Calibrating a Comprehensive Immune Age Metric to Analyze the Cross Sectional Age-Related Decline in Cardiorespiratory Fitness
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. DVS Sample
2.2. Cardiorespiratory Fitness Assessment
2.3. Immune Parameters
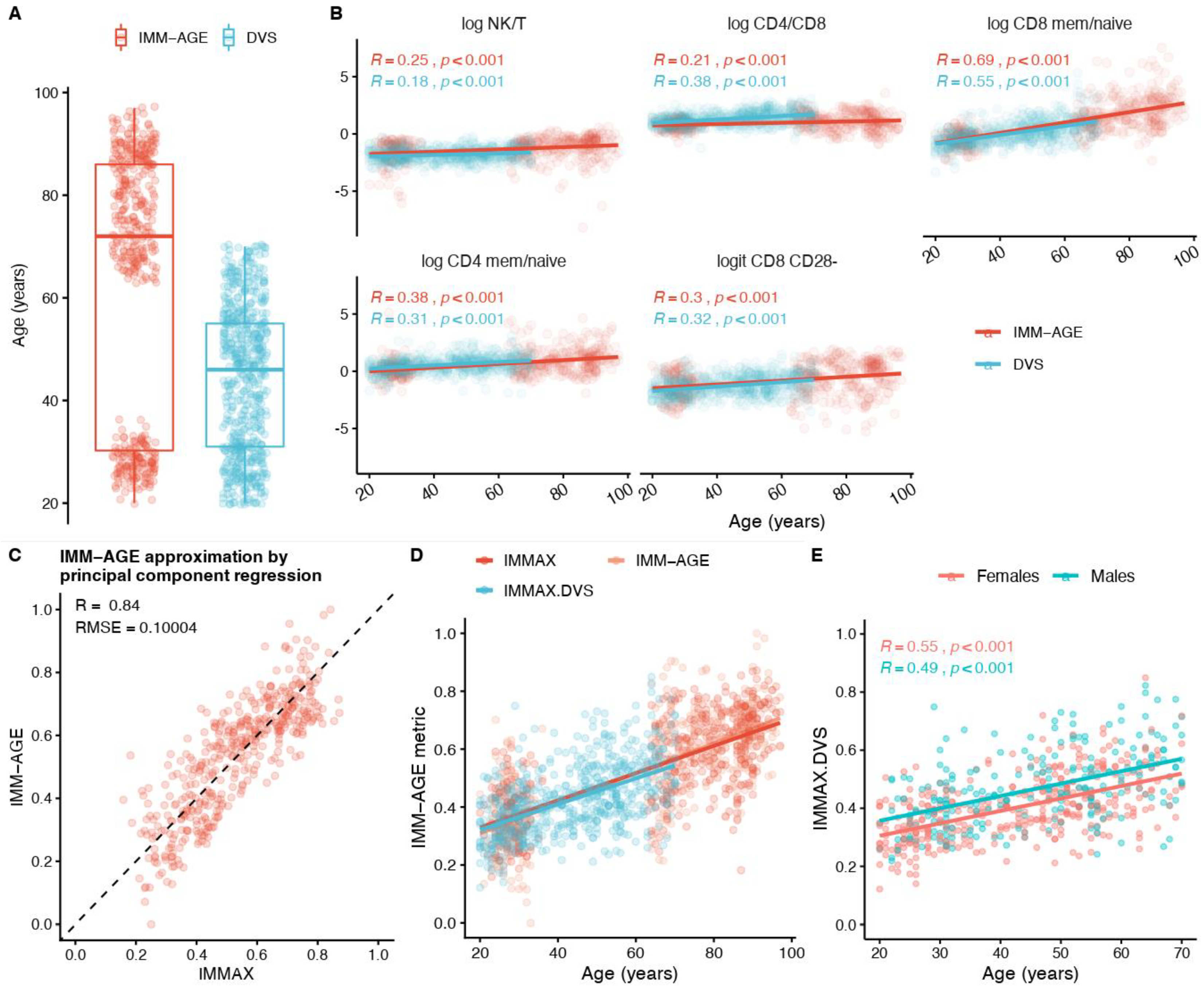
2.4. Approximating IMM-AGE by IMMAX
2.5. Statistical Analysis of Cardiorespiratory Fitness
3. Results
3.1. IMM-AGE Approximation
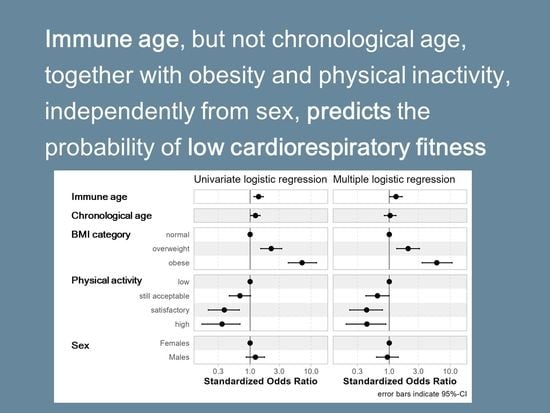
3.2. Associations with Cardiorespiratory Fitness
3.2.1. Univariate Analyses
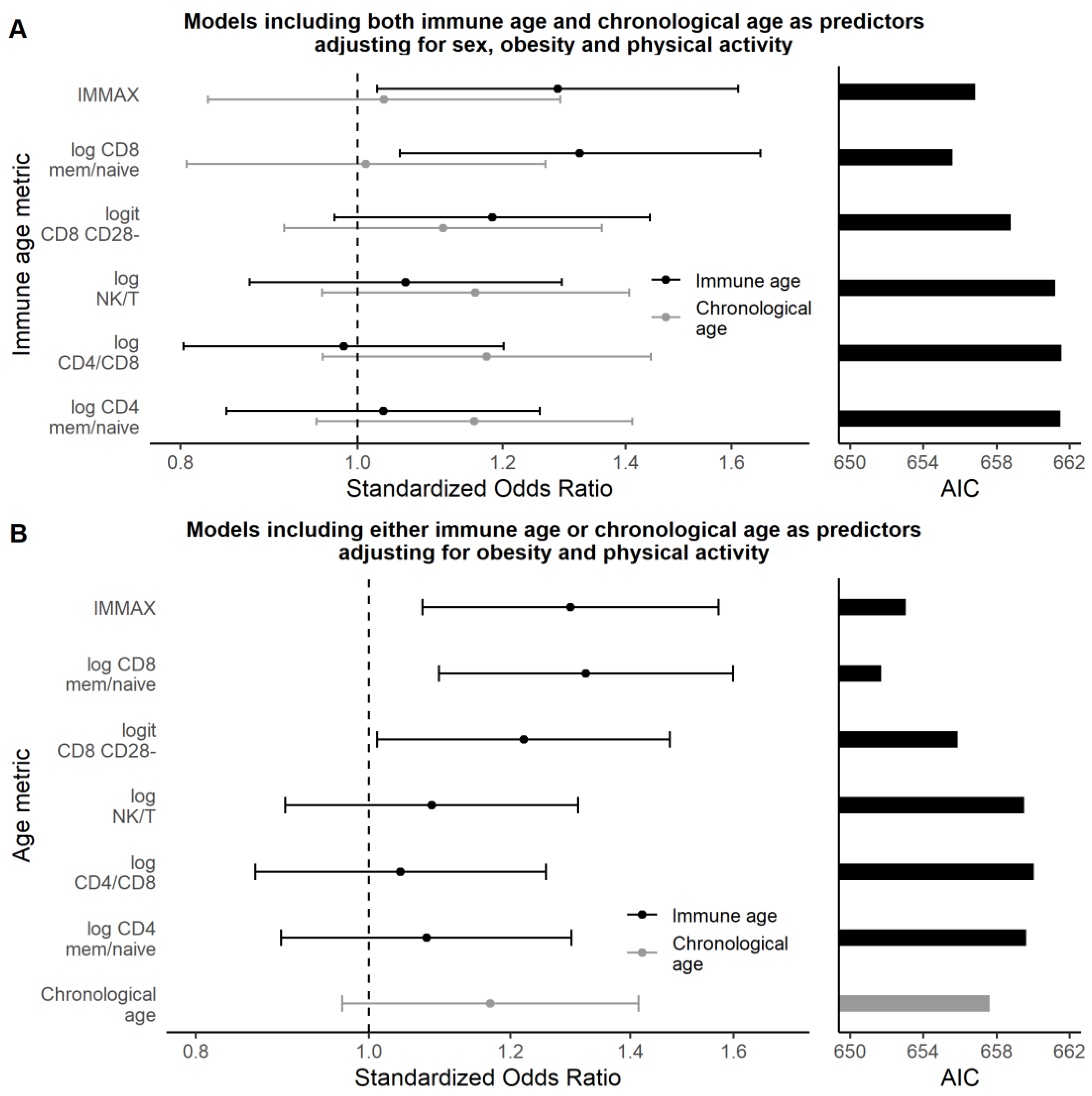
3.2.2. Multivariate Analyses
4. Discussion
4.1. Calibrated Immune Age Metric IMMAX
4.2. Cardiorespiratory Fitness and Immune Age
4.3. Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, J.; McAuley, P.; Lavie, C.J.; Despres, J.-P.; Arena, R.; Kokkinos, P. Physical Activity and Cardiorespiratory Fitness as Major Markers of Cardiovascular Risk: Their Independent and Interwoven Importance to Health Status. Prog. Cardiovasc. Dis. 2015, 57, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Al-Mallah, M.H.; Sakr, S.; Al-Qunaibet, A. Cardiorespiratory Fitness and Cardiovascular Disease Prevention: An Update. Curr. Atheroscler. Rep. 2018, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N.; Kohl, H.W., III; Paffenbarger, R.S., Jr.; Clark, D.G.; Cooper, K.H.; Gibbons, L.W. Physical Fitness and All-Cause Mortality: A Prospective Study of Healthy Men and Women. JAMA 1989, 262, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Imboden, M.T.; Harber, M.P.; Whaley, M.H.; Finch, W.H.; Bishop, D.L.; Kaminsky, L.A. Cardiorespiratory Fitness and Mortality in Healthy Men and Women. J. Am. Coll. Cardiol. 2018, 72, 2283–2292. [Google Scholar] [CrossRef]
- Burdorf, A.; Robroek, S. Promoting work ability through exercise programmes. Lancet Public Health 2019, 4, e316–e317. [Google Scholar] [CrossRef] [Green Version]
- Kenny, G.P.; Groeller, H.; McGinn, R.; Flouris, A.D. Age, human performance, and physical employment standards. Appl. Physiol. Nutr. Metab. 2016, 41, S92–S107. [Google Scholar] [CrossRef] [Green Version]
- Kenny, G.P.; Yardley, J.E.; Martineau, L.; Jay, O. Physical work capacity in older adults: Implications for the aging worker. Am. J. Ind. Med. 2008, 51, 610–625. [Google Scholar] [CrossRef]
- Smolander, J.; Blair, S.N.; Kohl, H.W.I. Work Ability, Physical Activity, and Cardiorespiratory Fitness: 2-year Results From Project Active. J. Occup. Environ. Med. 2000, 42, 906–910. [Google Scholar] [CrossRef]
