Radiation-Induced Rescue Effect: Insights from Microbeam Experiments
Abstract
:Simple Summary
Abstract
1. Introduction
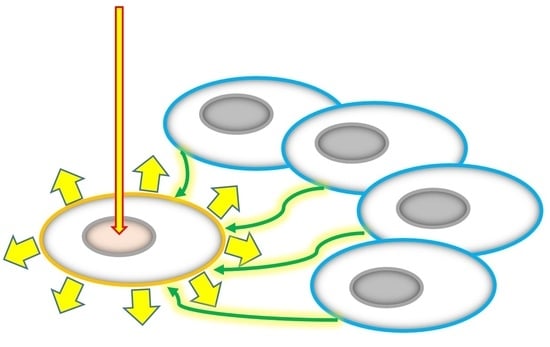
2. Radiation-Induced Rescue Effect (RIRE)
2.1. Discovery of RIRE
2.2. RIRE and Bilateral Bystander Response (BBR)
2.3. RIRE for Different Types of Ionizing Radiations and Cell Lines/Organism
2.4. Mechanisms Underlying RIRE
2.4.1. Involvement of Cyclic Adenosine Monophosphate (cAMP)
2.4.2. Involvement of Activation of Nuclear Factor Kappa B (NF-κB) Pathway
2.4.3. Involvement of NF-κB and COX-2 Signaling Pathways
2.4.4. Induction of Autophagy and Interleukin 6 (IL-6) Secretion in Bystander Cells
2.4.5. Involvement of a Poly (ADP-Ribose) Polymerase 1 (PARP1)–NF-κB Positive Feedback Loop
2.4.6. Involvement of Nitric Oxide (NO)
2.4.7. Scheme for Activation of NF-κB Pathway in RIRE
2.5. Mechanisms Underlying Bilateral Bystander Response (BBR)
3. Unique Features of Microbeam Radiations
3.1. Unique Features of Microbeam Radiations
3.1.1. Small Beam Size to Target Individual Cells and Individual Cell Nuclei
3.1.2. Mixing of Co-Cultured Targeted and Non-Targeted Cells
3.1.3. Well-Defined Irradiation Areas
3.1.4. Delivery of Ultra-High Local Doses and Dose Rates
3.2. RIRE Studies Using SPICE at NIRS
3.2.1. RIRE between Lung Adenocarcinoma (A549) Cells and Human Lung Normal Fibroblast (WI38) Cells [44]
3.2.2. RIRE between Lung Adenocarcinoma (A549) Cells and Human Lung Normal Fibroblast (WI38) Cells [52]
3.2.3. RIRE between Nonstem-like Cells and Stem-like Cells in Human Fibrosarcoma HT1080 [53]
3.2.4. RIRE between Cells in Zebrafish Embryos (Danio rerio) Irradiated at the Two-Cell Stage [54]
3.3. RIRE Studies Using Synchrotron X-ray Microbeam Irradiation System at the BL-27B Station in the Photon Factory, KEK [17]
4. Discussion
4.1. Mechanisms Underlying RIRE
4.2. Generalized Rescue Scheme (GRS)
4.3. Radiation Dose Response of RIRE
4.4. Radiation-Induced Field Size Effect (RIFSE)
4.5. FLASH Effect
4.6. In Vivo Studies on RIRE—Zebrafish Embryos
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagasawa, H.; Little, J.B. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992, 52, 6394–6396. [Google Scholar] [PubMed]
- Azzam, E.I.; de Toledo, S.M.; Gooding, T.; Little, J.B. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat. Res. 1998, 150, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Seymour, C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int. J. Radiat. Biol. 1997, 71, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.B.; McNeill, F.E.; Byun, S.H.; Prestwich, W.V.; Mothersill, C.; Seymour, C.; Armstrong, A.; Fernandez, C. Ultra-violet light emission from HPV-G cells irradiated with low LET radiation from 90Y; Consequences for radiation induced bystander effects. Dose Response 2013, 11, 498–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, M.; McNeill, F.E.; Seymour, C.; Rainbow, A.J.; Mothersill, C.E. An observed effect of ultraviolet radiation emitted from beta-irradiated HaCaT cells upon non-beta-irradiated bystander cells. Radiat. Res. 2015, 183, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Seymour, C. Radiation induced bystander effects: Past history and future directions. Radiat. Res. 2001, 155, 759–767. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C. Radiation induced bystander effects—Implications for cancer. Nat. Rev. 2004, 4, 158–164. [Google Scholar] [CrossRef]
