Effect of Heat Stress on Some Physiological and Anatomical Characteristics of Rice (Oryza sativa L.) cv. KDML105 Callus and Seedling
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Conditions
2.2. MDA Content
- Vf = final volume
- Ve = volume of TCA used for extraction
- Va = volume of supernatants used in absorbance detection
- FW = fresh weight of samples
2.3. Electrolyte Leakage
2.4. Chlorophyll Content
- V = volume of 80% acetone
- W = sample fresh weight
2.5. Peeling Technique
2.6. Statistical Analysis
3. Results
3.1. Seedling Growth and Development
3.2. Callus Induction of KDML105 under Heat Stress
3.3. Physiological Response of KDML105 Rice Seedlings to Heat Stress
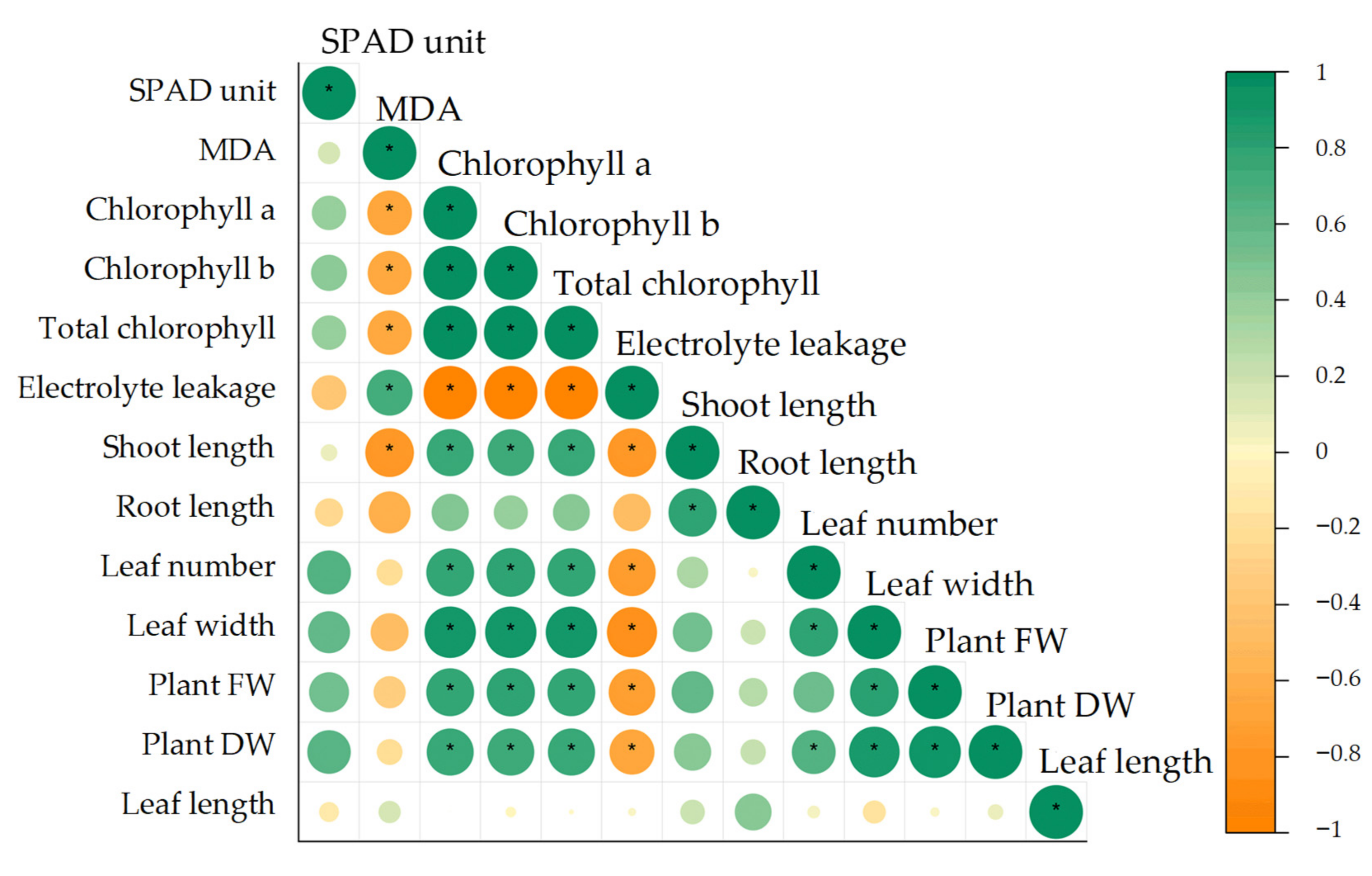
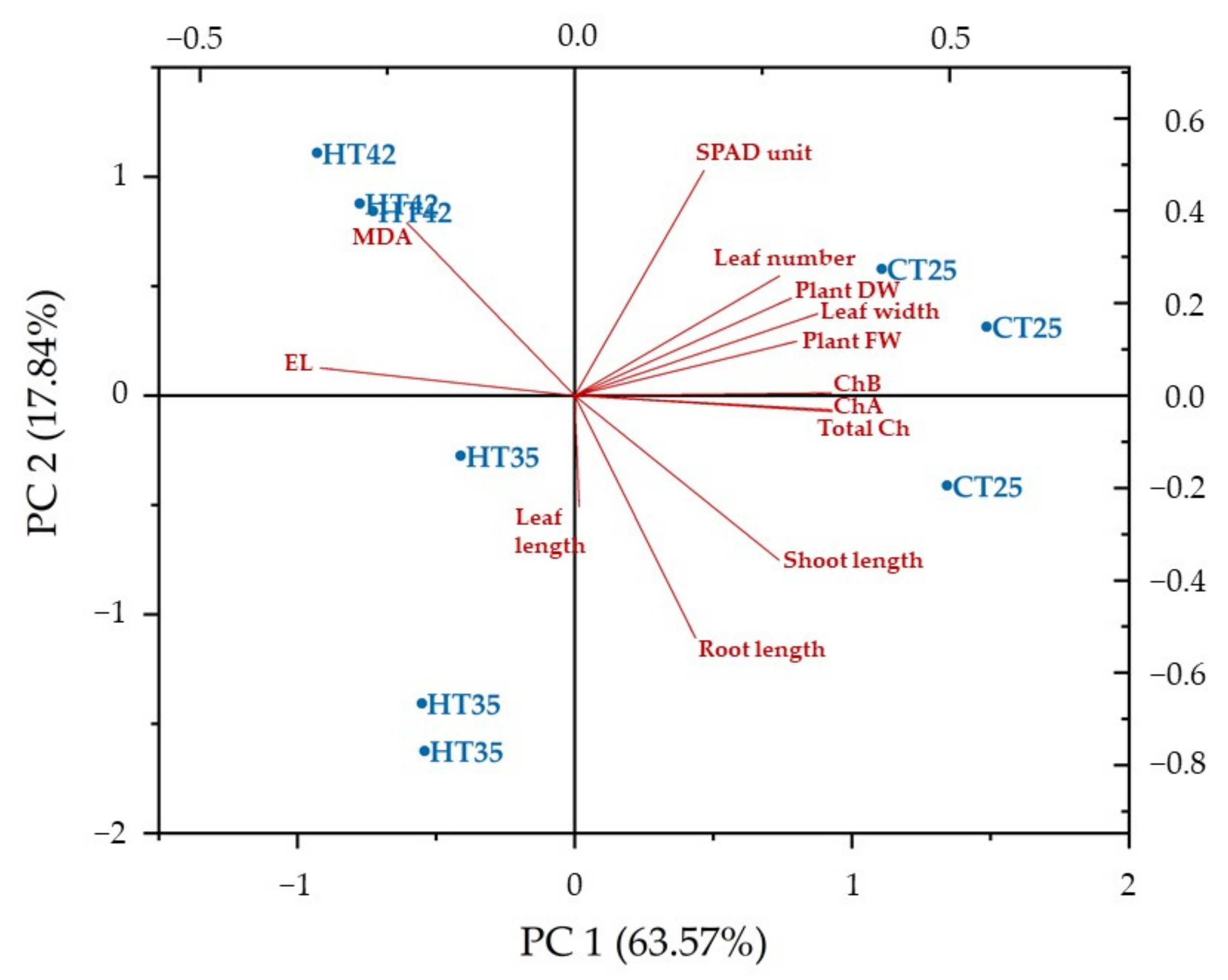
3.4. Pearson’s Correlation, Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA) for Growth and Physiological Parameters
3.5. Leaf Surface Anatomy of KDML105 Rice Seedlings Grown under Heat Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senapeng, P.; Prahadchai, T.; Guayjarernpanishk, P.; Park, J.S.; Busababodhin, P. Spatial modeling of extreme temperature in northeast Thailand. Atmosphere 2022, 13, 589. [Google Scholar] [CrossRef]
- Bandumula, N. Rice production in Asia: Key to global food security. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 88, 1323–1328. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; McCarl, B.; Chang, C.C. Climate change, sea level rise and Rice: Global market implications. Clim. Chang. 2012, 110, 543–560. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Wang, C.; Linderholm, H.W.; Fu, Y.; Cai, W.; Xu, J.; Zhuang, L.; Wu, M.; Shi, Y.; Wang, G.; et al. The negative impact of increasing temperatures on rice yields in southern China. Sci. Total Environ. 2022, 820, 153262. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef]
- Thai Meteorological Department. Map of Maximum Temperature in Thailand. 2022. Available online: https://www3.tmd.go.th/climate/hightemperaturemap (accessed on 21 October 2022).