- Tengland, P.A. The concept of work ability. J. Occup. Rehabil. 2011, 21, 275–285. [Google Scholar] [CrossRef]
- Ezzatvar, Y.; Calatayud, J.; Andersen, L.L.; Ramos Vieira, E.; López-Bueno, R.; Casaña, J. Muscular Fitness and Work Ability among Physical Therapists. Int. J. Environ. Res. Public Health 2021, 18, 1722. [Google Scholar] [CrossRef]
- Mänttäri, S.; Oksa, J.; Lusa, S.; Korkiakangas, E.; Punakallio, A.; Oksanen, T.; Laitinen, J. Interventions to promote work ability by increasing physical activity among workers with physically strenuous jobs: A scoping review. Scand. J. Public Health 2021, 49, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Grabara, M.; Nawrocka, A.; Powerska-Didkowska, A. The relationship between physical activity and work ability—A cross-sectional study of teachers. Int. J. Occup. Med. Environ. Health 2018, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.A.; Niemiro, G.; Harridge, S.D.R.; Simpson, R.J.; Lord, J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 2019, 19, 563–572. [Google Scholar] [CrossRef]
- Spielmann, G.; Bigley, A.B.; LaVoy, E.C.; Simpson, R.J. Aging Immunity and the Impact of Physical Exercise. In Immunology of Aging; Massoud, A., Rezaei, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 369–397. [Google Scholar] [CrossRef]
- Spielmann, G.; McFarlin, B.K.; O’Connor, D.P.; Smith, P.J.W.; Pircher, H.; Simpson, R.J. Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav. Immun. 2011, 25, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Smart, T.F.F.; Doleman, B.; Hatt, J.; Paul, M.; Toft, S.; Lund, J.N.; Phillips, B.E. The role of resistance exercise training for improving cardiorespiratory fitness in healthy older adults: A systematic review and meta-analysis. Age Ageing 2022, 51, afac143. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Rosengren, A. Obesity and cardiovascular health: The size of the problem. Eur. Heart J. 2021, 42, 3404–3406. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; Arena, R.; Myers, J.; Peterman, J.E.; Bonikowske, A.R.; Harber, M.P.; Medina Inojosa, J.R.; Lavie, C.J.; Squires, R.W. Updated Reference Standards for Cardiorespiratory Fitness Measured with Cardiopulmonary Exercise Testing: Data from the Fitness Registry and the Importance of Exercise National Database (FRIEND). Mayo Clin. Proc. 2022, 97, 285–293. [Google Scholar] [CrossRef]