- Goldberg, Z.; Lehnert, B.E. Radiation induced effects in unirradiated cells: A review and implications in cancer. Int. J. Oncol. 2002, 21, 337–349. [Google Scholar] [CrossRef]
- Little, J.B. Cellular radiation effects and the bystander response. Mutat. Res. 2006, 597, 113–118. [Google Scholar] [CrossRef]
- Morgan, W.F.; Sowa, M.B. Non-targeted bystander effects induced by ionizing radiation. Mutat. Res. 2007, 616, 159–164. [Google Scholar] [CrossRef]
- Hei, T.K.; Zhou, H.; Ivanov, V.N.; Hong, M.; Lieberman, H.B.; Brenner, D.J.; Amundson, S.A.; Geard, C.R. Mechanism of radiation induced bystander effects: A unifying model. J. Pharm. Pharmacol. 2008, 60, 943–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonanno, M.; Gonon, G.; Pandey, B.N.; Azzam, E.I. The intercellular communications mediating radiation-induced bystander effects and their relevance to environmental, occupational, and therapeutic exposures. Int. J. Radiat. Biol. 2022, 27, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Palomo, C.; Nikitaki, Z.; Djonov, V.; Georgakilas, A.G.; Martin, O.A. Non-targeted effects of synchrotron radiation: Lessons from experiments at the Australian and European synchrotrons. Appl. Sci. 2022, 12, 2079. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Y.; Han, W.; Chiu, S.K.; Zhu, L.; Wu, L.; Yu, K.N. Rescue effects in radiobiology: Unirradiated bystander cells assist irradiated cells through intercellular signal feedback. Mutat. Res. 2011, 706, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.K.K.; Fung, Y.K.; Han, W.; Yu, K.N. Rescue effects: Irradiated cells helped by unirradiated bystander cells. Int. J. Mol. Sci. 2015, 16, 2591–2609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.N. Radiation-induced rescue effect. J. Radiat. Res. 2019, 60, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Ojima, M.; Ito, A.; Usami, N.; Ohara, M.; Suzuki, K.; Kai, M. Field size effects on DNA damage and proliferation in normal human cell populations irradiated with X-ray microbeams. Sci. Rep. 2021, 11, 7001. [Google Scholar] [CrossRef]
- Coggle, J.E.; Hansen, L.S.; Wells, J.; Charles, M.W. Nonstochastic effects of different energy beta emitters on the mouse skin. Radiat. Res. 1984, 99, 336–345. [Google Scholar] [CrossRef]
- Peel, D.M.; Hopewell, J.W.; Wells, J.; Charles, M.W. Nonstochastic effects of different energy beta emitters on pig skin. Radiat Res. 1984, 99, 372–382. [Google Scholar] [CrossRef]
- Yu, K.N. Role of radiation-induced rescue effect in radiation field size effect. Radiat. Phys. Chem. 2022, 200, 110143. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Beyreuther, E.; Brand, M.; Hans, S.; Hideghéty, K.; Karsch, L.; Leßmann, E.; Schürer, M.; Szabó, E.R.; Pawelke, J. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation. Radiother. Oncol. 2019, 139, 46–50. [Google Scholar] [CrossRef]
- Weiss, H.; Epp, E.R.; Heslin, J.M.; Ling, C.C.; Santomasso, A. Oxygen depletion in cells irradiated at ultra-high dose-rates and at conventional dose-rates. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1974, 26, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hendry, J.H.; Moore, J.V.; Hodgson, B.W.; Keene, J.P. The constant low oxygen concentration in all the target cells for mouse tail radionecrosis. Radiat. Res. 1982, 92, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother. Oncol. 2017, 124, 365–369. [Google Scholar] [CrossRef]
