Simple Summary
Pentamerous radial symmetrical echinoderm adults develop from bilaterally symmetrical larvae and are a great model for understanding the origin and evolution of deuterostome nervous systems. Neuropeptides are important neuronal signaling molecules that regulate diverse behavioral and physiological processes in animals including echinoderms. In this study, we revealed the remarkable complexity of embryonic and larval nervous systems, identified the neuropeptide profile, and quantified the expressions of specific neuropeptide precursor genes in Apostichopus japonicus embryo and larvae. Collectively, this research will enable us to have a more comprehensive understanding of the holothuroid embryonic and larval nervous system and gain insights into the potential functions of neuropeptidergic systems in holothuroid larvae.
Abstract
Here, we described the complex nervous system at five early developmental stages (blastula, gastrula, auricularia, doliolaria and pentactula) of a holothurian species with highly economic value, Apostichopus japonicus. The results revealed that the nervous system of embryos and larvae is mainly distributed in the anterior apical region, ciliary bands or rings, and the feeding and attachment organs, and that serotonergic immunoreactivity was not observed until the embryo developed into the late gastrula; these are evolutionarily conserved features of echinoderm, hemichordate and protostome larvae. Furthermore, based on available transcriptome data, we reported the neuropeptide precursors profile at different embryonic and larval developmental stages. This analysis showed that 40 neuropeptide precursors present in adult sea cucumbers were also identified at different developmental stages of embryos and larvae, and only four neuropeptide precursors (SWYG precursor 2, GYWKDLDNYVKAHKT precursor, Neuropeptide precursor 14-like precursor, GLRFAmprecursor-like precursor) predicted in adults were absent in embryos and larvae. Combining the quantitative expression of ten specific neuropeptide precursor genes (NPs) by qRT-PCR, we revealed the potential important roles of neuropeptides in embryo development, feeding and attachment in A. japonicus larvae. In conclusion, this work provides novel perspectives on the diverse physiological functions of neuropeptides and contributes to understanding the evolution of neuropeptidergic systems in echinoderm embryos and larvae.
1. Introduction
Echinoderms have attracted attention from many scholars because of their key evolutionary status, strong regenerative ability, and morphological diversity [1]. Adult echinoderms, which display a radially symmetric body and radial nervous systems, have often been viewed as atavistic. The development of their body plan is closely associated with the development of the nervous system [2]. The central nervous system of adult echinoderms includes radial nerve cords (RNCs) and a circumoral nerve ring (CNR) in the oral (mouth) region [3,4]. In most echinoderms, RNCs can be further subdivided into ectoneural and hyponeural systems [3,5]. The peripheral nervous system (enteric nervous system, connective tissue plexus, and the neural circuitry of the podia or arm) that connects the RNC with other organs, is considered to be the inner entoneural system [3,6,7,8]. In echinoids, holothuroids, asteroids, and ophiuroids, the ecto and hyponeural components are dominant whereas in crinoids the inner entoneural system is the main part of the adult nervous system [8,9].
Unlike their pentaradial adults, the echinoderm larval nervous system usually shows bilateral serotonergic neurons and nerve tracts along the ciliary bands, serotonin-positive cells first appear at the gastrula stage in holothuroids, echinoids, asteroids and ophuiroids, playing a role in embryogenesis and swimming [2,10,11,12,13,14]. Nerve structures, such as neurons located in the apical organ and tracts of axons associated with the ciliary bands were identified during the larval development stage in echinoderms, which matches reports for hemichordate larvae [12,15,16]. In recent years, the development of genomic resources and molecular methods have allowed great progress for understanding the mechanisms of neurogenesis in the larvae of many echinoderm species, including the echinoids Strongylocentrotus purpuratus, Paracentrotus lividus, the asteroids Patiria miniata, Asterias rubens, the ophiuroids Amphipholis kochii, the crinoids Antedon mediterranea, Metacrinus rotundus, Anneissia japonica, and also the holothuroids Apostichopus californicus, Apostichopus parvimensis and Apostichopus japonicus [2,13,17,18,19,20,21,22]. These studies all indicated that the larval nervous system shows unexpected diversity in cell and fiber types and their distribution in both central and peripheral nervous components [2,13,17,18,19,20,21,22]. Among echinoderms, the planktotrophic holothuroid sea cucumbers retain both the ancestral body plan and the ancestral nervous system developmental pattern of echinoderms: a lack of neural precursor migration in the embryo and a feeding initiation stage-auricularia followed by a doliolaria stage [11,23]. Apostichopus japonicus, a classical planktotrophic sea cucumber, has multiple larval strategies and its life cycle can be divided into eight major stages: fertilization (0 hpf (hours post fertilization)), blastula (14 hpf), gastrula (24 hpf), auricularia (48 hpf), doliolaria (11 dpf (days post fertilization)), pentactula (12 dpf), juvenile (16 dpf), and adult [24]. Although some advances have been made to elucidate certain developmental stages of the holothuroids’ nervous system [2,21,25], a comprehensive study of the nervous system from the blastula to pentactula stages has still not been conducted.
Neuropeptides are considered to be the oldest neuronal signaling molecules in metazoans, which makes them optimal tracers for neuroendocrine activity [26,27,28]. Studies of marine invertebrate neuropeptide systems have been revealed in Mollusca, annelids, marine arthropods (crustaceans) and echinoderms [29,30,31,32,33,34,35,36,37,38,39]. In echinoderms, the recent development of RNA high-throughout sequencing technology has allowed strong advances in the identification and characterization of neuropeptides in adults [40,41,42,43,44,45,46,47,48,49]. Neuropeptides in adult echinoderms have been proven to play important roles in muscle contractility, feeding and reproduction [50,51,52,53,54,55,56,57,58,59,60,61,62]. In comparison with adults, little is known about neuropeptide localization and function during the larval stages of most echinoderms, especially in holothuroids. Limited studies of echinoids and asteroids indicated the potential function of neuropeptides in larval locomotion, feeding, digestive system, attachment, and metamorphosis [19,52,63,64].
The foundation for the present study was our recent identification of 44 neuropeptide precursor transcripts in the CNR of adult A. japonicus, which represents the most comprehensive resource to date for sea cucumber neuropeptide research [48]. However, the anatomy and nervous system of larvae are completely different from adult animals. Hence, we aimed first to investigate the comprehensive landscape of the nervous system from blastula to pentactula by immunofluorescence (IF). Secondly, we aimed to describe whether neuropeptide precursors predicted in the adult nervous system were also expressed in sea cucumber embryos and larvae by analyzing the published transcriptome database at different developmental stages, and further explore the expression of ten specific neuropeptides precursor genes (NPs): A. japonicus Kisspeptin-type precursor (AjKPP), A. japonicus Gonadotropin-releasing hormone-type precursor (AjGnRHP), A. japonicus Calcitonin-type precursors (AjCTP1/2: AjCTP1 and AjCTP2), A. japonicus MPMNPADYFSRGTVYIPTRDS precursor (AjMS21P), A. japonicus Pedal peptide-type precursor 2 (AjPPLNP2), A. japonicus Vasopressin/oxytocin-type precursor (AjholotocinP), A. japonicus Thyrotropin-releasing hormone (TRH)-type precursor (AjTRHP), A. japonicus Bursicon alpha-type precursors (AjBAP), and A. japonicus Orexin-type precursors (AjOXP1 and AjOXP2) by quantitative real-time PCR (qRT-PCR). Nine of the NPs that we selected, except AjMS21P, were chosen because they have been reported in other echinoderms previously and our group has performed functional research on these and proven their importance in biological processes in adult sea cucumbers (unpublished data). The remaining NP (AjMS21P) has only been identified in A. japonicus to date and possibly plays important roles in adult sea cucumbers [48]. The present research will enable us to have a more comprehensive understanding of the embryonic and larval holothuroid nervous system and gain insights into the potential functions of neuropeptide systems in sea cucumber embryos and larvae.