- Zeiher, J.; Manz, K.; Kuntz, B.; Perumal, N.; Keil, T.; Mensink, G.B.M.; Finger, J.D. Individual and interpersonal correlates of cardiorespiratory fitness in adults—Findings from the German Health Interview and Examination Survey. Sci. Rep. 2020, 10, 445. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, T.; So, R.; Takahashi, M. Workers’ physical activity data contribute to estimating maximal oxygen consumption: A questionnaire study to concurrently assess workers’ sedentary behavior and cardiorespiratory fitness. BMC Public Health 2020, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, P.D.; Getzmann, S.; Bröde, P.; Burke, M.; Cadenas, C.; Capellino, S.; Claus, M.; Genç, E.; Golka, K.; Hengstler, J.G.; et al. Impact of Biological and Lifestyle Factors on Cognitive Aging and Work Ability in the Dortmund Vital Study: Protocol of an Interdisciplinary, Cross-sectional, and Longitudinal Study. JMIR Res. Protoc. 2022, 11, e32352. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, P.; Rieker, J.A.; Athanassiou, G.; Bröde, P.; Claus, M.; Golka, K.; Hengstler, J.; Kleinsorge, T.; Nitsche, M.; Reinders, J.; et al. A Systematic Analysis Of Biological And Environmental Factors Contributing To Work Ability Across The Working Lifespan: A Cross-Sectional Study (Preprint). JMIR Prepr. 2022. [Google Scholar] [CrossRef]
- Wood, T.R.; Jóhannsson, G.F. Metabolic health and lifestyle medicine should be a cornerstone of future pandemic preparedness. Lifestyle Med. 2020, 1, e2. [Google Scholar] [CrossRef]
- Rutledge, J.; Oh, H.; Wyss-Coray, T. Measuring biological age using omics data. Nat. Rev. Genet. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, S.; Zhou, W.; Schüssler-Fiorenza Rose, S.M.; Sailani, M.R.; Contrepois, K.; Avina, M.; Ashland, M.; Brunet, A.; Snyder, M. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nat. Med. 2020, 26, 83–90. [Google Scholar] [CrossRef]
- Levine, M.E. Modeling the Rate of Senescence: Can Estimated Biological Age Predict Mortality More Accurately Than Chronological Age? J. Gerontol. Ser. A 2012, 68, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef] [Green Version]
- Goudsmit, J.; van den Biggelaar, A.H.J.; Koudstaal, W.; Hofman, A.; Koff, W.C.; Schenkelberg, T.; Alter, G.; Mina, M.J.; Wu, J.W. Immune age and biological age as determinants of vaccine responsiveness among elderly populations: The Human Immunomics Initiative research program. Eur. J. Epidemiol. 2021, 36, 753–762. [Google Scholar] [CrossRef]