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific Fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Y.; Zhang, Q.; Cui, Y.; Xiang, J.; Chen, H.; Hu, G.; Chen, Y.; Wang, X.; Zhu, D.; et al. Comparative transcriptome analysis of panicle development under heat stress in two rice (Oryza sativa L.) cultivars differing in heat tolerance. PeerJ 2019, 7, e7595. [Google Scholar] [CrossRef] [Green Version]
- Rann, A.; Anusontpornperm, S.; Thanachit, S.; Sreewongchai, T. Response of KDML105 and RD41 rice varieties grown on a Typic Natrustalf to granulated pig manure and chemical fertilizers. Agric. Nat. Resour. 2016, 50, 104–113. [Google Scholar] [CrossRef]
- Borriboon, W.; Lontom, W.; Pongdontri, P.; Theerakulpisut, P.; Dongsansuk, A. Effects of Short- and Long-Term Temperature on Seed Germination, Oxidative Stress and Membrane Stability of Three Rice Cultivars (Dular; KDML105 and Riceberry). Pertanika J. Trop. Agric. Sci. 2018, 41, 151–162. [Google Scholar]
- Prasertthai, P.; Paethaisong, W.; Theerakulpisut, P.; Dongsansuk, A. High temperature alters leaf lipid membrane composition associated with photochemistry of PSII and membrane thermostability in rice seedlings. Plants 2022, 11, 1454. [Google Scholar] [CrossRef] [PubMed]
- Kondamudi, R.; Swamy, K.N.; Chakravarthy, D.V.; Vishnuprasanth, V.; Rao, Y.V.; Rao, P.R.; Sarla, N.; Subrahmanyam, D.; Voleti, S.R. Heat stress in Rice—Physiological mechanisms and adaptation strategies. In Crop Stress and Its Management: Perspectives and Strategies; Venkateswarlu, B., Arun, K.S., Chitra, S., Masheswari, M., Eds.; Springer: Berlin, Germany, 2012; pp. 193–224. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Chen, L. Effect of heat stress on growth and physiological traits of alfalfa (Medicago sativa L.) and a comprehensive evaluation for heat tolerance. Agronomy 2019, 9, 597. [Google Scholar] [CrossRef] [Green Version]
- Rosmaina; Utami, D.; Aryanti, E.; Zulfahmi. Impact of heat stress on germination and seedling growth of chili pepper (Capsicum annuum L.). IOP Conf. Ser. Earth Environ. Sci. 2021, 637, 012032. [Google Scholar] [CrossRef]