2. Materials and Methods
2.1. Embryo and Larval Culture
Typical early period embryos and larvae of A. japonicus (blastula (14 hpf), gastrula (24 hpf), late-gastrula (34 hpf), auricularia (48 hpf), doliolaria (11 dpf), and pentactula (12 dpf)) specimens were collected from Shandong oriental ocean sea cucumber breeding farm (Weihai, China) in May 2020. The embryos and larvae were cultured in filtered sea water (temperature: 20–21 °C, salinity: 32 ppt, dissolved oxygen level: 8 mg/L) under the density of 0.5~0.8 individual/ml (ind/mL) and all specimens were collected using a 60 μm filter. For immunostaining, the specimens were fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS) for 15 min at room temperature (RT) and were washed four times in PBS. The animals were dehydrated through a graded methanol series and then transferred into ice-cold methanol and stored at −20 °C until use [2,65]. For qRT-PCR analysis, the filtered samples (six biological replicates) were frozen in liquid nitrogen and stored at −80 °C for use. All animal care and use procedures were approved by the Institutional Animal Care and Use Committee of Ocean University of China (Permit Number: 20141201) and performed according to the Chinese Guidelines for the Care and Use of Laboratory Animals (GB/T 35892-2018).
2.2. Immunostaining
For nervous system immunostaining of echinoderms, acetylated α-tubulin present in axons and dendrites and neurotransmitter serotonin were used as primary antibodies in the present study [66,67,68,69,70]. Fixed A. japonicus larvae were transferred onto adhesion microscope slides (Citotest) and dried at 25 °C for 10 mins. Specimens were then circled with a Liquid Blocker pen and incubated with 3% H2O2 resolutions at room temperature (RT) for 25 min to pre-block. Blocking was carried out using 5% goat serum (Solarbio, Cat# SL038) for 30 min at RT followed by subsequent incubation with primary antibodies in PBST overnight at 4 °C. The primary antibodies were: mouse anti-acetylated α-tubulin (Sigma-Aldrich, Cat# T6793) used at 1:200 and rabbit anti-serotonin (ImmunoStar, Cat# 20080) used at 1:200. After incubation, primary antibodies were removed by three washes in PBST for 10 mins each at RT. Specimens were then incubated for 1 hour at RT with one of two secondary antibodies diluted 1:600 in PBS: Alexa Fluor 488-AffiniPure Goat Anti-Rabbit IgG (H+L) (Jackson, Cat# 111-545-003) or Rhodamine (TRITC)–conjugated Goat Anti-Mouse IgG(H+L) (Proteintech, Cat# SA00007-1). Nuclear staining was performed for 10 mins at RT using DAPI (Solarbio, Cat# C0065). To test the specificity of the antibodies, negative control treatments were carried out by omission of the primary antibody (Supplementary Figure S1). All immunostaining was imaged using a Fluorescence microscope system (Olympus BX53F).
2.3. Identification of Neuropeptide Precursors and Putative Neuropeptides in A. japonicus Embryonic and Larval Stages
The transcriptomes of A. japonicus at different developmental stages (blastula, gastrula, auricularia, pentactula) were downloaded from the NCBI database (accession numbers: SRR6075435-SRR6075438). As the original source reported, one replicate per stage (~100 embryos/larvae) was used for transcriptome sequencing, resulting in 230.8 million raw paired-end reads [71]. Low-quality reads were filtered using Trimmomatic v0.39 with the following parameters: “LEADING:30 TRAILING:30 SLIDINGWINDOW:5:30 AVGQUAL:34 MINLEN:21” [72]. The clean reads generated were applied for constructing de novo assemblies using Trinity v2.12.0 [73,74]. Finally, we obtained a total of 133,040, 172,308, 158,993, 134,712 transcripts in blastula, gastrula, auricularia and pentactula respectively and these four larval transcriptome libraries were used for local BLAST with Protein Query-Translated Subject BLAST (Version 2.12.0+). The data that support the findings of this study have been deposited into the CNGB Sequence Archive (CNSA) of the China National GeneBank DataBase (CNGBdb) [75,76] with accession number CNP0002851. To search for transcripts encoding putative neuropeptide or peptide hormone precursor proteins at different developmental stages, the sequences of neuropeptides or peptide hormone precursors previously identified in A. japonicus [48] were submitted individually as queries in a local blast search of the four transcriptome databases using Protein Query Translated Subject BLAST (Version 2.12.0+) with the e-value setting set to 0.01.
2.4. RNA Isolation, cDNA Synthesis and Full-Length Cloning of Putative Neuropeptide Precursor Genes (NPs)
Total RNA was isolated from A. japonicus embryos and larvae using Trizol (Takara, Japan, Code # 9109) according to the manufacturer’s instructions, and the RNA quality was determined via spectrophotometry using a NanoDrop 2000 (Thermo, Waltham, MA, USA) and 1% agarose gel electrophoresis. The full-length cDNA sequences encoding AjTRHP, AjPPLNP2, AjMS21P, AjCTP1, AjCTP2 and AjHolotocinP were amplified using a SMARTer® RACE 50/30 Kit (Clontech, Mountain View, CA, USA, Cat # 634858) as described in Wang et al. (2019) [77] and sequenced by BGI TECH SOLUTIONS (BEIJING LIUHE) CO., LIMITED Qingdao, China. Primer information is listed in Supplementary Table S1.
2.5. Quantitative Real-Time PCR (qRT-PCR) in Early Developmental Stages of A. japonicus
The samples at five stages were collected and RNA from six biological replicates (6 × 103–7 × 103 individuals/replicate per stage) was isolated. Relative transcript levels were determined using a TB Green® Premix Ex Taq™ (Tli RNaseH Plus) (Takara, Cat# RR420A) with a StepOnePlus (ABI Inc., Foster City, CA, USA). Each sample was run in triplicate. The specific primers for NPs were designed using Primer 6 software (Version 5.0) and are listed in the Supplementary Table S1. A special case is that of AjCTP1, which has only one more short-fragment exon as compared with AjCTP2, making it difficult to design specific primers to distinguish them. Therefore, the common part of the two sequences was applied to design a specific primer for detecting the total relative transcript levels of AjCTP1/2 (AjCTP1 + AjCTP2). β-actin (ACTB, PIK61412.1) and β-Tubulin (TUBB, PIK51093) were used as housekeeping genes for standardization, as previously validated [78]. The 2−ΔΔCT method was applied to analyze the comparative expression levels. All data are given as the mean ± S.E. (n = 6) and were analyzed using a one-way analysis of variance (ANOVA) followed by a Tukey post hoc test (SPSS 17.0, Inc., Chicago, IL, USA). The level of statistical significance was set at p < 0.05.
3. Results
3.1. Nervous System Profile of A. japonicus at Different Developmental Stages
To acquire a comprehensive characterization of the nervous system of A. japonicus over the developmental stages of embryos and larvae, we applied two regularly used metazoan nervous system markers, acetylated α-tubulin and serotonin, to profile the nervous system of A. japonicus by IF (Figure 1, Figure 2 and Figure 3) [13,21]. The embryo and larvae stages were divided according to Qiu et al. (2015) [24]. Names of the five early developmental stages (blastula, gastrula, auricularia, doliolaria, pentactula) body parts are as described in Figure 1, Figure 2 and Figure 3, and negative controls were performed and are shown in Supplementary Figure S1.
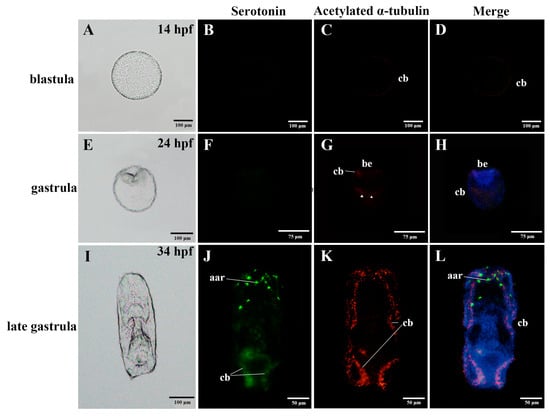
Figure 1.