- Higgins-Chen, A.T.; Thrush, K.L.; Levine, M.E. Aging biomarkers and the brain. Semin. Cell Dev. Biol. 2021, 116, 180–193. [Google Scholar] [CrossRef]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wong, G.; Hwang, Y.Y.; Larbi, A. The untwining of immunosenescence and aging. Semin. Immunopathol. 2020, 42, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Alpert, A.; Pickman, Y.; Leipold, M.; Rosenberg-Hasson, Y.; Ji, X.; Gaujoux, R.; Rabani, H.; Starosvetsky, E.; Kveler, K.; Schaffert, S.; et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 2019, 25, 487–495. [Google Scholar] [CrossRef]
- Brzezińska, A.; Magalska, A.; Szybińska, A.; Sikora, E. Proliferation and apoptosis of human CD8+CD28+ and CD8+CD28− lymphocytes during aging. Exp. Gerontol. 2004, 39, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Huff, W.X.; Kwon, J.H.; Henriquez, M.; Fetcko, K.; Dey, M. The Evolving Role of CD8+CD28− Immunosenescent T Cells in Cancer Immunology. Int. J. Mol. Sci. 2019, 20, 2810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goronzy, J.J.; Fang, F.; Cavanagh, M.M.; Qi, Q.; Weyand, C.M. Naive T Cell Maintenance and Function in Human Aging. J. Immunol. 2015, 194, 4073–4080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13, S422–S428. [Google Scholar] [CrossRef] [Green Version]
- Fagnoni, F.F.; Vescovini, R.; Passeri, G.; Bologna, G.; Pedrazzoni, M.; Lavagetto, G.; Casti, A.; Franceschi, C.; Passeri, M.; Sansoni, P. Shortage of circulating naive CD8+ T cells provides new insights on immunodeficiency in aging. Blood 2000, 95, 2860–2868. [Google Scholar] [CrossRef]
- Garrido-Rodríguez, V.; Herrero-Fernández, I.; Castro, M.J.; Castillo, A.; Rosado-Sánchez, I.; Galvá, M.I.; Ramos, R.; Olivas-Martínez, I.; Bulnes-Ramos, Á.; Cañizares, J.; et al. Immunological features beyond CD4/CD8 ratio values in older individuals. Aging 2021, 13, 13443–13459. [Google Scholar] [CrossRef]
- Wikby, A.; Månsson, I.A.; Johansson, B.; Strindhall, J.; Nilsson, S.E. The immune risk profile is associated with age and gender: Findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology 2008, 9, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, K.; Utsuyama, M.; Hayashi, Y.; Kitagawa, M.; Makinodan, T.; Fulop, T. Slower immune system aging in women versus men in the Japanese population. Immun. Ageing 2013, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasubramanian, R.; Meier, H.C.S.; Vivek, S.; Klopack, E.; Crimmins, E.M.; Faul, J.; Nikolich-Žugich, J.; Thyagarajan, B. Evaluation of T-cell aging-related immune phenotypes in the context of biological aging and multimorbidity in the Health and Retirement