- Loo, B.W.; Schuler, E.; Lartey, F.M.; Rafat, M.; King, G.J.; Trovati, S.; Koong, A.C.; Maxim, P.G. (P003) Delivery of ultra-rapid flash radiation therapy and demonstration of normal tissue sparing after abdominal irradiation of mice. Int. J. Radiat. Oncol. 2017, 98, E16. [Google Scholar] [CrossRef] [Green Version]
- Vozenin, M.C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin. Cancer Res. 2019, 25, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montay-Gruel, P.; Bouchet, A.; Jaccard, M.; Patin, D.; Serduc, R.; Aim, W.; Petersson, K.; Petit, B.; Bailat, C.; Bourhis, J.; et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother. Oncol. 2018, 129, 582–588. [Google Scholar] [CrossRef]
- Bourhis, J.; Montay-Gruel, P.; Gonçalves Jorge, P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Vozenin, M.C.; Hendry, J.H.; Limoli, C.L. Biological Benefits of Ultra-high Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin. Oncol. 2019, 31, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.M.L.; Donoghue, J.F.; Ventura, J.A.; Livingstone, J.; Bailey, T.; Day, L.R.J.; Crosbie, J.C.; Rogers, P.A.W. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci. Rep. 2018, 8, 12044. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yuan, D.; Xiao, L.; Tu, W.; Dong, C.; Liu, W.; Shao, C. The crosstalk between α-irradiated Beas-2B cells and its bystander U937 cells through MAPK and NF-κB signaling pathways. Mutat. Res. 2016, 783, 1–8. [Google Scholar] [CrossRef]
- Fu, J.; Wang, J.; Wang, X.; Wang, P.; Xu, J.; Zhou, C.; Bai, Y.; Shao, C. Signaling factors and pathways of α-particle irradiation induced bilateral bystander responses between Beas-2B and U937 cells. Mutat. Res. 2016, 789, 1–8. [Google Scholar] [CrossRef]
- Kong, E.Y.; Cheng, S.H.; Yu, K.N. Induction of autophagy and interleukin 6 secretion in bystander cells: Metabolic cooperation for radiation-induced rescue effect? J. Radiat. Res. 2018, 59, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palm, W.; Thompson, C.B. Nutrient acquisition strategies of mammalian cells. Nature 2017, 546, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef] [Green Version]
- Tardito, S.; Oudin, A.; Ahmed, S.U.; Fack, F.; Keunen, O.; Zheng, L.; Miletic, H.; Sakariassen, P.Ø.; Weinstock, A.; Wagner, A.; et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat. Cell Biol. 2015, 17, 1556–1568. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Trachootham, D.; Liu, J.; Chen, G.; Pelicano, H.; Garcia-Prieto, C.; Lu, W.; Burger, J.A.; Croce, C.M.; Plunkett, W.; et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat. Cell Biol. 2012, 14, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Sousa, C.M.; Biancur, D.E.; Wang, X.; Halbrook, C.J.; Sherman, M.H.; Zhang, L.; Kremer, D.; Hwang, R.F.; Witkiewicz, A.K.; Ying, H.; et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 2016, 536, 479–483. [Google Scholar] [CrossRef] [Green Version]
- Muranen, T.; Iwanicki, M.P.; Curry, N.L.; Hwang, J.; DuBois, C.D.; Coloff, J.L.; Hitchcock, D.S.; Clish, C.B.; Brugge, J.S.; Kalaany, N.Y. Starved epithelial cells uptake extracellular matrix for survival. Nat. Commun. 2017, 8, 13989. [Google Scholar] [CrossRef]