Anatomy diagrams and localization of the nervous system in the embryos of the sea cucumber A. japonicus, using immunofluorescence. (A–D) blastula; (E–H) gastrula; (I–L) late gastrula. Labels are: aar, anterior apical region; be, blastopore; cb, ciliary band. Triangles on (G) label the positive immunoreactions in the bottom half of the gastrula. Green, serotonin; Red, acetylated α-tubulin. Information of hpf (hours post fertilization) was labelled on the upper right of the anatomy diagrams.
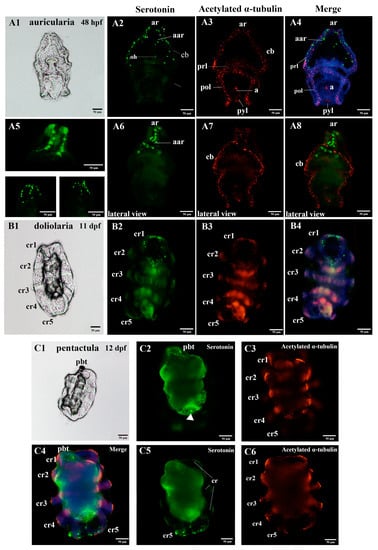
Figure 2.
Anatomy diagrams and localization of the nervous system in the larvae of the sea cucumber A. japonicus, using immunofluorescence. (A1–A8) auricularia; (B1–B4) doliolaria; (C1–C6) pentactula. (A5) is a high resolution picture of the anterior region of auricularia stained for serotonin (green). (A6–A8) are lateral views of the serotonin and acetylated α-tubulin staining. (C2,C3,C5,C6) are pictured by focusing on the different layers, respectively. The labels are: a, anus; aar, anterior apical region; ar, apical ridge; cb, ciliary band; cr, ciliary ring; oh, oral hood; pbt, primary buccal tentacle; pol, posterior loop; prl, preoral loop; pyl, pylorus. Green, serotonin; Red, acetylated α-tubulin. Information of hpf (hours post fertilization) and dpf (days post fertilization) is labelled at the upper right of the anatomy diagrams.
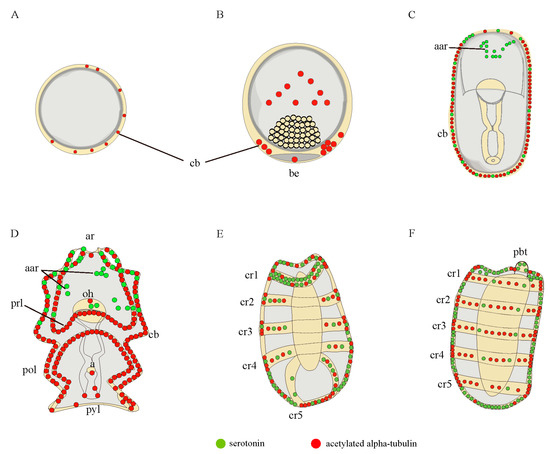
Figure 3.
A diagrammatic representation of the nervous system of A. japonicus embryos and larvae stained by serotonin (green) and acetylated α-tubulin (red). (A) blastula; (B) gastrula; (C) late gastrula; (D) auricularia; (E) doliolaria; (F) pentactula. The labels are: a, anus; aar, anterior apical region; ar, apical ridge; be, blastopore; cb, ciliary band; cr, ciliary ring; oh, oral hood; pbt, primary buccal tentacle; pol, posterior loop; prl, preoral loop; pyl, pylorus.
At the embryonic stage, serotonin-immunoreactions were not observed until the embryos developed into late-gastrula, where they were located in the anterior apical region, and along the ciliary bands (Figure 1B,D,F,H,J,L and Figure 3A–C). Expressions of acetylated α-tubulin were detected in the ciliary band at the blastula and late-gastrula stages, and were also identified in the ciliary band, blastopore and in the bottom half of the gastrula (Figure 1C,D,G,H,K,L and Figure 3A–C). Serotonin and acetylated α-tubulin were co-expressed in the ciliary band (Figure 1L and Figure 3C).
At the larvae stages, the auricularia larvae possessed two clusters of bilaterally serotonergic staining along the ciliary bands in the apical region and projected a fine axon-like structure in the front and lateral view (Figure 2A2,A5,A6 and Figure 3D). We also observed positive serotonergic staining around the oral hood (Figure 2A2 and Figure 3D). After the transition from auricularia to doliolaria, the ciliary band nerve tracts gradually moved with the rearranged ciliary bands and remained immunoreactive (Figure 2B2,B4 and Figure 3E). Positive serotonergic nerve immunoreactions were observed in five ciliary rings and formed a circle-like structure in the first ciliary ring (Figure 2B2,B4 and Figure 3E). When the doliolaria larvae developed into an early pentactula stage, the serotonergic immunoreactivity was detected in the ciliary rings, especially in the fifth ciliary ring and primary buccal tentacle (Figure 2C2,C4,C5 and Figure 3F).
In addition, acetylated α-tubulin staining was observed mainly over the ciliary band, pre-oral loop, and post-oral loop in auricularia. Positive immunoreactions were also identified in the apical ridge and around the anus and pylorus (Figure 2A3,A4,A7,A8 and Figure 3D). With the transformation in morphology, acetylated α-tubulin protein was clearly identified in the ciliary rings in the doliolaria and early pentactula stages (Figure 2B3,B4,C3,C4,C6 and Figure 3E,F). Colocalization of serotonin and acetylated α-tubulin were observed in the ciliary band and anterior apical region in auricularia, and ciliary rings in doliolaria and pentactula (Figure 2A4,A8,B4,C4 and Figure 3E,F).
3.2. Identification of Neuropeptide Precursor Transcripts in A. japonicus Embryo and Larvae
A total of 44 neuropeptide precursor transcripts predicted in adult A. japonicus [48] were submitted as queries for in silico tBLASTn analysis based on the transcriptomes of A. japonicus larvae from four developmental stages with the following accession numbers: SRR6075437 (blastula), SRR6075438 (gastrula), SRR6075435 (auricularia), and SRR6075436 (pentactula) with the e-value setting set to 0.01 [71]. The results showed that the transcriptional expression pattern of neuropeptide precursors is specific in different developmental stages (Figure 4), and the identity percentage of the hits for the results of tBLASTn-based analysis is shown in the Supplementary Data 1. For NPs belonging to known bilaterian neuropeptide families, only four neuropeptides were expressed in all four early developmental stages, whereas the Cholecystokinin-type precursor1 (CCKP1), Somatostatin-type precursors (SSP1 and SSP2) and Corazonin-type precursor (CRZP) were only present at the pentactula stage. One third of the NPs were not transcribed until the larvae developed into the auricularia stage, and Bursicon beta-type precursors (BBP) were only found in blastula. NP9 and NP15 of NPs that have, thus far, been expressed as specific to echinoderms appeared at all four stages. L-type SALMFamide precursor (L-SALMFaP) was absent only in gastrula larvae; whereas F-type SALMFamide precursor (F-SALMFaP) was absent at the blastula and pentactula stages. Some NPs, such as AN peptide precursor (ANPP) and NP23, were present only at the pentactula or auricularia stages. NP25 were not transcribed at the first two stages (blastula and gastrula). For NPs that were previously discovered in A. japonicus, none of the NPs were present at all four stages and the GLRFA precursor (GLRFA-P), GN19 precursor (GN19P) and MS21P were found at the late developmental stages (auricularia and pentactula stages). StichopinP was only expressed in the pentactula stage. Other novel putative neuropeptide precursors were found only when neuropeptide precursor 11-like precursor (NP11LP) were expressed in pentactula. Interestingly, four neuropeptide precursors (SWYGP2, GT15P, NP14LP, GLRFALP) predicted in adults were absent in embryos and larvae.
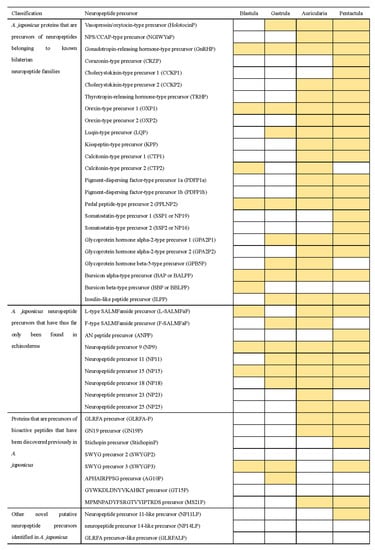
Figure 4.