Study. Immun. Ageing 2022, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.B.; Swardfager, W.; Maurya, P.K.; Graiff, M.Z.; Pedrini, M.; Asevedo, E.; Cassinelli, A.C.; Bauer, M.E.; Cordeiro, Q.; Scott, J.; et al. An immunological age index in bipolar disorder: A confirmatory factor analysis of putative immunosenescence markers and associations with clinical characteristics. Int. J. Methods Psychiatr. Res. 2018, 27, e1614. [Google Scholar] [CrossRef] [Green Version]
- Sayed, N.; Huang, Y.; Nguyen, K.; Krejciova-Rajaniemi, Z.; Grawe, A.P.; Gao, T.; Tibshirani, R.; Hastie, T.; Alpert, A.; Cui, L.; et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat. Aging 2021, 1, 598–615. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Garcia, D.; Blomberg, B.B. B Cell Immunosenescence. Annu. Rev. Cell Dev. Biol. 2020, 36, 551–574. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Guerville, F.; De Souto Barreto, P.; Ader, I.; Andrieu, S.; Casteilla, L.; Dray, C.; Fazilleau, N.; Guyonnet, S.; Langin, D.; Liblau, R.; et al. Revisiting the Hallmarks of Aging to Identify Markers of Biological Age. J. Prev. Alzheimer’s Dis. 2020, 7, 56–64. [Google Scholar] [CrossRef]
- Henrickson, S.E. Is your immune system over the hill? Sci. Immunol. 2019, 4, eaax8198. [Google Scholar] [CrossRef]
- Koff, W.C.; Williams, M.A. Covid-19 and Immunity in Aging Populations—A New Research Agenda. N. Engl. J. Med. 2020, 383, 804–805. [Google Scholar] [CrossRef]
- Pawelec, G. The human immunosenescence phenotype: Does it exist? Semin. Immunopathol. 2020, 42, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Maecker, H.T.; Brodin, P.; Nygaard, U.C.; Lyu, S.C.; Davis, M.M.; Nadeau, K.C.; Andorf, S. Aging and CMV discordance are associated with increased immune diversity between monozygotic twins. Immun. Ageing 2021, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Barletta, W.A. The Influence of SARS-CoV-2 Variants on National Case-Fatality Rates: Correlation and Validation Study. JMIRx Med. 2022, 3, e32935. [Google Scholar] [CrossRef] [PubMed]
- WHO. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. In WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Höltke, V.; Jakob, E. Lüdenscheider Aktivitätsfragebogen zum Risikofaktor Bewegungsmangel [Lüdenscheid Physical Activity Questionnaire Concerning the Risk Factor Inactivity]. Available online: https://www.gesundheitszentrum-hellersen.de/fileadmin/Sportklinik/Dokumente/Informationen/GZH_Aktivitaetsfragebogen.pdf (accessed on 14 July 2022).