- Mayers, J.R.; Wu, C.; Clish, C.B.; Kraft, P.; Torrence, M.E.; Fiske, B.P.; Yuan, C.; Bao, Y.; Townsend, M.K.; Tworoger, S.S.; et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med. 2014, 20, 1193–1198. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Eder, S.; Schauer, S.; Diwoky, C.; Temmel, H.; Guertl, B.; Gorkiewicz, G.; Tamilarasan, K.P.; Kumari, P.; Trauner, M.; et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 2011, 333, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Widel, M.; Przybyszewski, W.M.; Cieslar-Pobuda, A.; Saenko, Y.V.; Rzeszowska-Wolny, J. Bystander normal human fibroblasts reduce damage response in radiation targeted cancer cells through intercellular ROS level modulation. Mutat. Res. 2012, 731, 117–124. [Google Scholar] [CrossRef]
- Desai, S.; Kobayashi, A.; Konishi, T.; Oikawa, M.; Pandey, B.N. Damaging and protective bystander cross-talk between human lung cancer and normal cells after proton microbeam irradiation. Mutat. Res. 2014, 763–764, 39–44. [Google Scholar] [CrossRef]
- Pereira, S.; Malard, V.; Ravanat, J.-L.; Davin, A.-H.; Armengaud, J.; Foray, N.; Adam-Guillermin, C. Low doses of gamma-irradiation induce an early bystander effect in zebrafish cells which is sufficient to radioprotect cells. PLoS ONE 2014, 9, e92974. [Google Scholar] [CrossRef] [PubMed]
- Matsuya, Y.; Satou, Y.; Hamada, N.; Date, H.; Ishikawa, M.; Sato, T. DNA damage induction during localized chronic exposure to an insoluble radioactive microparticle. Sci. Rep. 2019, 9, 10365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathikonda, S.; Cheng, S.H.; Yu, K.N. Role of PARP1 regulation in radiation-induced rescue effect. J. Radiat. Res. 2020, 61, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Adrian, G.; Ceberg, C.; Carneiro, A.; Ekblad, L. Rescue Effect Inherited in Colony Formation Assays Affects Radiation Response. Radiat. Res. 2018, 189, 44–52. [Google Scholar] [CrossRef]
- He, M.; Dong, C.; Xie, Y.; Li, J.; Yuan, D.; Bai, Y.; Shao, C. Reciprocal bystander effect between α-irradiated macrophage and hepatocyte is mediated by cAMP through a membrane signaling pathway. Mutat. Res. 2014, 763–764, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.K.K.; Fung, Y.K.; Han, W.; Li, L.; Chiu, S.K.; Cheng, S.H.; Yu, K.N. Modulation of NF-κB in rescued irradiated cells. Radiat. Prot. Dosim. 2015, 167, 37–43. [Google Scholar] [CrossRef]
- Lam, R.K.K.; Han, W.; Yu, K.N. Unirradiated cells rescue cells exposed to ionizing radiation: Activation of NF-κB pathway in irradiated cells. Mutat. Res. 2015, 782, 23–33. [Google Scholar] [CrossRef]
- Kobayashi, A.; Tengku Ahmad, T.; Autsavapromporn, N.; Oikawa, M.; Homma-Takeda, S.; Furusawa, Y.; Wang, J.; Konishi, T. Enhanced DNA double-strand break repair of microbeam targeted A549 lung carcinoma cells by adjacent WI38 normal lung fibroblast cells via bi-directional signaling. Mutat. Res. 2017, 803–805, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kobayashi, A.; Fu, Q.; Yang, G.; Konishi, T.; Uchihori, Y.; Hei, T.K.; Wang, Y. Rescue of targeted nonstem-like cells from bystander stem-like cells in human fibrosarcoma HT1080. Radiat. Res. 2015, 184, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Choi, V.W.Y.; Yum, E.H.W.; Konishi, T.; Oikawa, M.; Cheng, S.H.; Yu, K.N. Triphasic low-dose response in zebrafish embryos irradiated by microbeam protons. J. Radiat. Res. 2012, 53, 475–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuya, Y.; Hamada, N.; Yachi, Y.; Satou, Y.; Ishikawa, M.; Date, H.; Sato, T. Inflammatory Signaling and DNA Damage Responses after Local Exposure to an Insoluble Radioactive Microparticle. Cancers 2022, 14, 1045. [Google Scholar] [CrossRef]