- Begcy, K.; Sandhu, J.; Walia, H. Transient heat stress during early seed development primes germination and seedling establishment in Rice. Front. Plant Sci. 2018, 9, 1768. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Shi, Z.; Chang, T.G.; Chen, F.; Zhao, H.; Song, Q.; Wang, M.; Wang, Y.; Zhou, Z.; Wang, C.; Zhou, S.C.; et al. Morphological and physiological factors contributing to early vigor in the elite rice cultivar 9311. Sci. Rep. 2020, 10, 14813. [Google Scholar] [CrossRef]
- Giri, A.; Heckathorn, S.; Mishra, S.; Krause, C. Heat stress decreases levels of nutrient uptake and assimilation proteins in tomato roots. Plants 2017, 6, 6. [Google Scholar] [CrossRef]
- Alhaithloul, H.A. Impact of combined heat and drought stress on the potential growth responses of the Desert Grass Artemisia sieberi alba: Relation to biochemical and molecular adaptation. Plants 2019, 8, 416. [Google Scholar] [CrossRef]
- Xu, Y.; Chu, C.; Yao, S. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Feng, B.; Liu, P.; Li, G.; Dong, S.T.; Wang, F.H.; Kong, L.A.; Zhang, J.W. Effect of heat stress on the photosynthetic characteristics in flag leaves at the grain-filling stage of different heat-resistant winter wheat varieties. J. Agron. Crop Sci. 2013, 200, 143–155. [Google Scholar] [CrossRef]
- Sánchez-Reinoso, A.D.; Garcés-Varón, G.; Restrepo-Díaz, H. Biochemical and physiological characterization of three rice cultivars under different daytime temperature conditions. Chil. J. Agric. Res. 2014, 74, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.-L.; Chen, J.-H.; He, N.-Y.; Guo, F.-Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Gull, S.; Ali, M.M.; Yousef, A.F.; Ercisli, S.; Kalaji, H.M.; Telesiński, A.; Auriga, A.; Wróbel, J.; Radwan, N.S.; et al. Heat stress mitigation in tomato (Solanum lycopersicum L.) through foliar application of gibberellic acid. Sci. Rep. 2022, 12, 11324. [Google Scholar] [CrossRef]
- Rai, A.N.; Saini, N.; Yadav, R.; Suprasanna, P. A potential seedling-stage evaluation method for heat tolerance in Indian mustard (Brassica juncea L. Czern and Coss). 3 Biotech 2020, 10, 114. [Google Scholar] [CrossRef]