- Farnad, L.; Ghasemian-Shirvan, E.; Mosayebi-Samani, M.; Kuo, M.-F.; Nitsche, M.A. Exploring and optimizing the neuroplastic effects of anodal transcranial direct current stimulation over the primary motor cortex of older humans. Brain Stimul. 2021, 14, 622–634. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Falkenstein, M. Lifelong physical activity and executive functions in older age assessed by memory based task switching. Neuropsychologia 2015, 73, 195–207. [Google Scholar] [CrossRef]
- Getzmann, S.; Falkenstein, M.; Gajewski, P.D. Long-Term Cardiovascular Fitness Is Associated with Auditory Attentional Control in Old Adults: Neuro-Behavioral Evidence. PLoS ONE 2013, 8, e74539. [Google Scholar] [CrossRef] [Green Version]
- Finger, J.D.; Gößwald, A.; Härtel, S.; Müters, S.; Krug, S.; Hölling, H.; Kuhnert, R.; Bös, K. Messung der kardiorespiratorischen Fitness in der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) [Measurement of cardiorespiratory fitness in the German Health Interview and Examination Survey for Adults (DEGS1)]. Bundesgesundheitsblatt-Gesundh. Gesundh. 2013, 56, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Andersen, K.L.; Shephard, R.J.; Denolin, H.; Varnauskas, E.; Masironi, R.; World Health Organization. Fundamentals of exercise testing. Available online: https://apps.who.int/iris/handle/10665/40145 (accessed on 20 September 2022).
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise Standards for Testing and Training. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Platen, P. Biologische Grundlagen: Beurteilung der körperlichen Leistungsfähigkeit. In Lehrbuch der Sportmedizin; Rost, R., Ed.; Deutscher Ärzteverlag: Köln, Germany, 2001; pp. 48–66. [Google Scholar]
- Claus, M.; Dychus, N.; Ebel, M.; Damaschke, J.; Maydych, V.; Wolf, O.T.; Kleinsorge, T.; Watzl, C. Measuring the immune system: A comprehensive approach for the analysis of immune functions in humans. Arch. Toxicol. 2016, 90, 2481–2495. [Google Scholar] [CrossRef]
- van den Boogaart, K.G.; Tolosana-Delgado, R. Analyzing Compositional Data with R; Springer: Berlin/Heidelberg, Germany, 2013; p. 255. [Google Scholar] [CrossRef]
- Liland, K.H.; Mevik, B.-H.; Wehrens, R. pls: Partial Least Squares and Principal Component Regression; R Package Version 2.8-1; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Smithson, M.; Verkuilen, J. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol. Methods 2006, 11, 54–71. [Google Scholar] [CrossRef] [Green Version]