Blast-based identification of neuropeptide precursors in A. japonicus embryos and larvae. The shading indicates that the transcripts of NP were identified.
3.3. Quantitative Analysis of Specific Neuropeptide Precursors in A. japonicus Embryos and Larvae
The expression levels of NP genes in the embryonic (blastula, gastrula) and larvae (auricularia, doliolaria, and pentactula) stages (six biological replicates) were detected using quantitative real-time PCR (qRT-PCR) (Figure 5). The ten NP genes were present with different expression patterns, and are basically consistent with those in the transcriptome. Few expressions of AjKPP were detected in the first three stages, which was significantly lower than that in the doliolaria and pentactula (p < 0.05) (Figure 5A), and AjGnRHP was significantly highly expressed in the blastula stage (p < 0.05) (Figure 5B). AjCTP1/2 was barely expressed until the larvae developed into thee pentactula stage (Figure 5C). AjMS21P (Figure 5D) and AjPPLNP2 (Figure 5E) had a dominant expression in auricularia larvae, which was obviously higher than that at the gastrula, doliolaria and pentactula stages (p < 0.05). Similar with AjPPLNP2 and AjMS21P, AjHolotocinP had a peak expression at the auricularia stage, but there was no difference among the five stages (p > 0.05) (Figure 5F). For AjTRHP, the expression levels at the gastrula and auricularia stages were significantly higher than that at the blastula, doliolaria and pentactula stages (p < 0.05) (Figure 5G). AjBAP was significantly highly expressed in embryos (blastula and gastrula) than in larvae, especially in the blastula (Figure 5H). AjOXP1 and AjOXP2 showed different expression pattern. AjOXP1 had the highest expression at the gastrula stage and the lowest expression at the blastula stage (Figure 5I), whereas expression of AjOXP2 at the pentactula stage was significantly higher than those at the blastula stage (Figure 5J). Overall, ten NP genes showed a complex transcriptional pattern (Figure 5K,L).
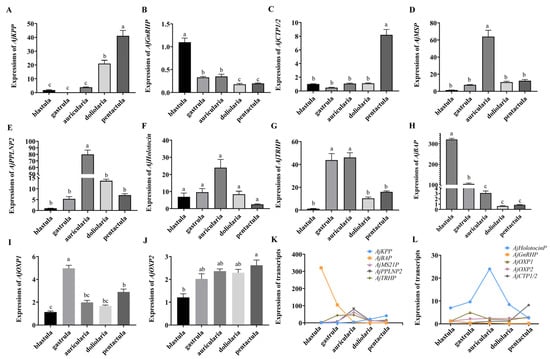
Figure 5.
Relative transcriptional level of NP genes in the embryos and larvae of the sea cucumber A. japonicus revealed by quantitative real-time PCR (qRT-PCR). (A) AjKPP; (B) AjGnRHP; (C) AjCTP1/2; (D) AjMS21P; (E) AjPPLNP2; (F) AjHolotocinP; (G) AjTRHP; (H) AjBAP; (I) AjOXP1; (J) AjOXP2; (K) Comprehensive analysis of five NP genes (AjKPP, AjBAP, AjMS21P, AjPPLNP2, AjTRHP) expression; (L) Comprehensive analysis of five NP genes (AjHolotocinP, AjGnRHP, AjOXP1, AjOXP2, AjCTP1/2) expression. Different lowercase letters indicate significant differences between the different stages (p < 0.05).
4. Discussion
4.1. Nervous System Complexity at Early Developmental Stages
Serotonergic neurons are the first neurons to differentiate in most echinoderm and hemichordate species and are thought to be primary sensory neurons with short apical dendritic poles and basal axonal projections [12,16]. In the late gastrula stage, we observed serotonin-positive immunoreactions in the anterior apical region, that matches the discovery in echinoderms, hemichordates, and protostomes with swimming larvae [2,12,13,18,79,80,81]. Interestingly, serotonergic immunoreactions were also identified in the ciliary band of A. japonicus embryos and larvae, supporting the potential role of serotonin in the modulation of ciliary beating and metamorphosis. Similarly, the nervous system of echinoderm larvae, like those of the starfish Asterina pectinifera and the sea urchin S. purpuratus, appears to be centered on the ciliary band, whose function may be to sense environmental cues [15,82,83].
Unlike asteroids [13,16], early serotonergic cells do not migrate in the auricularia as the ciliary band forms and a serotonergic nerve tract connects the left and right ciliary band tracts at the apical ridge of the auricularia in holothurian species [2,25] including in our present study. Ciliary bands change into ciliary rings during the transformation from auricularia to doliolaria and retain immunostaining in A. japonicus. Consistent with the previous study in A. japonicus [2], the rearrangement of ciliary bands followed the rearrangement of the larval nervous system. It was clearer in the larvae stained by an acetylated α-tubulin antibody, which is widely used as a pan-neuronal marker labeling neurites and cilia [69,70,84]. Acetylated α-tubulin was found in the ciliary band or ring at all developmental stages in A. japonicus and regularly spaced within the ciliary rings in doliolaria and early pentactula larvae, which is consistent with the dipleurula-type larvae of sea urchin, starfish, brittle star and feather star echinoderms, and evolutionarily closely related cephalochordates and hemichordates [13,18,21,69,70,85]. Our observation supports the possible principal role of the ciliary band in locomotion and feeding, which is possibly controlled by the nervous system in echinoderm larvae [16,86,87]. Nerve components were also observed in several organs, such as the oral hood, anus, and pylorus. Generally, positive staining along with the ciliary bands and around the organs, such as the oral hood, anus, and pylorus, make up the peripheral nervous system in larvae, which has also been reported in other echinoderms [12,19].
At the initial stage of the adult nervous system, the early pentactula stage in A. japonicus, we found intense serotonergic immunoreactions in the anterior-most region, which is consistent with the observations in metamorphosing larvae of the asteroid, A. kochii, and the pentacrinoid larvae of the crinoid A. mediterranea [18,85]. In the present study, we found that serotonin-immunoreactions mainly gathered at the anterior region in all investigated larvae after the late gastrula stage and were obvious in the first ciliary ring where nerve rings formed in doliolaria and the early pentactula larvae of A. japonicus [88,89]. This supports the hypothesis of anterior neurons as a subset of apical organ neurons that are considered to be the central nervous system of unattachment larvae [12]. Therefore, the common ancestor of echinoderms may have possessed a central integrated nervous organ during the larvae stage to regulate biological processes including development, feeding, swimming and attachments [8,13,18]. However, the detailed regulatory pattern and pathway of the larval nervous system remain to be further studied.
4.2. Neuropeptides at Early Developmental Stages
Neuropeptides are present across the bilaterians, suggesting that these ancient molecules play a vital role in the function and evolution of nervous systems [28,90]. Recent studies have revealed multiple neuropeptides at the early developmental stages in echinoid and asteroid species [19,64]; however, little work has been carried out on holothurians. Here, the available transcriptome data gives us a chance to report the first identification of 40 NPs at different developmental stages of the embryos and larvae of A. japonicus, and note that four neuropeptide precursors (SWYGP2, GT15P, NP14LP, GLRFALP) predicted in adults were absent in embryo and larvae [48]. The differences in neuropeptide variety at different developmental stages results, perhaps, from two reasons: (1) the quality of the genome and transcriptome is not good enough to make the correct assemble of transcripts; (2) the neuropeptides really do not express at this developmental stage, so it is impossible for them to play a role at this stage.
