Myofascial Trigger Points and Central Sensitization Signs, but No Anxiety, Are Shown in Women with Dysmenorrhea: A Case-Control Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
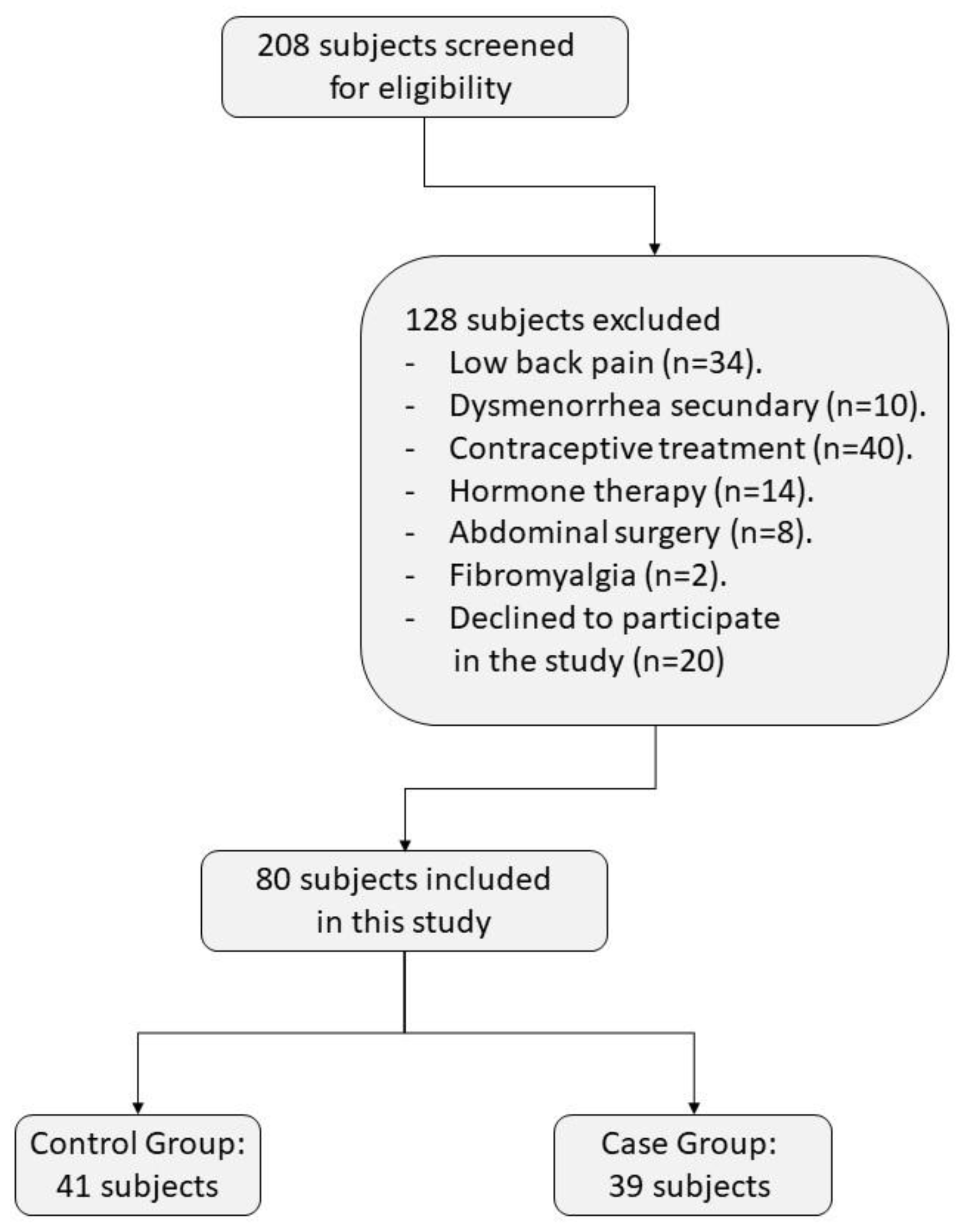
2.2. Participants
2.3. Ethical Considerations
2.4. Procedure
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Payne, L.A.; Seidman, L.C.; Sim, M.S.; Rapkin, A.J.; Naliboff, B.D.; Zeltzer, L.K. Experimental evaluation of central pain processes in young women with primary dysmenorrhea. Pain 2019, 160, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Sharghi, M.; Mansurkhani, S.M.; Larky, D.A.; Kooti, W.; Niksefat, M.; Firoozbakht, M.; Behzadifar, M.; Azami, M.; Servatyari, K.; Jouybari, L. An update and systematic review on the treatment of primary dysmenorrhea. JBRA Assist. Reprod. 2019, 23, 51–57. [Google Scholar] [CrossRef]
- Hu, Z.; Tang, L.; Chen, L.; Kaminga, A.C.; Xu, H. Prevalence and Risk Factors Associated with Primary Dysmenorrhea among Chinese Female University Students: A Cross-sectional Study. J. Pediatr. Adolesc. Gynecol. 2020, 33, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Fayyaz, K.; Javed, U.; Usman, M.; Malik, R.; Arif, N.; Kaleem, A. Prevalence of Dysmenorrhea and Determinants of Pain Intensity Among University-Age Women. Pain Med. 2021, 22, 2851–2862. [Google Scholar] [CrossRef] [PubMed]
- Del Forno, S.; Arena, A.; Pellizzone, V.; Lenzi, J.; Raimondo, D.; Cocchi, L.; Paradisi, R.; Youssef, A.; Casadio, P.; Seracchioli, R. Assessment of levator hiatal area using 3D/4D transperineal ultrasound in women with deep infiltrating endometriosis and superficial dyspareunia treated with pelvic floor muscle physiotherapy: Randomized controlled trial. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2021, 57, 726–732. [Google Scholar] [CrossRef]
- Momma, R.; Nakata, Y.; Sawai, A.; Takeda, M.; Natsui, H.; Mukai, N.; Watanabe, K. Comparisons of the Prevalence, Severity, and Risk Factors of Dysmenorrhea between Japanese Female Athletes and Non-Athletes in Universities. Int. J. Environ. Res. Public Health 2021, 19, 52. [Google Scholar] [CrossRef]
- Söderman, L.; Edlund, M.; Marions, L. Prevalence and impact of dysmenorrhea in Swedish adolescents. Acta Obstet. Gynecol. Scand. 2019, 98, 215–221. [Google Scholar] [CrossRef]
- Durand, H.; Monahan, K.; McGuire, B.E. Prevalence and Impact of Dysmenorrhea Among University Students in Ireland. Pain Med. 2021, 22, 2835–2845. [Google Scholar] [CrossRef]
- Iacovides, S.; Avidon, I.; Baker, F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef]
- Barcikowska, Z.; Rajkowska-Labon, E.; Grzybowska, M.E.; Hansdorfer-Korzon, R.; Zorena, K. Inflammatory Markers in Dysmenorrhea and Therapeutic Options. Int. J. Environ. Res. Public Health 2020, 17, 1191. [Google Scholar] [CrossRef]
- Ferries-Rowe, E.; Corey, E.; Archer, J.S. Primary Dysmenorrhea: Diagnosis and Therapy. Obstet. Gynecol. 2020, 136, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.Y. Primary dysmenorrhea: Advances in pathogenesis and management. Obstet. Gynecol. 2006, 108, 428–441. [Google Scholar] [CrossRef] [PubMed]