- Tulkova, E.; Kabashnikova, L. Malondialdehyde content in the leaves of small-leaved linden tilia cordata and Norway maple acer platanoides under the influence of volatile organic compounds. Plant Biosyst. 2021, 156, 619–627. [Google Scholar] [CrossRef]
- Niu, Y.; Xiang, Y. An overview of biomembrane functions in plant responses to high-temperature stress. Front. Plant Sci. 2018, 9, 915. [Google Scholar] [CrossRef] [Green Version]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and Metabolic Adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Su, X.; Wei, F.; Huo, Y.; Xia, Z. Comparative physiological and molecular analyses of two contrasting flue-cured tobacco genotypes under progressive drought stress. Front. Plant Sci. 2017, 8, 827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafqat, W.; Mazrou, Y.S.; Sami-ur-Rehman; Nehela, Y.; Ikram, S.; Bibi, S.; Naqvi, S.A.; Hameed, M.; Jaskani, M.J. Effect of three water regimes on the physiological and anatomical structure of stem and leaves of different citrus rootstocks with distinct degrees of tolerance to drought stress. Horticulturae 2021, 7, 554. [Google Scholar] [CrossRef]
- Wang, R.; Ding, S.; Hu, X.; Zhang, Y. Stability of chlorophyll–protein complex (photosystem II) in processed spinach: Effect of high hydrostatic pressure. Int. J. Food Prop. 2017, 20 (Suppl. S3), S3177–S3188. [Google Scholar] [CrossRef] [Green Version]
- Sarabi, B.; Ghashghaie, J. Evaluating the physiological and biochemical responses of melon plants to NaCl salinity stress using supervised and unsupervised statistical analysis. Plant Stress 2022, 4, 100067. [Google Scholar] [CrossRef]
- Yamamoto, Y. Quality Control of Photosystem II: The mechanisms for avoidance and tolerance of light and heat stresses are closely linked to membrane fluidity of the thylakoids. Front. Plant Sci. 2016, 7, 1136. [Google Scholar] [CrossRef] [Green Version]