Among the NPs identified in embryos and larvae, a few of them (10/40) were expressed in the blastula, indicating that neuropeptidergic systems appear from the early stage of embryonic development. Thirteen of them were not identified until the auricularia stage of A. japonicus, including NPS/CCAP-type precursor (NGIWYamide precursor, NGIWYaP), Cholecystokinin-type precursor 2 (CCKP2), TRHP, Orexin-type precursor 2 (OXP2), Kisspeptin-type precursor (KPP), Calcitonin-type precursor 1 (CTP1), Pigment-dispersing factor-type precursors (PDFP1a and PDFP1b), Glycoprotein hormone alpha-2-type precursor 2 (GPA2P2), NP25, GLRFA-P, GN19P, MS21P. Our qRT-PCR analysis also revealed peak expressions of AjTRHP, AjPPLNP2, AjHolotocinP and AjMS21P at the auricularia larvae stage. (Figure 5). Auricularia is a key stage at which the larvae begin to have feeding and digestive tissues and organs [24]. Therefore, we speculated that these NPs may play an important role in the feeding process. In addition, previous studies have also reported that the expression of TRH was involved in regulating feeding behavior in sea urchin larvae [19]. Holotocin, as a member of the VP/OT family, plays ancient roles in regulating feeding and has been experimentally proven to be involved in feeding in A. rubens [91]. Interestingly, AjMS21 was identified as a myoactive peptide in adults [92,93,94], suggesting that it may be involved in the regulation of feeding behavior by controlling the relaxation and contraction of feeding organs throughout the sea cucumber lifecycle. All of the above further support the putative roles of these neuropeptides in regulating larval feeding.
Attachment and metamorphosis are one of the most important early life strategies in marine invertebrates including echinoderms, which are regulated by neurotransmitters and neuropeptides [8,64,95,96,97,98]. In our present study, CRZP, Cholecystokinin-type precursor 1 (CCKP1), SSP1, SSP2, ANPP, StichopinP and NP11LP were found only in the pentactula stage, which indicates a potential for these NPs in the attachment of larvae. We also observed the expression of AjCTP and AjKPP in the early pentactula stage to be significantly higher than in other stages (p < 0.05) (Figure 5A,C). Previous studies also revealed the presence of CTP in the adhesive disk, which may participate in the permanent or temporary attachment of starfish A. rubens [64]. Therefore, the expressions of these NPs in the A. japonicus pentactula stage presumably reflect the potential physiological roles of these neuropeptides in mediating the process of attachment.
AjGnRHP and AjBAP showed statistically significant differences in transcript levels at the embryo stage, suggesting their potential roles in embryo development. Previous studies also indicated that GnRH improves blastula formation and the quality of embryos, further supporting our suggestion that GnRH is involved in embryo development [99,100]. It is also noteworthy that different subtypes belonging to the same NP family were mostly (7/9) identified at different larval stages, whereas SSP1/2 and PDFP1a/1b were identified at the same developmental stages, which suggests the potential complex regulatory patterns of these neuropeptide families.
5. Conclusions
In conclusion, this study reveals the remarkable complexity of the embryonic and larval nervous system of A. japonicus, reported the NPs at early stages, and detected the quantitative expression of ten specific NPs in echinoderm embryos and larvae. By describing the complex nervous system in A. japonicus larvae, we provide new insights into the neurophysiology of echinoderm embryos and larvae. Our present study also revealed the potential roles of neuropeptides in regulating physiological activities including embryo development, feeding and attachment. Future classification of nerve cells and functional studies of neuropeptides will continue to be carried out in larvae and adult deuterostome including our echinoderm species A. japonicus, which will broaden our view about the diverse physiological functions of neuropeptides in these animals and contribute to our understanding of the evolution of neuropeptidergic systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology11101538/s1, Figure S1: Negative controls of nervous system immunostaining in embryo and larvae of A. japonicus by incubating with 1xPBS to verify the specificity of the primary antibody. DAPI was used for nuclear staining (blue). (A) blastula; (B) gastrula; (C) late-gastrula; (D) auricularia; (E) doliolaria; (F) pentactula; Table S1: Primer sequences used in the RACE and qRT-PCR amplifications.; Data S1: The identity percentage of the hits for the results of tBLASTn-based analysis.
Author Contributions
Conceptualization, M.C.; methodology and software, Y.Z., X.C. and Y.W.; validation, Y.Z., H.L. and X.C.; data curation, Y.W.; writing—original draft preparation, Y.Z.; writing—review and editing, M.C. and K.B.S.; visualization, Y.Z. and X.C.; supervision, M.C. and K.B.S.; project administration, M.C.; funding acquisition, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31972767 and 42276103).
Institutional Review Board Statement
The study was approved by the Ocean University of China Institutional Animal Care and Use Committee (OUC-IACUC) prior to the initiation of the study (Permit Number: 20141201). All experiments and relevant methods were carried out in accordance with the approved guidelines and regulations of OUC-IACUC (GB/T 35892-2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets presented in this study can be found in the online repositories of the CNGB Sequence Archive (CNSA) of the China National GeneBank DataBase (CNGBdb) (CNP0002851) at https://db.cngb.org/search/project/CNP0002851/, accessed on 30 March 2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arnone, M.I.; Andrikou, C.; Annunziata, R. Echinoderm systems for gene regulatory studies in evolution and development. Curr. Opin. Genet. Dev. 2016, 39, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Murabe, N.; Amemiya, S.; Nakajima, Y. Nervous system development of the sea cucumber Stichopus japonicus. Dev. Biol. 2006, 292, 205–212. [Google Scholar] [CrossRef]
- Garcia-Arraras, J.E.; Rojas-Soto, M.; Jimenez, L.B.; Diaz-Miranda, L. The enteric nervous system of echinoderms: Unexpected complexity revealed by neurochemical analysis. J. Exp. Biol. 2001, 204, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Mashanov, V.S.; Zueva, O.R.; Heinzeller, T.; Dolmatov, I.Y. Ultrastructure of the circumoral nerve ring and the radial nerve cords in holothurians (Echinodermata). Zoomorphology 2006, 125, 27–38. [Google Scholar] [CrossRef]
- Hyman, L.H. The Invertebrates; Mc Graw-Hill Publications: New York, NY, USA, 1955. [Google Scholar]
- Diaz-Balzac, C.A.; Abreu-Arbelo, J.E.; Garcia-Arraras, J.E. Neuroanatomy of the tube feet and tentacles in Holothuria glaberrima (Holothuroidea, Echinodermata). Zoomorphology 2010, 129, 33–43. [Google Scholar] [CrossRef]
- Diaz-Balzac, C.A.; Lazaro-Pena, M.I.; Vazquez-Figueroa, L.D.; Diaz-Balzac, R.J.; Garcia-Arraras, J.E. Holothurian Nervous System Diversity Revealed by Neuroanatomical Analysis. PLoS ONE 2016, 11, e0151129. [Google Scholar] [CrossRef]