- Lander, H.M.; Hajjar, D.P.; Hempstead, B.L.; Mirza, U.A.; Chait, B.T.; Campbell, S.; Quilliam, L.A. A molecular redox switch on p21ras: Structural basis for the nitric oxide-p21ras interaction. J. Biol. Chem. 1997, 272, 4323–4326. [Google Scholar] [CrossRef] [Green Version]
- Pahan, K.; Liu, X.; McKinney, M.J.; Wood, C.; Sheikh, F.G.; Raymond, J.R. Expression of a dominant-negative mutant of p21ras inhibits induction of nitric oxide synthase and activation of nuclear factor-κB in primary astrocytes. J. Neurochem. 2000, 74, 2288–2295. [Google Scholar] [CrossRef] [Green Version]
- Anrather, J.; Csizmadia, V.; Soares, M.P.; Winkler, H. Regulation of NF-κB RelA phosphorylation and transcriptional activity by p21ras and protein kinase Cζ in primary endothelial cells. J. Biol. Chem. 1999, 274, 13594–13603. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H.; Hayashi, S.; Hatashita, M.; Shioura, H.; Ohtsubo, T.; Kitai, R.; Ohnishi, T.; Yukawa, O.; Furusawa, Y.; Kano, E. Induction of radioresistance to accelerated carbon-ion beams in recipient cells by nitric oxide excreted from irradiated donor cells of human glioblastoma. Int. J. Radiat. Biol. 2000, 76, 1649–1657. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hayashi, S.; Hatashita, M.; Ohnishi, K.; Shioura, H.; Ohtsubo, T.; Kitai, R.; Ohnishi, T.; Kano, E. Induction of radioresistance by a nitric oxide-mediated bystander effect. Radiat. Res. 2001, 155, 387–396. [Google Scholar] [CrossRef]
- Shao, C.; Furusawa, Y.; Aoki, M.; Matsumoto, H.; Ando, K. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int. J. Radiat. Biol. 2002, 78, 837–844. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hamada, N.; Takahashi, A.; Kobayashi, Y.; Ohnishi, T. Vanguards of paradigm shift in radiation biology: Radiation-induced adaptive and bystander responses. J. Radiat. Res. 2007, 48, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H.; Tomita, M.; Otsuka, K.; Hatashita, M. A new paradigm in radioadaptive response developing from microbeam research. J. Radiat. Res. 2009, 50 (Suppl. A), A67–A79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, H.; Tomita, M.; Otsuka, K.; Hatashita, M.; Hamada, N. Nitric oxide is a key molecule serving as a bridge between radiation-induced bystander and adaptive responses. Curr. Mol. Pharmacol. 2011, 4, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Maeda, M.; Kobayashi, K.; Matsumoto, H. Dose response of soft X-Ray-induced bystander cell killing affected by p53 status. Radiat. Res. 2013, 179, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Kobayashi, K.; Matsumoto, H.; Usami, N.; Tomita, M. X-ray-induced bystander responses reduce spontaneous mutations in V79 cells. J. Radiat. Res. 2013, 54, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Connelly, L.; Palacios-Callender, M.; Ameixa, C.; Moncada, S.; Hobbs, A.J. Biphasic regulation of NF-κB activity underlies the Pro- and Anti-Inflammatory actions of nitric oxide. J. Immunol. 2001, 166, 3873–3881. [Google Scholar] [CrossRef] [Green Version]