- Filho, E.G.; Braga, L.N.; Silva, L.M.; Miranda, F.R.; Silva, E.O.; Canuto, K.M.; Miranda, M.R.; de Brito, E.S.; Zocolo, G.J. Physiological changes for drought resistance in different species of Phyllanthus. Sci. Rep. 2018, 8, 15141. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Liu, H.; Hua, L.; Luo, Q.; Lin, Y.; He, P.; Feng, S.; Liu, J.; Ye, Q. Differential responses of stomata and photosynthesis to elevated temperature in two co-occurring subtropical forest tree species. Front. Plant Sci. 2018, 9, 467. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.F.; Zhao, B.; Xu, J.J.; Liang, W.; Huang, W.M.; Li, H.H. Effects of heat stress on changes in physiology and anatomy in two cultivars of Rhododendron. S. Afr. J. Bot. 2017, 112, 338–345. [Google Scholar] [CrossRef]
- Carrera, C.S.; Solís, S.M.; Ferrucci, M.S.; Vega, C.C.R.; Galati, B.G.; Ergo, V.; Andrade, F.H.; Lascano, R.H. Leaf structure and ultrastructure changes induced by heat stress and drought during seed filling in field-grown soybean and their relationship with grain yield. An. Acad. Bras. Cienc. 2021, 93, e20191388. [Google Scholar] [CrossRef]
- Zhou, R.; Kjaer, K.H.; Rosenqvist, E.; Yu, X.; Wu, Z.; Ottosen, C.-O. Physiological response to heat stress during seedling and anthesis stage in tomato genotypes differing in heat tolerance. J. Agron. Crop Sci. 2016, 203, 68–80. [Google Scholar] [CrossRef]
- Gu, K.; Geng, X.M.; Yue, Y.; Ozaki, Y. Contribution of keeping more stable anatomical structure under high temperature to heat resistance of Rhododendron seedlings. J. Fac. Agric. Kyushu Univ. 2016, 61, 273–279. [Google Scholar] [CrossRef]
- Benderradji, L.; Brini, F.; Kellou, K.; Ykhlef, N.; Djekoun, A.; Masmoudi, K.; Bouzerzour, H. Callus induction, proliferation, and plantlets regeneration of two bread wheat (Triticum aestivum L.) genotypes under saline and heat stress conditions. ISRN Agron. 2012, 2012, 367851. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Song, L.; Wang, X.; Bi, Y. Effect of abscisic acid on heat stress tolerance in the calli from two ecotypes of Phragmites communis. Biol. Plant. 2010, 54, 607–613. