, Elevated Plus-Maze Test, and Vogel’s Conflict Test. Bull. Exp. Biol. Med. 2013, 155, 295–297. [Google Scholar] [CrossRef]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Ehninger, D.; Kempermann, G. Paradoxical effects of learning the Morris water maze on adult hippocampal neurogenesis in mice may be explained by a combination of stress and physical activity. Genes, Brain Behav. 2005, 5, 29–39. [Google Scholar] [CrossRef]
- Van Der Lee, S.; Boot, L.M. Spontaneous pseudopregnancy in mice. Acta Physiol. Pharmacol. Neerl. 1955, 4, 443. [Google Scholar]
- Van Der Lee, S.; Boot, L.M. Spontaneous pseudopregnancy in mice. II. Acta Physiol. Pharmacol. Neerl. 1956, 5, 213–214. [Google Scholar]
- Whitten, W.K. Modification of the oestrus cycle of the mouse by external stimuli associated with the male. J. Endocrinol. 1956, 13, 399–404. [Google Scholar] [CrossRef]
- Whitten, W.K. Modification of the oestrus cycle of the mouse by external stimuli associated with the male: Changes in the oestrus cycle determined by vaginal smears. J. Endocrinol. 1958, 17, 307–313. [Google Scholar] [CrossRef]
- Bruce, H.M. An Exteroceptive Block to Pregnancy in the Mouse. Nature 1959, 184, 105. [Google Scholar] [CrossRef]
- Parkes, A.S.; Bruce, H.M. Olfactory Stimuli in Mammalian Reproduction. Science 1961, 134, 1049–1054. [Google Scholar] [CrossRef]
- Koyama, S.; Soini, H.A.; Wager-Miller, J.; Alley, W.R.; Pizzo, M.J.; Rodda, C.; Alberts, J.R.; Crystal, J.D.; Lai, C.; Foley, J.; et al. Cross-generational impact of a male murine pheromone 2-sec-butyl-4,5-dihydrothiazole in female mice. Proc. R. Soc. B Boil. Sci. 2015, 282, 20151074. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic Acids in the Brain: Gangliosides and Polysialic Acid in Nervous System Development, Stability, Disease, and Regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef]
- Chen, H.; Qu, Z.; Liu, W. Effects of Simulated Mobile Phone Electromagnetic Radiation on Fertilization and Embryo Development. Fetal Pediatr. Pathol. 2016, 36, 123–129. [Google Scholar] [CrossRef]
- Rijn, C.M.V.; Krijnen, H.; Menting-Hermeling, S.; Coenen, A.M.L. Decapitation in Rats: Latency to Unconsciousness and the ‘Wave of Death’. PLoS ONE 2011, 6, e16514. [Google Scholar] [CrossRef]
- Ramos-Ortolaza, D.L.; Doreste-Mendez, R.J.; Alvarado-Torres, J.K.; Torres-Reveron, A. Ovarian hormones modify anxiety behavior and glucocorticoid receptors after chronic social isolation stress. Behav. Brain Res. 2017, 328, 115–122. [Google Scholar] [CrossRef]
- Gouveia, A.; dos Santos, U.D.; Felisbino, F.E.; de Afonseca, T.L.; Antunes, G.; Morato, S. Influence of the estrous cycle on the behavior of rats in the elevated T-maze. Behav. Process. 2004, 67, 167–171. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudakov, S.K.; Bogdanova, N.G.; Nazarova, G.A. RETRACTED: Cross-Generational Impact of Epigenetic Male Influence on Physical Activity in Rat. Biology 2022, 11, 1606. https://doi.org/10.3390/biology11111606
Sudakov SK, Bogdanova NG, Nazarova GA. RETRACTED: Cross-Generational Impact of Epigenetic Male Influence on Physical Activity in Rat. Biology. 2022; 11(11):1606. https://doi.org/10.3390/biology11111606
Chicago/Turabian StyleSudakov, Sergey K., Natalia G. Bogdanova, and Galina A. Nazarova. 2022. "RETRACTED: Cross-Generational Impact of Epigenetic Male Influence on Physical Activity in Rat" Biology 11, no. 11: 1606. https://doi.org/10.3390/biology11111606
APA StyleSudakov, S. K., Bogdanova, N. G., & Nazarova, G. A. (2022). RETRACTED: Cross-Generational Impact of Epigenetic Male Influence on Physical Activity in Rat. Biology, 11(11), 1606. https://doi.org/10.3390/biology11111606