- Clancy, R.M.; Amin, A.R.; Abramson, S.B. The role of nitric oxide in inflammation and immunity. Arthritis. Rheum. 1998, 41, 1141–1151. [Google Scholar] [CrossRef]
- Konishi, T.; Oikawa, M.; Suya, N.; Ishikawa, T.; Maeda, T.; Kobayashi, A.; Shiomi, N.; Kodama, K.; Hamano, T.; Homma-Takeda, S.; et al. SPICE-NIRS microbeam: A focused vertical system for proton irradiation of a single cell for radiobiological research. J. Radiat. Res. 2013, 54, 736–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, E.Y.; Choi, V.W.Y.; Cheng, S.H.; Yu, K.N. Some properties of the signals involved in unirradiated zebrafish embryos rescuing α-particle irradiated zebrafish embryos. Int. J. Radiat. Biol. 2014, 90, 1133–1142. [Google Scholar] [CrossRef]
- Choi, V.W.Y.; Lam, R.K.K.; Chong, E.Y.W.; Cheng, S.H.; Yu, K.N. Designing experimental setup and procedures for studying alpha-particle-induced adaptive response in zebrafish embryos in vivo. Nucl. Instrum. Methods B 2010, 268, 651–656. [Google Scholar] [CrossRef]
- Yum, E.H.W.; Choi, V.W.Y.; Nikezic, D.; Li, V.W.T.; Cheng, S.H.; Yu, K.N. Alpha-particle-induced bystander effects between zebrafish embryos in vivo. Radiat. Meas. 2009, 44, 1077–1080. [Google Scholar] [CrossRef]
- Choi, V.W.Y.; Ng, C.Y.P.; Kobayashi, A.; Konishi, T.; Oikawa, M.; Cheng, S.H.; Yu, P.K.N. Response of 5 hpf zebrafish embryos to low-dose microbeam protons. J. Radiat. Res. 2014, 55 (Suppl. 1), i113. [Google Scholar] [CrossRef] [Green Version]
- Kong, E.Y.; Cheng, S.H.; Yu, K.N. Biphasic and triphasic dose responses in zebrafish embryos to low-dose 150 kV X-rays with different hardness. J. Radiat. Res. 2016, 57, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, M.V.; Smilenov, L.B.; Hall, E.J.; Panyutin, I.G.; Bonner, W.M.; Sedelnikova, O.A. Ionizing radiation induces DNA double-strand breaks in bystander primary human fibroblasts. Oncogene 2005, 24, 7257–7265. [Google Scholar] [CrossRef] [Green Version]
- Schültke, E.; Balosso, J.; Breslin, T.; Cavaletti, G.; Djonov, V.; Esteve, F.; Grotzer, M.; Hildebrandt, G.; Valdman, A.; Laissue, J. Microbeam radiation therapy—Grid therapy and beyond: A clinical perspective. Br. J. Radiol. 2017, 90, 20170073. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef] [Green Version]
- Choi, V.W.Y.; Ng, C.Y.P.; Cheng, S.H.; Yu, K.N. α-Particle irradiated zebrafish embryos rescued by bystander unirradiated zebrafish embryos. Environ. Sci. Technol. 2012, 46, 226–231. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McGarry, C.K.; Trainor, C.; McMahon, S.J.; O’Sullivan, J.M.; Schettino, G.; Hounsell, A.R.; Prise, K.M. Dose, dose-rate and field size effects on cell survival following exposure to non-uniform radiation fields. Phys. Med. Biol. 2012, 57, 3197–3206. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Parsons, J.L. FLASH radiotherapy: Current knowledge and future insights using proton-beam therapy. Int. J. Mol. Sci. 2020, 21, 6492. [Google Scholar] [CrossRef] [PubMed]
- Esplen, N.; Mendonca, M.S.; Bazalova-Carter, M. Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy: A topical review. Phys. Med. Biol. 2020, 65, 23TR03. [Google Scholar] [CrossRef]