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.; Budzinski, I.G.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Mostafiz, S.B.; Wagiran, A.; Johan, N.S.; Abdullah Zulkifli, N.S.; Ng, J.M. The effects of temperature on callus induction and regeneration in selected Malaysian rice cultivar Indica. Sains Malays. 2018, 47, 2647–2655. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, P.; Kumar, S.; Saxena, S.N.; Khandelwal, V.; Rizwan, M. Heat treatment affects regeneration, protein expression and genetic make-up of Vigna aconitifolia (Jacq.) Marechal. Ann. Agrar. Sci. 2018, 16, 116–120. [Google Scholar] [CrossRef]
- Haque, M.; Islam, S.M.; Subramaniam, S. Effects of salt and heat pre-treatment factors on efficient regeneration in barley (Hordeum vulgare L.). 3 Biotech 2017, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Ngcala, M.G.; Goche, T.; Brown, A.P.; Chivasa, S.; Ngara, R. Heat stress triggers differential protein accumulation in the extracellular matrix of Sorghum cell suspension cultures. Proteomes 2020, 8, 29. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.-J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
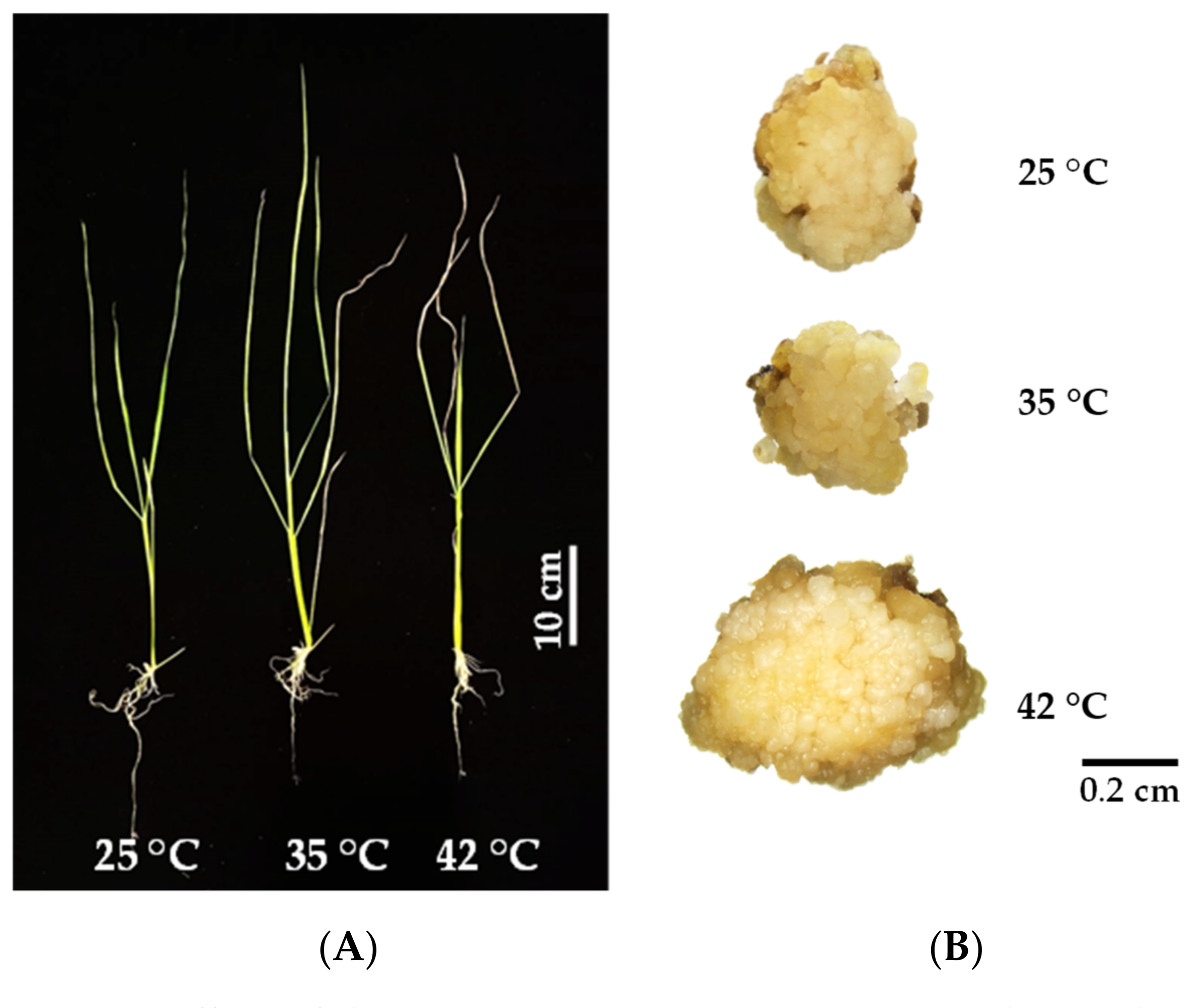


| Characteristics | Temperatures (°C) | ||
|---|---|---|---|
| 25 | 35 | 42 | |
| Growth of seedlings | |||
| Survival percentage (%) | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| Shoot length (cm) | 27.20 ± 0.41 a | 26.39 ± 0.18 a | 24.09 ± 0.09 b |
| Root length (cm) | 6.16 ± 0.64 a | 6.29 ± 0.26 a | 4.53 ± 0.33 b |
| Leaf number | 6.00 ± 0.38 a | 4.25 ± 0.14 b | 4.75 ± 0.25 b |
| Leaf width (cm) | 0.27 ± 0.00 a | 0.19 ± 0.00 b | 0.21 ± 0.00 c |
| Leaf length (cm) | 14.93 ± 1.20 a | 16.57 ± 0.28 a | 14.35 ± 3.70 a |
| Fresh weight (mg) | 373.84 ± 75.13 a | 172.42 ± 16.63 b | 189.12 ± 19.91 b |
| Dry weight (mg) | 60.75 ± 7.01 a | 32.08 ± 2.42 b | 41.50 ± 3.19 b |
| Physiological characteristics | |||
| SPAD value | 29.17 ± 0.02 a | 25.00 ± 2.00 b | 27.83 ± 0.07 ab |
| MDA (µmole mg FW−1) | 0.02 ± 0.00 b | 0.09 ± 0.04 b | 0.30 ± 0.07 a |