- López-Liria, R.; Torres-Álamo, L.; Vega-Ramírez, F.A.; García-Luengo, A.V.; Aguilar-Parra, J.M.; Trigueros-Ramos, R.; Rocamora-Pérez, P. Efficacy of Physiotherapy Treatment in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 7832. [Google Scholar] [CrossRef]
- Carroquino-Garcia, P.; Jiménez-Rejano, J.J.; Medrano-Sanchez, E.; de la Casa-Almeida, M.; Diaz-Mohedo, E.; Suarez-Serrano, C. Therapeutic Exercise in the Treatment of Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. Phys. Ther. 2019, 99, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Romero-Morales, C.; de la Cueva-Reguera, M.; Miñambres-Vallejo, B.; Ruiz-Ruiz, B.; Calvo-Lobo, C.; Casado-Hernández, I.; López-López, D.; Abuín-Porras, V. Ultrasound Assessment of the Abdominal Wall Muscles in Women with and without Primary Dysmenorrhea: A Cross-Sectional Study. Diagnostics 2020, 10, 166. [Google Scholar] [CrossRef]
- Grinberg, K.; Weissman-Fogel, I.; Lowenstein, L.; Abramov, L.; Granot, M. How Does Myofascial Physical Therapy Attenuate Pain in Chronic Pelvic Pain Syndrome? Pain Res. Manag. 2019, 2019, 6091257. [Google Scholar] [CrossRef]
- Ross, V.; Detterman, C.; Hallisey, A. Myofascial Pelvic Pain: An Overlooked and Treatable Cause of Chronic Pelvic Pain. J. Midwifery Womens Health 2021, 66, 148–160. [Google Scholar] [CrossRef]
- Fernández-Carnero, J.; Gilarranz-De-Frutos, L.; León-Hernández, J.V.; Pecos-Martin, D.; Alguacil-Diego, I.; Gallego-Izquierdo, T.; Martín-Pintado-Zugasti, A. Effectiveness of Different Deep Dry Needling Dosages in the Treatment of Patients with Cervical Myofascial Pain: A Pilot RCT. Am. J. Phys. Med. Rehabil. 2017, 96, 726–733. [Google Scholar] [CrossRef]
- Pastore, E.A.; Katzman, W.B. Recognizing myofascial pelvic pain in the female patient with chronic pelvic pain. J. Obstet. Gynecol. Neonatal Nurs. JOGNN 2012, 41, 680–691. [Google Scholar] [CrossRef]
- Llamas-Ramos, R.; Pecos-Martín, D.; Gallego-Izquierdo, T.; Llamas-Ramos, I.; Plaza-Manzano, G.; Ortega-Santiago, R.; Cleland, J.; Fernández-de-las-Peñas, C. Comparison of the short-term outcomes between trigger point dry needling and trigger point manual therapy for the management of chronic mechanical neck pain: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 2014, 44, 852–861. [Google Scholar] [CrossRef]
- Ferragut-Garcías, A.; Plaza-Manzano, G.; Rodríguez-Blanco, C.; Velasco-Roldán, O.; Pecos-Martín, D.; Oliva-Pascual-Vaca, J.; Llabrés-Bennasar, B.; Oliva-Pascual-Vaca, Á. Effectiveness of a Treatment Involving Soft Tissue Techniques and/or Neural Mobilization Techniques in the Management of Tension-Type Headache: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2017, 98, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pintado-Zugasti, A.; Pecos-Martin, D.; Rodríguez-Fernández, Á.L.; Alguacil-Diego, I.M.; Portillo-Aceituno, A.; Gallego-Izquierdo, T.; Fernandez-Carnero, J. Ischemic Compression After Dry Needling of a Latent Myofascial Trigger Point Reduces Postneedling Soreness Intensity and Duration. PM&R 2015, 7, 1026–1034. [Google Scholar]