- Diffenderfer, E.S.; Verginadis, I.I.; Kim, M.M.; Shoniyozov, K.; Velalopoulou, A.; Goia, D.; Putt, M.; Hagan, S.; Avery, S.; Teo, K.; et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Jolly, S.; Owen, H.; Schippers, M.; Welsch, C. Technical challenges for FLASH proton therapy. Phys. Med. 2020, 78, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.; McCauley, S.; Vairamani, K.; Speth, J.; Girdhani, S.; Abel, E.; Sharma, R.A.; Perentesis, J.P.; Wells, S.I.; Mascia, A.; et al. FLASH proton pencil beam scanning irradiation minimizes radiation-induced leg contracture and skin toxicity in mice. Cancers 2021, 13, 1012. [Google Scholar] [CrossRef]
- Kroll, F.; Brack, F.E.; Bernert, C.; Bock, S.; Bodenstein, E.; Brüchner, K.; Cowan, T.E.; Gaus, L.; Gebhardt, R.; Helbig, U.; et al. Tumour irradiation in mice with a laser-accelerated proton beam. Nat. Phys. 2022, 18, 316–322. [Google Scholar] [CrossRef]
- Patriarca, A.; Fouillade, C.; Auger, M.; Martin, F.; Pouzoulet, F.; Nauraye, C.; Heinrich, S.; Favaudon, V.; Meyroneinc, S.; Dendale, R.; et al. Experimental set-up for FLASH proton irradiation of small animals using a clinical system. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.N.; Cheng, S.H. In vivo studies of α-particle radiation effects using zebrafish embryos. In Advances in Biomedical Sciences and Engineering; Tjong, S.C., Ed.; Bentham Science Publishers: Oak Park, IL, USA, 2009; pp. 263–283. [Google Scholar]
- Choi, V.W.Y.; Yu, K.N. Embryos of the zebrafish Danio rerio in studies of non-targeted effects of ionizing radiation. Cancer Lett. 2015, 356, 91–104. [Google Scholar] [CrossRef]
- Berry, J.P.; Gantar, M.; Gibbs, D.L.; Schmale, M.C. The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Comp. Biochem. Physiol. C 2007, 145, 61–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, W.S.; Roy, V.A.L.; Yu, K.N. Review on toxic effects of Di(2-ethylhexyl) phthalate on zebrafish embryos. Toxics 2021, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Terriente, J.; Pujades, C. Use of zebrafish embryos for small molecule screening related to cancer. Dev. Dynam. 2013, 242, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Barbazuk, W.B.; Korf, I.; Kadavi, C.; Heyen, J.; Tate, S.; Wun, E.; Bedell, J.A.; McPherson, J.D.; Johnson, S.L. The syntenic relationship of the zebrafish and human genomes. Genome Res. 2000, 10, 1351–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Mimeault, M.; Batra, S.K. Emergence of zebrafish models in oncology for validating novel anticancer drug targets and nanomaterials. Drug Discov. Today 2013, 18, 128–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10943–10951. [Google Scholar] [CrossRef]




| Radiation Type | Doses to Irradiated Cells | Bystander/Rescued Cells | Rescue Responses/ Experimental End Points | Authors/Year/Ref |
|---|---|---|---|---|
| Broad-beam photons: 6 MV X-rays | 2/4 Gy | NHDF/ | micronucleus formation and extent of apoptosis reduced in irradiated cells | Widel et al., 2012 [43] |
| Me45 | ||||
| Broad-beam photons: 137Cs γ-rays | 70/550 mGy | ZF4/ZF4 | γ-H2AX foci reduced in irradiated cells | Pereira et al., 2014 [45] |
| Broad-beam photons: 200 kV X-rays (filtered) | 2 Gy | HeLa/HeLa | autophagy accumulation reduced in irradiated cells | Kong et al., 2018 [34] |
| Broad-beam photons: 6 MV X-rays | 1 Gy | (1) WI-38/ WI-38 | γ-H2AX foci/nucleus reduced in irradiated cells | Matsuya et al., 2019 [46] |
| (2) HBEC-3KT/ HBEC-3KT | ||||