- Díaz-Balzac, C.A.; García-Arrarás, J.E. Echinoderm nervous system. Oxf. Res. Encycl. Neurosci. 2018. [Google Scholar] [CrossRef]
- Heinzeller, T.; Welsch, U. The echinoderm nervous system and its phylogenetic interpretation. In Brain Evolution and Cognition; Roth, G., Wullimann, M.F., Eds.; John Wiley and Sons: New York, NY, USA, 2001; pp. 41–75. [Google Scholar]
- Buznikov, G.A.; Peterson, R.E.; Nikitina, L.A.; Bezuglov, V.V.; Lauder, J.M. The pre-nervous serotonergic system of developing sea urchin embryos and larvae: Pharmacologic and immunocytochemical evidence. Neurochem. Res. 2005, 30, 825–837. [Google Scholar] [CrossRef]
- Bishop, C.D.; Burke, R.D. Ontogeny of the holothurian larval nervous system: Evolution of larval forms. Dev. Genes Evol. 2007, 217, 585–592. [Google Scholar] [CrossRef]
- Byrne, M.; Nakajima, Y.; Chee, F.C.; Burke, R.D. Apical organs in echinoderm larvae: Insights into larval evolution in the Ambulacraria. Evol. Dev. 2007, 9, 432–445. [Google Scholar] [CrossRef]
- Carter, H.F.; Thompson, J.R.; Elphick, M.R.; Oliveri, P. The development and neuronal complexity of bipinnaria larvae of the sea star Asterias rubens. Integr. Comp. Biol. 2021, 61, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Sillar, K.T.; Reith, C.A.; McDearmid, J.R. Development and Aminergic Neuromodulation of a Spinal Locomotor Network Controlling Swimming in Xenopus Larvae. Ann. N. Y. Acad. Sci. 1998, 860, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Kaneko, H.; Murray, G.; Burke, R.D. Divergent patterns of neural development in larval echinoids and asteroids. Evol. Dev. 2004, 6, 95–104. [Google Scholar] [CrossRef]
- Hinman, V.F.; Burke, R.D. Embryonic neurogenesis in echinoderms. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e316. [Google Scholar] [CrossRef]
- Nakano, H.; Nakajima, Y.; Amemiya, S. Nervous system development of two crinoid species, the sea lily Metacrinus rotundus and the feather star Oxycomanthus japonicus. Dev. Genes Evol. 2009, 219, 565–576. [Google Scholar] [CrossRef]
- Mercurio, S.; Gattoni, G.; Messinetti, S.; Sugni, M.; Pennati, R. Nervous system characterization during the development of a basal echinoderm, the feather star Antedon mediterranea. J. Comp. Neurol. 2019, 527, 1127–1139. [Google Scholar] [CrossRef]
- Wood, N.J.; Mattiello, T.; Rowe, M.L.; Ward, L.; Perillo, M.; Arnone, M.I.; Elphick, M.R.; Oliveri, P. Neuropeptidergic systems in pluteus larvae of the sea uchin Strongylocentrotus purpuratus: Neurochemical complexity in a "simple" nervous system. Front. Endocrinol. 2018, 9, 628. [Google Scholar] [CrossRef]
- Zueva, O.; Khoury, M.; Heinzeller, T.; Mashanova, D.; Mashanov, V. The complex simplicity of the brittle star nervous system. Front. Zool. 2018, 15, 1. [Google Scholar] [CrossRef]
- Formery, L.; Orange, F.; Formery, A.; Yaguchi, S.; Lowe, C.J.; Schubert, M.; Croce, J.C. Neural anatomy of echinoid early juveniles and comparison of nervous system organization in echinoderms. J. Comp. Neurol. 2021, 529, 1135–1156. [Google Scholar] [CrossRef]
- Paganos, P.; Voronov, D.; Musser, J.; Arendt, D.; Arnone, M.I. Single cell RNA sequencing of the Strongylocentrotus purpuratus larva reveals the blueprint of major cell types and nervous system of a non-chordate deuterostome. Elife 2021, 10, e70416. [Google Scholar] [CrossRef]
- Hodin, J.; Heyland, A.; Mercier, A.; Pernet, B.; Cohen, D.L.; Hamel, J.-F.; Allen, J.D.; McAlister, J.S.; Byrne, M.; Cisternas, P.; et al. Culturing echinoderm larvae through metamorphosis. In Echinoderms; Part, A., Kathy, R., Amro, H., Eds.; Academic Press: Amsterdam, The Netherlands, 2019; pp. 125–169. [Google Scholar] [CrossRef]
- Qiu, T.; Zhang, T.; Hamel, J.F.; Mercier, A. Development, settlement, and post-settlement growth. In The Sea Cucumber Apostichopus Japonicus: History, Biology and Aquaculture; Yang, H.S., Hamel, J.F., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; Volume 39, pp. 111–131. [Google Scholar] [CrossRef]
- Burke, R.D.; Brand, D.G.; Bisgrove, B.W. Structure of the nervous system of the auricularia larva Ofparasticopus californicus. Biol. Bull. 1986, 170, 450–460. [Google Scholar] [CrossRef]
- Hökfelt, T.; Broberger, C.; Xu, Z.Q.D.; Sergeyev, V.; Ubink, R.; Diez, M. Neuropeptides—Overview. Neuropharmacology 2000, 39, 1337–1356. [Google Scholar] [CrossRef]
- Watanabe, H.; Fujisawa, T.; Holstein, T.W. Cnidarians and the evolutionary origin of the nervous system. Dev. Growth Differ. 2009, 51, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Elphick, M.R.; Mirabeau, O.; Larhammar, D. Correction: Evolution of neuropeptide signalling systems. J. Exp. Biol. 2018, 221, 19. [Google Scholar] [CrossRef]
- Braubach, O.R.; Dickinson, A.J.; Evans, C.C.; Croll, R.P. Neural control of the velum in larvae of the gastropod, Ilyanassa obsoleta. J. Exp. Biol. 2006, 209, 4676–4689. [Google Scholar] [CrossRef]
- Dyachuk, V.; Odintsova, N. Development of the larval muscle system in the mussel Mytilus trossulus (Mollusca, Bivalvia). Dev. Growth Differ. 2009, 51, 69–79. [Google Scholar] [CrossRef]
- Kiss, T. Diversity and abundance: The basic properties of neuropeptide action in molluscs. Gen. Comp. Endocrinol. 2011, 172, 10–14. [Google Scholar] [CrossRef]
- Veenstra, J.A. Neuropeptide evolution: Neurohormones and neuropeptides predicted from the genomes of Capitella teleta and Helobdella robusta. Gen. Comp. Endocrinol. 2011, 171, 160–175. [Google Scholar] [CrossRef]
- Dickinson, P.S.; Qu, X.; Stanhope, M.E. Neuropeptide modulation of pattern-generating systems in crustaceans: Comparative studies and approaches. Curr. Opin. Neurobiol. 2016, 41, 149–157. [Google Scholar] [CrossRef]
- Kerbl, A.; Conzelmann, M.; Jékely, G.; Worsaae, K. High diversity in neuropeptide immunoreactivity patterns among three closely related species of Dinophilidae (Annelida). J. Comp. Neurol. 2017, 525, 3596–3635. [Google Scholar] [CrossRef]
- Semmens, D.C.; Elphick, M.R. The evolution of neuropeptide signalling: Insights from echinoderms. Brief. Funct. Genom. 2017, 16, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Cropper, E.C.; Jing, J.; Vilim, F.S.; Barry, M.A.; Weiss, K.R. Multifaceted expression of peptidergic modulation in the feeding system of Aplysia. ACS Chem. Neurosci. 2018, 9, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, A.; Alexander, J.L.; Swain, M.T.; Webster, S.G.; Wilcockson, D.C. Transcriptomic analysis of crustacean neuropeptide signaling during the moult cycle in the green shore crab, Carcinus maenas. BMC Genom. 2018, 19, 711. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, L.; Wu, J.; Liu, H.; Zheng, L.; Lü, Z.; Chi, C. An FMRFamide neuropeptide in cuttlefish Sepia pharaonis: Identification, characterization, and potential function. Molecules 2020, 25, 1636. [Google Scholar] [CrossRef]
- Zhang, Y.; Yañez-Guerra, L.A.; Tinoco, A.B.; Escudero Castelán, N.; Egertová, M.; Elphick, M.R. Somatostatin-type and allatostatin-C–type neuropeptides are paralogous and have opposing myoregulatory roles in an echinoderm. Proc. Natl. Acad. Sci. USA 2022, 119, e2113589119. [Google Scholar] [CrossRef]