- Pecos-Martín, D.; Montañez-Aguilera, F.J.; Gallego-Izquierdo, T.; Urraca-Gesto, A.; Gómez-Conesa, A.; Romero-Franco, N.; Plaza-Manzano, G. Effectiveness of dry needling on the lower trapezius in patients with mechanical neck pain: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, M.L.L.S.; Braz, C.A.; Rosa-e-Silva, J.C.; Candido-dos-Reis, F.J.; Nogueira, A.A.; Poli-Neto, O.B. Anaesthetic injection versus ischemic compression for the pain relief of abdominal wall trigger points in women with chronic pelvic pain. BMC Anesthesiol. 2015, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-M.; Liu, L. Wet needling of myofascial trigger points in abdominal muscles for treatment of primary dysmenorrhoea. Acupunct. Med. 2014, 32, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Hammi, C.; Schroeder, J.D.; Yeung, B. Trigger Point Injection; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- López-Ruiz, M.; Losilla, J.M.; Monfort, J.; Portell, M.; Gutiérrez, T.; Poca, V.; Garcia-Fructuoso, F.; Llorente, J.; Garcia-Fontanals, A.; Deus, J. Central sensitization in knee osteoarthritis and fibromyalgia: Beyond depression and anxiety. PLoS ONE 2019, 14, e0225836. [Google Scholar] [CrossRef]
- Van Griensven, H.; Schmid, A.; Trendafilova, T.; Low, M. Central Sensitization in Musculoskeletal Pain: Lost in Translation? J. Orthop. Sports Phys. Ther. 2020, 50, 592–596. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Arias-Buría, J.L.; Ortega-Santiago, R.; De-la-Llave-Rincón, A.I. Understanding central sensitization for advances in management of carpal tunnel syndrome. F1000Research 2020, 9, F1000 Faculty Rev-605. [Google Scholar] [CrossRef]
- Stratton, P.; Khachikyan, I.; Sinaii, N.; Ortiz, R.; Shah, J. Association of chronic pelvic pain and endometriosis with signs of sensitization and myofascial pain. Obstet. Gynecol. 2015, 125, 719–728. [Google Scholar] [CrossRef]
- Alfonsin, M.M.; Chapon, R.; de Souza, C.A.B.; Genro, V.K.; Mattia, M.M.C.; Cunha-Filho, J.S. Correlations among algometry, the visual analogue scale, and the numeric rating scale to assess chronic pelvic pain in women. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 3, 100037. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Galán-del-Río, F.; Fernández-Carnero, J.; Pesquera, J.; Arendt-Nielsen, L.; Svensson, P. Bilateral widespread mechanical pain sensitivity in women with myofascial temporomandibular disorder: Evidence of impairment in central nociceptive processing. J. Pain 2009, 10, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Staud, R. Is it all central sensitization? Role of peripheral tissue nociception in chronic musculoskeletal pain. Curr. Rheumatol. Rep. 2010, 12, 448–454. [Google Scholar] [CrossRef]
- Fernández-Carnero, J.; Fernández-de-Las-Peñas, C.; de la Llave-Rincón, A.I.; Ge, H.-Y.; Arendt-Nielsen, L. Widespread mechanical pain hypersensitivity as sign of central sensitization in unilateral epicondylalgia: A blinded, controlled study. Clin. J. Pain 2009, 25, 555–561. [Google Scholar] [CrossRef] [PubMed]