| Electrolyte leakage (%) | 28.24 ± 0.56 c | 40.25 ± 0.27 b | 44.71 ± 0.10 a |
| Chlorophyll a (mg/g tissue) | 0.1812 ± 0.0001 a | 0.1305 ± 0.0021 b | 0.1194 ± 0.0016 c |
| Chlorophyll b (mg/g tissue) | 0.0780 ± 0.0013 a | 0.0614 ± 0.0001 b | 0.0583 ± 0.0003 c |
| Total chlorophyll (mg/g tissue) | 0.2592 ± 0.0011 a | 0.1919 ± 0.0035 b | 0.1776 ± 0.0031 c |
| Callus induction | |||
| Survival percentage (%) | 91.67 ± 8.33 a | 66.67 ± 8.33 a | 66.67 ± 8.33 a |
| Callus width (cm) | 0.34 ± 0.05 a | 0.46 ± 0.02 a | 0.54 ± 0.13 a |
| Callus length (cm) | 0.64 ± 0.09 a | 0.96 ± 0.25 a | 0.80 ± 0.17 a |
| Fresh weight (mg) | 31.80 ± 0.01 a | 54.80 ± 0.01 a | 73.2 ± 0.09 a |
| Dry weight (mg) | 7.00 ± 0.00 a | 10.8 ± 0.00 a | 13.2 ± 0.01 a |
| Parameter | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 |
|---|---|---|---|---|---|---|---|---|
| SPAD unit | 0.495 | 0.742 | 0.171 | 0.050 | 0.413 | 0.033 | −0.034 | −0.010 |
| MDA | −0.645 | 0.571 | 0.470 | 0.023 | −0.019 | 0.088 | 0.171 | −0.006 |
| Chlorophyll a | 0.988 | −0.053 | −0.056 | −0.093 | −0.056 | 0.036 | 0.040 | −0.047 |
| Chlorophyll b | 0.985 | 0.008 | −0.084 | −0.107 | −0.042 | −0.071 | 0.065 | −0.004 |
| Total chlorophyll | 0.986 | −0.047 | −0.047 | −0.103 | −0.078 | 0.012 | 0.058 | −0.049 |
| Electrolyte leakage | −0.977 | 0.091 | 0.106 | 0.133 | −0.067 | 0.031 | −0.043 | 0.010 |
| Shoot length | 0.783 | −0.542 | 0.028 | 0.125 | 0.257 | −0.106 | −0.007 | 0.008 |
| Root length | 0.462 | −0.799 | 0.246 | 0.096 | 0.080 | 0.256 | 0.071 | 0.027 |
| Leaf number | 0.785 | 0.395 | 0.114 | −0.460 | −0.035 | 0.007 | 0.006 | 0.052 |
| Leaf width | 0.932 | 0.269 | −0.101 | 0.039 | −0.112 | 0.144 | −0.108 | 0.049 |
| Leaf length | 0.852 | 0.179 | 0.163 | 0.405 | −0.111 | −0.167 | 0.089 | 0.049 |
| Plant DW | 0.831 | 0.321 | 0.266 | 0.314 | −0.143 | 0.075 | −0.099 | −0.044 |
| Plant FW | 0.017 | −0.367 | 0.899 | −0.192 | −0.048 | −0.103 | −0.085 | −0.007 |
| Variance (%) | 63.570 | 17.844 | 9.718 | 4.530 | 2.365 | 1.217 | 0.640 | 0.117 |
| CV (%) | 63.570 | 81.415 | 91.132 | 95.662 | 98.027 | 99.243 | 99.883 | 100.000 |