- Rowe, M.L.; Elphick, M.R. The neuropeptide transcriptome of a model echinoderm, the sea urchin Strongylocentrotus purpuratus. Gen. Comp. Endocrinol. 2012, 179, 331–344. [Google Scholar] [CrossRef]
- Yamano, K.; Fujiwara, A.; Nakamura, A.; Yoshikuni, M. In vitro induction of oocyte maturation in the Japanese sea cucumber Apostichopus japonicus by cubifrin and the developmental ability of the eggs. Fish. Sci. 2013, 79, 823–832. [Google Scholar] [CrossRef]
- Rowe, M.L.; Achhala, S.; Elphick, M.R. Neuropeptides and polypeptide hormones in echinoderms: New insights from analysis of the transcriptome of the sea cucumber Apostichopus japonicus. Gen. Comp. Endocrinol. 2014, 197, 43–55. [Google Scholar] [CrossRef]
- Semmens, D.C.; Mirabeau, O.; Moghul, I.; Pancholi, M.R.; Wurm, Y.; Elphick, M.R. Transcriptomic identification of starfish neuropeptide precursors yields new insights into neuropeptide evolution. Open Biol. 2016, 6, 150224. [Google Scholar] [CrossRef]
- Smith, M.K.; Wang, T.; Suwansa-Ard, S.; Motti, C.A.; Elizur, A.; Zhao, M.; Rowe, M.L.; Hall, M.R.; Elphick, M.R.; Cummins, S.F. The neuropeptidome of the Crown-of-Thorns Starfish, Acanthaster planci. J. Proteom. 2017, 165, 61–68. [Google Scholar] [CrossRef]
- Zandawala, M.; Moghul, I.; Yanez Guerra, L.A.; Delroisse, J.; Abylkassimova, N.; Hugall, A.F.; O’Hara, T.D.; Elphick, M.R. Discovery of novel representatives of bilaterian neuropeptide families and reconstruction of neuropeptide precursor evolution in ophiuroid echinoderms. Open Biol. 2017, 7, 42–104. [Google Scholar] [CrossRef] [PubMed]
- Monroe, E.B.; Annangudi, S.P.; Wadhams, A.A.; Richmond, T.A.; Yang, N.; Southey, B.R.; Romanova, E.V.; Schoofs, L.; Baggerman, G.; Sweedler, J.V. Exploring the sea urchin neuropeptide landscape by mass spectrometry. J. Am. Soc. Mass Spectrom. 2018, 29, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Suwansa-Ard, S.; Chaiyamoon, A.; Talarovicova, A.; Tinikul, R.; Tinikul, Y.; Poomtong, T.; Elphick, M.R.; Cummins, S.F.; Sobhon, P. Transcriptomic discovery and comparative analysis of neuropeptide precursors in sea cucumbers (Holothuroidea). Peptides 2018, 99, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Talarovicova, A.; Zheng, Y.; Storey, K.B.; Elphick, M.R. Neuropeptide precursors and neuropeptides in the sea cucumber Apostichopus japonicus: A genomic, transcriptomic and proteomic analysis. Sci. Rep. 2019, 9, 8829. [Google Scholar] [CrossRef] [PubMed]
- Chieu, H.D.; Suwansa-Ard, S.; Wang, T.; Elizur, A.; Cummins, S.F. Identification of neuropeptides in the sea cucumber Holothuria leucospilota. Gen. Comp. Endocrinol. 2019, 283, 113229. [Google Scholar] [CrossRef]
- Kato, S.; Tsurumaru, S.; Taga, M.; Yamane, T.; Shibata, Y.; Ohno, K.; Fujiwara, A.; Yamano, K.; Yoshikuni, M. Neuronal peptides induce oocyte maturation and gamete spawning of sea cucumber, Apostichopus japonicus. Dev. Biol. 2009, 326, 169–176. [Google Scholar] [CrossRef]
- Mita, M.; Yoshikuni, M.; Ohno, K.; Shibata, Y.; Paul-Prasanth, B.; Pitchayawasin, S.; Isobe, M.; Nagahama, Y. A relaxin-like peptide purified from radial nerves induces oocyte maturation and ovulation in the starfish, Asterina pectinifera. Proc. Natl. Acad. Sci. USA 2009, 106, 9507–9512. [Google Scholar] [CrossRef]
- Perillo, M.; Arnone, M.I. Characterization of insulin-like peptides (ILPs) in the sea urchin Strongylocentrotus purpuratus: Insights on the evolution of the insulin family. Gen. Comp. Endocr. 2014, 205, 68–79. [Google Scholar] [CrossRef]
- Haraguchi, S.; Ikeda, N.; Abe, M.; Tsutsui, K.; Mita, M. Nucleotide sequence and expression of relaxin-like gonad-stimulating peptide gene in starfish Asterina pectinifera. Gen. Comp. Endocr. 2016, 227, 115–119. [Google Scholar] [CrossRef]
- Lin, M.; Mita, M.; Egertova, M.; Zampronio, C.G.; Jones, A.M.; Elphick, M.R. Cellular localization of relaxin-like gonad-stimulating peptide expression in Asterias rubens: New insights into neurohormonal control of spawning in starfish. J. Comp. Neurol. 2017, 525, 1599–1617. [Google Scholar] [CrossRef]
- Tinoco, A.B.; Semmens, D.C.; Patching, E.C.; Gunner, E.F.; Egertova, M.; Elphick, M.R. Characterization of NGFFYamide signaling in starfish reveals roles in regulation of feeding behavior and locomotory systems. Front. Endocrinol. 2018, 9, 507. [Google Scholar] [CrossRef]
- Yanez-Guerra, L.A.; Delroisse, J.; Barreiro-Iglesias, A.; Slade, S.E.; Scrivens, J.H.; Elphick, M.R. Discovery and functional characterisation of a luqin-type neuropeptide signalling system in a deuterostome. Sci. Rep. 2018, 8, 7220. [Google Scholar] [CrossRef] [PubMed]
- Chieu, H.D.; Turner, L.; Smith, M.K.; Wang, T.; Nocillado, J.; Palma, P.; Suwansa-Ard, S.; Elizur, A.; Cummins, S.F. Aquaculture breeding enhancement: Maturation and spawning in sea cucumbers using a recombinant relaxin-like gonad-stimulating peptide. Front. Genet. 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Chaiyamoon, A.; Tinikul, R.; Nontunha, N.; Chaichotranunt, S.; Poomtong, T.; Sobhon, P.; Tinikul, Y. Characterization of TRH/GnRH-like peptides in the sea cucumber, Holothuria scabra, and their effects on oocyte maturation. Aquaculture 2020, 518, 734814. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, L.; Fan, X.; Guo, X.; Liu, X.; Yang, H. The effect of pedal peptide-type neuropeptide on locomotor behavior and muscle physiology in the sea cucumber Apostichopus japonicus. Front. Physiol. 2020, 11, 559348. [Google Scholar] [CrossRef]
- Wang, T.; Cao, Z.; Shen, Z.; Yang, J.; Chen, X.; Yang, Z.; Xu, K.; Xiang, X.; Yu, Q.; Song, Y. Existence and functions of a kisspeptin neuropeptide signaling system in a non-chordate deuterostome species. Elife 2020, 9, e53370. [Google Scholar] [CrossRef]
- Zhang, Y.; Yanez Guerra, L.A.; Egertova, M.; Zampronio, C.G.; Jones, A.M.; Elphick, M.R. Molecular and functional characterization of somatostatin-type signalling in a deuterostome invertebrate. Open Biol. 2020, 10, 200172. [Google Scholar] [CrossRef]
- Tinoco, A.B.; Barreiro-Iglesias, A.; Guerra, L.A.Y.; Delroisse, J.; Zhang, Y.; Gunner, E.F.; Zampronio, C.G.; Jones, A.M.; Egertová, M.; Elphick, M.R. Ancient role of sulfakinin/cholecystokinin-type signalling in inhibitory regulation of feeding processes revealed in an echinoderm. Elife 2021, 10, e65667. [Google Scholar] [CrossRef]
- Beer, A.J.; Moss, C.; Thorndyke, M. Development of serotonin-like and SALMFamide-like immunoreactivity in the nervous system of the sea urchin Psammechinus miliaris. Biol. Bull. 2001, 200, 268–280. [Google Scholar] [CrossRef]
- Mayorova, T.D.; Tian, S.; Cai, W.; Semmens, D.C.; Odekunle, E.A.; Zandawala, M.; Badi, Y.; Rowe, M.L.; Egertova, M.; Elphick, M.R. Localization of neuropeptide gene expression in larvae of an echinoderm, the starfish Asterias rubens. Front. Neurosci. 2016, 10, 553. [Google Scholar] [CrossRef]
- Thompson, J.R.; Paganos, P.; Benvenuto, G.; Arnone, M.I.; Oliveri, P. Post-metamorphic skeletal growth in the sea urchin Paracentrotus lividus and implications for body plan evolution. EvoDevo 2021, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M.; Keyser, P. Acetylation of alpha-tubulin in cultured neurons and the induction of alpha-tubulin acetylation in PC12 cells by treatment with nerve growth factor. J. Neurosci. 1987, 7, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Cáceres, A. The expression of acetylated microtubules during axonal and dendritic growth in cerebellar macroneurons which develop in vitro. Dev. Brain Res. 1989, 49, 205–213. [Google Scholar] [CrossRef]