- de Tommaso, M.; Sciruicchio, V.; Delussi, M.; Vecchio, E.; Goffredo, M.; Simeone, M.; Barbaro, M.G.F. Symptoms of central sensitization and comorbidity for juvenile fibromyalgia in childhood migraine: An observational study in a tertiary headache center. J. Headache Pain 2017, 18, 59. [Google Scholar] [CrossRef]
- Michaelides, A.; Zis, P. Depression, anxiety and acute pain: Links and management challenges. Postgrad. Med. 2019, 131, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Bajalan, Z.; Moafi, F.; MoradiBaglooei, M.; Alimoradi, Z. Mental health and primary dysmenorrhea: A systematic review. J. Psychosom. Obstet. Gynaecol. 2019, 40, 185–194. [Google Scholar] [CrossRef]
- Pakpour, A.H.; Kazemi, F.; Alimoradi, Z.; Griffiths, M.D. Depression, anxiety, stress, and dysmenorrhea: A protocol for a systematic review. Syst. Rev. 2020, 9, 65. [Google Scholar] [CrossRef]
- Faramarzi, M.; Salmalian, H. Association of psychologic and nonpsychologic factors with primary dysmenorrhea. Iran. Red Crescent Med. J. 2014, 16, e16307. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E.; Buela-Casal, G.; Guillén, A.; Nicolás, R.; Cubero, S. Cuestionario de Ansiedad Estado-Rasgo Adaptación Española; Consulting Psychologists Press, Inc.: Palo Alto, CA, USA, 1982. [Google Scholar]
- Patil, S.; Daniel, G.; Vyas, R.; Tailor, Y.; Howell, M.; Ahmed, T.; Reutter, C.; Shrikhande, A. Neuromuscular treatment approach for women with chronic pelvic pain syndrome improving pelvic pain and functionality. Neurourol. Urodyn. 2022, 41, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Abuín-Porras, V.; Maldonado-Tello, P.; de la Cueva-Reguera, M.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; López-López, D.; Navarro-Flores, E.; Romero-Morales, C. Comparison of Lateral Abdominal Musculature Activation during Expiration with an Expiratory Flow Control Device Versus the Abdominal Drawing-in Maneuver in Healthy Women: A Cross-Sectional Observational Pilot Study. Medicina 2020, 56, 84. [Google Scholar] [CrossRef]
- Slater, H.; Paananen, M.; Smith, A.J.; O’Sullivan, P.; Briggs, A.M.; Hickey, M.; Mountain, J.; Karppinen, J.; Beales, D. Heightened cold pain and pressure pain sensitivity in young female adults with moderate-to-severe menstrual pain. Pain 2015, 156, 2468–2478. [Google Scholar] [CrossRef] [PubMed]
- As-Sanie, S.; Harris, R.E.; Harte, S.E.; Tu, F.F.; Neshewat, G.; Clauw, D.J. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet. Gynecol. 2013, 122, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Dougados, M.; Perrot, S. Fibromyalgia and central sensitization in chronic inflammatory joint diseases. Jt. Bone Spine 2017, 84, 511–513. [Google Scholar] [CrossRef]
- Clark, J.R.; Nijs, J.; Yeowell, G.; Holmes, P.; Goodwin, P.C. Trait Sensitivity, Anxiety, and Personality Are Predictive of Central Sensitization Symptoms in Patients with Chronic Low Back Pain. Pain Pract. 2019, 19, 800–810. [Google Scholar] [CrossRef]