| Broad-beam photons: 200 kV X-rays (filtered) | 2 Gy | (1) HeLa/HeLa | mRNA expression levels of 53BP1 and PARP1, fluorescent intensity of PARP1 reduced in irradiated cells | Pathikonda et al., 2020 [47] |
| (2) MCF7/MCF7 | ||||
| (3) CNE-2/CNE-2 | ||||
| (4) HCT116/ HCT116 | ||||
| Broad-beam | 1 mGy to 10 Gy | (1) WI-38/ WI-38 | nuclear γ-H2AX foci in irradiated cells under non-uniform exposure decreased when compared to those in cells under uniform irradiation | Matsuya et al., 2019 [46] |
| (β-particles + γ-ray photons) from 137Cs- and 134Cs-bearing microparticle | ||||
| (2) HBEC-3KT/HBEC-3KT | ||||
| Broad-beam | 20/40 cGy | (1) NHLF/NHLF | 53BP1 foci, micronucleus formation and extent of apoptosis reduced, while survival enhanced in irradiated cells | Chen et al., 2011 [14] |
| α-particles | (2) HeLa/HeLa | |||
| Broad-beam | 40 cGy | HL-7702/U937 | micronucleus formation reduced in irradiated cells | He et al., 2014 [49] |
| α-particles | ||||
| Broad-beam | 5 cGy | HeLa/HeLa | 53BP1 foci reduced in irradiated cells | Lam et al., 2015 [50,51] |
| α-particles | ||||
| Microbeam-protons | ~300 Gy | WI38/A549 | γ-H2AX foci fluorescence intensity per nucleus reduced in irradiated cells | Desai et al., 2014 [44] |
| Microbeam-protons | ~300 Gy | WI38/A549 | γ-H2AX foci fluorescence per nucleus reduced in irradiated cells | Kobayashi et al., 2017 [52] |
| Microbeam-protons | ~300 Gy | CSCs/NSCCs | relative fluorescence unit of nucleus 53BP1 foci reduced in irradiated cells | Liu et al., 2015 [53] |
| of HT1080 | ||||
| Microbeam-protons | ~300 Gy | Cells/cells in vivo on two-cell stage zebrafish embryos (Danio rerio) | apoptotic signals similar to and different from background for 1-cell and two-cell irradiation of two-cell stage embryos, respectively | Choi et al., 2012 [54] |
| Microbeam-X-ray: synchrotron X-ray microbeam | 1 Gy | MRC-5/MRC-5 | 53BP1 foci/cell decreased in small-size fields surrounded by non-irradiated cells | Ojima et al., 2021 [17] |
| Irradiation Conditions | Normalized Values | p Value (cf. Control Samples) |
|---|---|---|
| 10 × 1 (Exp 1) | 0.242 | 0.123 |
| 10 × 1 (Exp 2) | 0.048 | 0.397 |
| 10 × 2 (Exp 1) | 0.352 | 0.069 # |
| 10 × 2 (Exp 2) | 0.446 | 0.027 * |
| 20 × 1 | 0.053 | 0.30 |
| 20 × 2 | 0.060 | 0.189 |
| 40 × 1 | –0.028 | 0.450 |
| 40 × 2 | 0.631 | 0.034 * |
| 50 × 1 | 0.429 | 0.095 |
| 50 × 2 | 0.192 | 0.210 |
| 80 × 1 | 0.152 | 0.479 |
| 80 × 2 | 0.363 | 0.050 * |
| 100 × 1 | –0.014 | 0.451 |
| 100 × 2 | –0.220 | 0.066 # |
| 160 × 1 | 0.335 | 0.017 * |
| 200 × 1 | –0.189 | 0.116 |
| 200 × 2 | –0.298 | 0.022 * |
| 300 × 1 | –0.014 | 0.464 |
| 300 × 2 | 0.311 | 0.0968 |
| 2000 × 1 | 0.592 | 0.029 * |
| 2000 × 2 | 0.283 | 0.0645 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.N. Radiation-Induced Rescue Effect: Insights from Microbeam Experiments. Biology 2022, 11, 1548. https://doi.org/10.3390/biology11111548
Yu KN. Radiation-Induced Rescue Effect: Insights from Microbeam Experiments. Biology. 2022; 11(11):1548. https://doi.org/10.3390/biology11111548
Chicago/Turabian StyleYu, Kwan Ngok. 2022. "Radiation-Induced Rescue Effect: Insights from Microbeam Experiments" Biology 11, no. 11: 1548. https://doi.org/10.3390/biology11111548
APA StyleYu, K. N. (2022). Radiation-Induced Rescue Effect: Insights from Microbeam Experiments. Biology, 11(11), 1548. https://doi.org/10.3390/biology11111548



_Kwan_Ngok_Yu.png)