- Venables, W.N.; Ripley, B.D. Generalized Linear Models. In Modern Applied Statistics with S; Springer: New York, NY, USA, 2002; pp. 183–210. [Google Scholar] [CrossRef]
- Burnham, K.; Anderson, D.; Huyvaert, K. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Finger, J.D.; Banzer, W.; Baumeister, S.E.; Brandes, M.; Bös, K.; Gabrys, L.; Gößwald, A.; Härtel, S.; Kluttig, A.; Kuhnert, R.; et al. Referenzwerte für die kardiorespiratorische Fitness der allgemeinen Bevölkerung: Die Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) 2008–2011 [Reference Values for Cardiorespiratory Fitness of the General Population: The German National Health Interview and Examination Survey for Adults (DEGS1) 2008–2011]. Gesundheitswesen 2021, 83, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Noonan, V.; Dean, E. Submaximal Exercise Testing: Clinical Application and Interpretation. Phys. Ther. 2000, 80, 782–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björkman, F.; Ekblom, Ö.; Ekblom-Bak, E.; Bohman, T. The ability of a submaximal cycle ergometer test to detect longitudinal changes in VO2max. BMC Sport. Sci. Med. Rehabil. 2021, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jadhav, R.R.; Gustafson, C.E.; Le Saux, S.; Ye, Z.; Li, X.; Tian, L.; Weyand, C.M.; Goronzy, J.J. Distinct Age-Related Epigenetic Signatures in CD4 and CD8 T Cells. Front. Immunol. 2020, 11, 585168. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun. Ageing 2012, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenk, A.; Esser, T.; Belen, S.; Gunasekara, N.; Joisten, N.; Winker, M.T.; Weike, L.; Bloch, W.; Heidenreich, A.; Herden, J.; et al. Distinct distribution patterns of exercise-induced natural killer cell mobilisation into the circulation and tumor tissue of patients with prostate cancer. Am. J. Physiol. Cell Physiol. 2022, 323, C879–C884. [Google Scholar] [CrossRef]
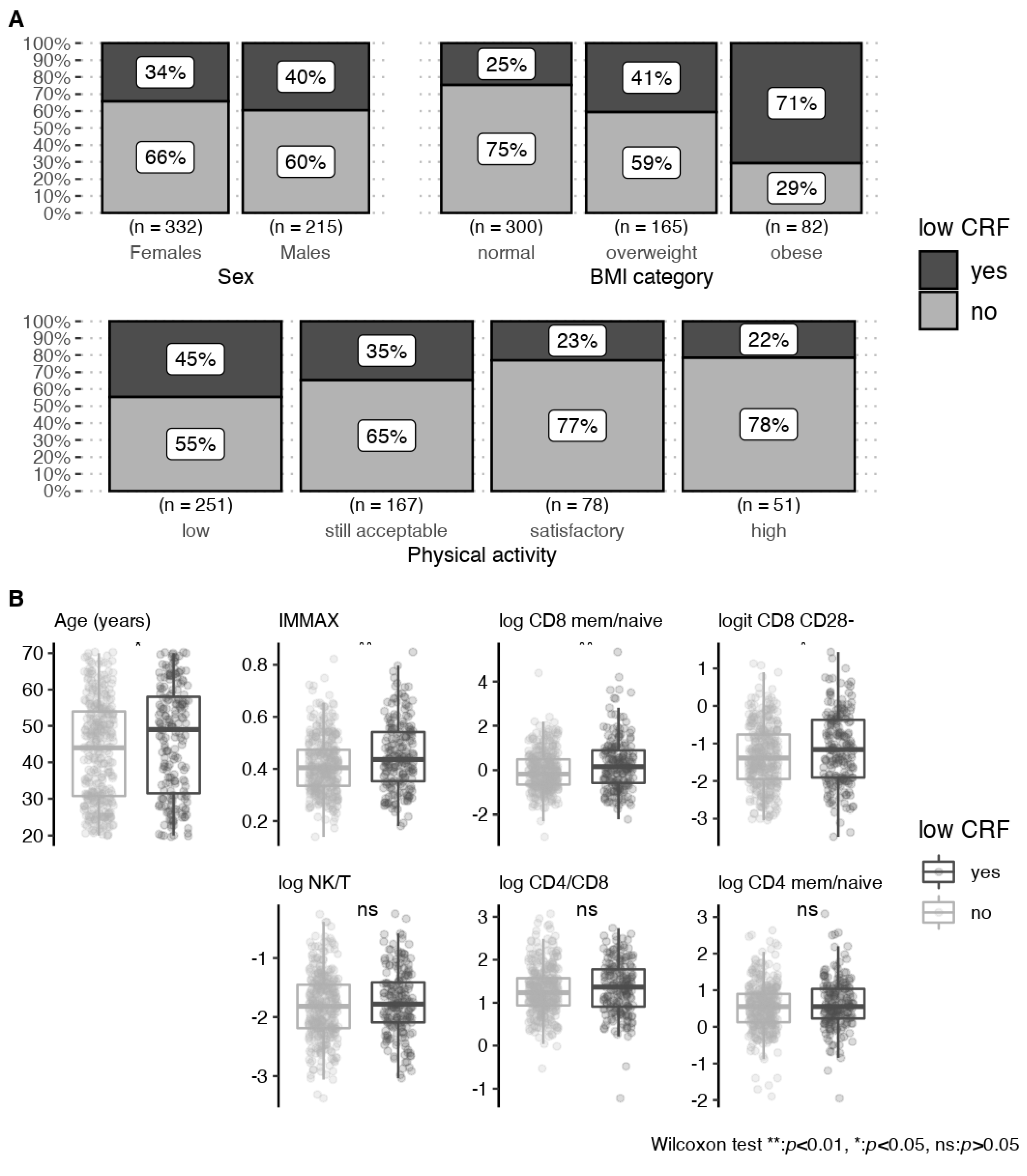
| Characteristic | Overall n = 597 | Females n = 368 | Males n = 229 |
|---|---|---|---|
| Age (years) | 44 (14) | 43 (14) | 46 (14) |
| Body height (m) | 1.73 (0.09) | 1.68 (0.07) | 1.82 (0.07) |
| Bodymass(kg) | 77 (17) | 70 (15) | 88 (16) |
| #missing | 3 | 2 | 1 |
| BMI category | |||
| normal | 323 (54%) | 228 (62%) | 95 (42%) |
| overweight | 181 (30%) | 90 (25%) | 91 (40%) |
| obese | 90 (15%) | 48 (13%) | 42 (18%) |
| #missing | 3 | 2 | 1 |
| Physical activity score | |||
| low | 268 (47%) | 181 (51%) | 87 (39%) |
| still acceptable | 174 (30%) | 101 (29%) | 73 (33%) |