- Clark, J.R.; Yeowell, G.; Goodwin, P.C. Trait anxiety and sensory processing profile characteristics in patients with non-specific chronic low back pain and central sensitisation—A pilot observational study. J. Bodyw. Mov. Ther. 2018, 22, 909–916. [Google Scholar] [CrossRef]
- Wood, L.R.J.; Peat, G.; Thomas, E.; Duncan, R. Knee osteoarthritis in community-dwelling older adults: Are there characteristic patterns of pain location? Osteoarthr. Cartil. 2007, 15, 615–623. [Google Scholar] [CrossRef]
| Data | Dysmenorrhea (n = 39) | Control (n = 41) | p-Value |
|---|---|---|---|
| Age, y | 24.00 ± 6.00 † | 23.00 ± 9.50 † | 0.764 ‡ |
| Weight, kg | 59.79 ± 9.57 * | 58.34 ± 11.50 * | 0.467 ** |
| Height, cm | 166.76 ± 6.55 * | 163.07 ± 6.21 * | 0.011 ** |
| BMI, kg/m2 | 21.49 ± 3.37 * | 21.89 ± 2.78 * | 0.558 ** |
| Data | Dysmenorrhea (n = 39) | Control (n = 41) | p-Value |
|---|---|---|---|
| NRS, average | 6.41 ± 1.35 * | 3.75 ± 1.37 * | <0.001 ** |
| NRS, maximum | 8.35 ± 0.95 * | 5.65 ± 1.68 * | <0.001 ** |
| Left RA PPT, kg/m2 | 1.58 ± 0.40 * | 2.40 ± 0.54 † | <0.001 ‡ |
| Right RA PPT, kg/m2 | 1.56 ± 0.36 * | 2.38 ± 0.62 * | <0.001 ** |
| Left TA PPT, kg/m2 | 2.82 ± 0.79 * | 4.50 ± 1.07 † | <0.001 ‡ |
| Right TA PPT, kg/m2 | 3.23 ± 1.23 † | 4.49 ± 0.72 * | <0.001 ‡ |
| STAI, anxiety-state | 12.00 ± 14.00 † | 12.90 ± 9.10 * | 0.352 ‡ |
| STAI, anxiety-trait | 22.00 ± 9.00 † | 18.34 ± 9.13 * | 0.053 ‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyos-Calderon, Y.-T.; Martínez-Merinero, P.; Nunez-Nagy, S.; Pecos-Martín, D.; Calvo-Lobo, C.; Romero-Morales, C.; Abuín-Porras, V.; Serrano-Imedio, A. Myofascial Trigger Points and Central Sensitization Signs, but No Anxiety, Are Shown in Women with Dysmenorrhea: A Case-Control Study. Biology 2022, 11, 1550. https://doi.org/10.3390/biology11111550
Hoyos-Calderon Y-T, Martínez-Merinero P, Nunez-Nagy S, Pecos-Martín D, Calvo-Lobo C, Romero-Morales C, Abuín-Porras V, Serrano-Imedio A. Myofascial Trigger Points and Central Sensitization Signs, but No Anxiety, Are Shown in Women with Dysmenorrhea: A Case-Control Study. Biology. 2022; 11(11):1550. https://doi.org/10.3390/biology11111550
Chicago/Turabian StyleHoyos-Calderon, Yennyt-Tatiana, Patricia Martínez-Merinero, Susana Nunez-Nagy, Daniel Pecos-Martín, César Calvo-Lobo, Carlos Romero-Morales, Vanesa Abuín-Porras, and Ana Serrano-Imedio. 2022. "Myofascial Trigger Points and Central Sensitization Signs, but No Anxiety, Are Shown in Women with Dysmenorrhea: A Case-Control Study" Biology 11, no. 11: 1550. https://doi.org/10.3390/biology11111550
APA StyleHoyos-Calderon, Y.-T., Martínez-Merinero, P., Nunez-Nagy, S., Pecos-Martín, D., Calvo-Lobo, C., Romero-Morales, C., Abuín-Porras, V., & Serrano-Imedio, A. (2022). Myofascial Trigger Points and Central Sensitization Signs, but No Anxiety, Are Shown in Women with Dysmenorrhea: A Case-Control Study. Biology, 11(11), 1550. https://doi.org/10.3390/biology11111550