- Gavilán, B.; Perea-Atienza, E.; Martínez, P. Xenacoelomorpha: A case of independent nervous system centralization? Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150039. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Loesel, R.; Purschke, G.; Schmidt-Rhaesa, A.; Scholtz, G.; Stach, T.; Vogt, L.; Wanninger, A.; Brenneis, G.; Döring, C. Invertebrate neurophylogeny: Suggested terms and definitions for a neuroanatomical glossary. Front. Zool. 2010, 7, 29. [Google Scholar] [CrossRef]
- Zieger, E.; Candiani, S.; Garbarino, G.; Croce, J.C.; Schubert, M. Roles of retinoic acid signaling in shaping the neuronal architecture of the developing amphioxus nervous system. Mol. Neurobiol. 2018, 55, 5210–5229. [Google Scholar] [CrossRef]
- Boyko, A.V.; Girich, A.S.; Eliseikina, M.G.; Maslennikov, S.I.; Dolmatov, I.Y. Reference assembly and gene expression analysis of Apostichopus japonicus larval development. Sci. Rep. 2019, 9, 1131. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Chen, F.Z.; You, L.J.; Yang, F.; Wang, L.N.; Guo, X.Q.; Gao, F.; Hua, C.; Tan, C.; Fang, L.; Shan, R.Q.; et al. CNGBdb: China National GeneBank DataBase. Hereditas 2020, 42, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, F.; Gao, F.; Li, L.; Liu, K.; You, L.; Hua, C.; Yang, F.; Liu, W.; Peng, C.; et al. CNSA: A data repository for archiving omics data. Database 2020, 2020, baaa055. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, M.; Yin, Y.; Storey, K.B. MiR-200-3p is potentially involved in cell cycle arrest by regulating cyclin a during aestivation in Apostichopus japonicus. Cells 2019, 8, 843. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, M.; Wang, T.; Sun, L.; Xu, D.; Yang, H. Selection of reference genes for qRT-PCR analysis of gene expression in sea cucumber Apostichopus japonicus during aestivation. Chin. J. Oceanol. Limnol. 2014, 32, 1248–1256. [Google Scholar] [CrossRef]
- Nakajima, Y.; Burke, R.D.; Noda, Y. The structure and development of the apical ganglion in the sea urchin pluteus larvae of Strongylocentrotus droebachiensis and Mespilia globulus. Dev. Growth Differ. 1993, 35, 531–538. [Google Scholar] [CrossRef]
- Chee, F.; Byrne, M. Development of the larval serotonergic nervous system in the sea star Patiriella regularis as revealed by confocal imaging. Biol. Bull. 1999, 197, 123–131. [Google Scholar] [CrossRef]
- Hay-Schmidt, A. The evolution of the serotonergic nervous system. Proc. Natl. Acad. Sci. USA 2000, 267, 1071–1079. [Google Scholar] [CrossRef]
- Conzelmann, M.; Offenburger, S.-L.; Asadulina, A.; Keller, T.; Münch, T.A.; Jékely, G. Neuropeptides regulate swimming depth of Platynereis larvae. Proc. Natl. Acad. Sci. USA 2011, 108, E1174–E1183. [Google Scholar] [CrossRef]
- Garner, S.; Zysk, I.; Byrne, G.; Kramer, M.; Moller, D.; Taylor, V.; Burke, R.D. Neurogenesis in sea urchin embryos and the diversity of deuterostome neurogenic mechanisms. Development 2016, 143, 286–297. [Google Scholar] [CrossRef]
- Arikawa, K.; Williams, D.S. Acetylated alpha-tubulin in the connecting cilium of developing rat photoreceptors. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2145–2149. [Google Scholar]
- Hirokawa, T.; Komatsu, M.; Nakajima, Y. Development of the nervous system in the brittle star Amphipholis kochii. Dev. Genes Evol. 2008, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.D. Development of the larval nervous system of the sand dollar, Dendraster excentricus. Cell Tissue Res. 1983, 229, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Lacalli, T.; Gilmour, T.; West, J. Ciliary band innervation in the bipinnaria larva of Pisaster ochraceus. Trans. R. Soc. B Biol. Sci. 1990, 330, 371–390. [Google Scholar] [CrossRef]
- Lacalli, T.C. Ventral neurons in the anterior nerve cord of amphioxus larvae. II. Further data on the pacemaker circuit. J. Morphol. 2003, 257, 212–218. [Google Scholar] [CrossRef]
- Lacalli, T.C. Protochordate body plan and the evolutionary role of larvae: Old controversies resolved? Can. J. Zool. 2005, 83, 216–224. [Google Scholar] [CrossRef]
- Jekely, G.; Melzer, S.; Beets, I.; Kadow, I.C.G.; Koene, J.; Haddad, S.; Holden-Dye, L. The long and the short of it—A perspective on peptidergic regulation of circuits and behaviour. J. Exp. Biol. 2018, 221, jeb166710. [Google Scholar] [CrossRef]
- Odekunle, E.A.; Semmens, D.C.; Martynyuk, N.; Tinoco, A.B.; Garewal, A.K.; Patel, R.R.; Blowes, L.M.; Zandawala, M.; Delroisse, G.; Slade, S.E.; et al. Ancient role of vasopressin/oxytocin-type neuropeptides as regulators of feeding revealed in an echinoderm. BMC Biol. 2019, 17, 60. [Google Scholar] [CrossRef]
- Elphick, M.R. The protein precursors of peptides that affect the mechanics of connective tissue and/or muscle in the echinoderm Apostichopus japonicus. PLoS ONE 2012, 7, e44492. [Google Scholar] [CrossRef]
- Lin, M.; Egertová, M.; Zampronio, C.G.; Jones, A.M.; Elphick, M.R. Pedal peptide/orcokinin-type neuropeptide signaling in a deuterostome: The anatomy and pharmacology of starfish myorelaxant peptide in Asterias rubens. J. Comp. Neurol. 2017, 525, 3890–3917. [Google Scholar] [CrossRef]
- Lin, M.; Egertova, M.; Zampronio, C.G.; Jones, A.M.; Elphick, M.R. Functional characterization of a second pedal peptide/orcokinin-type neuropeptide signaling system in the starfish Asterias rubens. J. Comp. Neurol. 2018, 526, 858–876. [Google Scholar] [CrossRef]
- Whittington, I.D.; Cribb, B.W. Adhesive secretions in the Platyhelminthes. Adv. Parasitol. 2001, 48, 101–224. [Google Scholar] [CrossRef] [PubMed]
- Flammang, P.; Santos, R.; Haesaerts, D. Echinoderm adhesive secretions: From experimental characterization to biotechnological applications. In Echinodermata; Progress in Molecular and Subcellular Biology (Marine Molecular Biotechnology); Matranga, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 39, pp. 201–220. [Google Scholar] [CrossRef]
- Terenina, N.; Kreshchenko, N.; Mochalova, N.; Movsesyan, S. Serotonin and neuropeptide FMRFamide in the attachment organs of trematodes. Helminthologia 2018, 55, 185. [Google Scholar] [CrossRef] [PubMed]
- Zieger, E.; Robert, N.S.; Calcino, A.; Wanninger, A. Ancestral role of ecdysis-related neuropeptides in animal life cycle transitions. Curr. Biol. 2021, 31, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Mulrenin, E.M.; Witkin, J.W.; Silverman, A.J. Embryonic development of the gonadotropin-releasing hormone (GnRH) system in the chick: A spatio-temporal analysis of GnRH neuronal generation, site of origin, and migration. Endocrinology 1999, 140, 422–433. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gurbuz, A.S.; Gode, F.; Uzman, M.S.; Ince, B.; Kaya, M.; Ozcimen, N.; Ozcimen, E.E.; Acar, A. GnRH agonist triggering affects the kinetics of embryo development: A comparative study. J. Ovarian Res. 2016, 9, 22. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).