| satisfactory | 79 (14%) | 42 (12%) | 37 (17%) |
| high | 53 (9%) | 29 (8%) | 24 (11%) |
| #missing | 23 | 15 | 8 |
| PWC130 (W/kg) | 1.61 (0.47) | 1.52 (0.42) | 1.77 (0.50) |
| #missing | 92 | 59 | 33 |
| low CRF events | 211 (37%) | 122 (35%) | 89 (40%) |
| #missing | 25 | 20 | 5 |
| Immunosenescence biomarker | |||
| IMMAX | 0.43 (0.12) | 0.40 (0.11) | 0.47 (0.13) |
| log NK/T | −1.81 (0.55) | −1.91 (0.53) | −1.64 (0.54) |
| log CD4/CD8 | 1.31 (0.59) | 1.32 (0.55) | 1.31 (0.64) |
| log CD8 mem/naive | 0.06 (1.03) | −0.11 (0.94) | 0.35 (1.12) |
| log CD4 mem/naive | 0.57 (0.68) | 0.45 (0.64) | 0.75 (0.69) |
| logit CD8 CD28- | −1.26 (0.88) | −1.36 (0.83) | −1.11 (0.94) |
| Predictor | OR a | 95% CI a | p-Value | q-Value b |
|---|---|---|---|---|
| Sex | 0.22 | 0.24 | ||
| Females c | — | — | ||
| Males | 1.25 | 0.88–1.78 | ||
| BMI category | <0.001 | <0.001 | ||
| Normal c | — | — | ||
| overweight | 2.09 | 1.39–3.14 | ||
| obese | 7.38 | 4.34–12.9 | ||
| Physical activity | <0.001 | 0.001 | ||
| Low c | — | — | ||
| still acceptable | 0.66 | 0.44–0.99 | ||
| satisfactory | 0.37 | 0.20–0.66 | ||
| high | 0.34 | 0.16–0.67 | ||
| Age (standardized) | 1.25 | 1.05–1.50 | 0.012 | 0.020 |
| Immune age metric (standardized) | ||||
| IMMAX | 1.37 | 1.15–1.64 | <0.001 | 0.001 |
| log CD8 mem/naive | 1.36 | 1.14–1.64 | <0.001 | 0.001 |
| logit CD8 CD28- | 1.28 | 1.07–1.52 | 0.006 | 0.013 |
| log NK/T | 1.15 | 0.96–1.37 | 0.12 | 0.17 |
| log CD4 mem/naive | 1.13 | 0.95–1.35 | 0.17 | 0.21 |
| log CD4/CD8 | 1.10 | 0.92–1.31 | 0.29 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bröde, P.; Claus, M.; Gajewski, P.D.; Getzmann, S.; Golka, K.; Hengstler, J.G.; Wascher, E.; Watzl, C. Calibrating a Comprehensive Immune Age Metric to Analyze the Cross Sectional Age-Related Decline in Cardiorespiratory Fitness. Biology 2022, 11, 1576. https://doi.org/10.3390/biology11111576
Bröde P, Claus M, Gajewski PD, Getzmann S, Golka K, Hengstler JG, Wascher E, Watzl C. Calibrating a Comprehensive Immune Age Metric to Analyze the Cross Sectional Age-Related Decline in Cardiorespiratory Fitness. Biology. 2022; 11(11):1576. https://doi.org/10.3390/biology11111576
Chicago/Turabian StyleBröde, Peter, Maren Claus, Patrick D. Gajewski, Stephan Getzmann, Klaus Golka, Jan G. Hengstler, Edmund Wascher, and Carsten Watzl. 2022. "Calibrating a Comprehensive Immune Age Metric to Analyze the Cross Sectional Age-Related Decline in Cardiorespiratory Fitness" Biology 11, no. 11: 1576. https://doi.org/10.3390/biology11111576