| Eigenvalue | 8.264 | 2.320 | 1.263 | 0.589 | 0.307 | 0.158 | 0.083 | 0.015 |
| Characteristics | Temperatures (°C) | ||
|---|---|---|---|
| 25 | 35 | 42 | |
| Stomatal length (µm) | 11.24 ± 0.19 b | 13.35 ± 0.55 a | 11.76 ± 0.41 b |
| Stomatal width (µm) | 8.97 ± 0.15 a | 9.93 ± 0.45 a | 8.88 ± 0.19 a |
| Stomatal density | 37.00 ± 0.58 a | 34.33 ± 1.20 a | 39.33 ± 3.84 a |
| Short-epidermal cell length (µm) | 25.13 ± 0.93 a | 28.35 ± 1.02 a | 28.44 ± 1.64 a |
| Short-epidermal cell width (µm) | 5.95 ± 0.00 a | 6.83 ± 0.37 a | 5.82 ± 0.90 a |
| Long-epidermal cell length (µm) | 59.61 ± 3.37 a | 61.15 ± 4.99 a | 72.44 ± 4.76 a |
| Long-epidermal cell width (µm) | 6.75 ± 0.20 a | 7.19 ± 0.19 a | 6.61 ± 0.40 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taratima, W.; Chuanchumkan, C.; Maneerattanarungroj, P.; Trunjaruen, A.; Theerakulpisut, P.; Dongsansuk, A. Effect of Heat Stress on Some Physiological and Anatomical Characteristics of Rice (Oryza sativa L.) cv. KDML105 Callus and Seedling. Biology 2022, 11, 1587. https://doi.org/10.3390/biology11111587
Taratima W, Chuanchumkan C, Maneerattanarungroj P, Trunjaruen A, Theerakulpisut P, Dongsansuk A. Effect of Heat Stress on Some Physiological and Anatomical Characteristics of Rice (Oryza sativa L.) cv. KDML105 Callus and Seedling. Biology. 2022; 11(11):1587. https://doi.org/10.3390/biology11111587
Chicago/Turabian StyleTaratima, Worasitikulya, Chantima Chuanchumkan, Pitakpong Maneerattanarungroj, Attachai Trunjaruen, Piyada Theerakulpisut, and Anoma Dongsansuk. 2022. "Effect of Heat Stress on Some Physiological and Anatomical Characteristics of Rice (Oryza sativa L.) cv. KDML105 Callus and Seedling" Biology 11, no. 11: 1587. https://doi.org/10.3390/biology11111587
APA StyleTaratima, W., Chuanchumkan, C., Maneerattanarungroj, P., Trunjaruen, A., Theerakulpisut, P., & Dongsansuk, A. (2022). Effect of Heat Stress on Some Physiological and Anatomical Characteristics of Rice (Oryza sativa L.) cv. KDML105 Callus and Seedling. Biology, 11(11), 1587. https://doi.org/10.3390/biology11111587






