Na,K-ATPase Kinetics and Oxidative Stress in Kidneys of Zucker Diabetic Fatty (fa/fa) Rats Depending on the Diabetes Severity—Comparison with Lean (fa/+) and Wistar Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Characterization of Oxidative Status and Consequences of Increased Glycemia
2.3. Plasma Membrane Fraction Preparation
2.4. Na,K-ATPase Enzyme Kinetics
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of the Experimental Animals
3.2. Oxidative Status and Consequences of Increased Glycemia
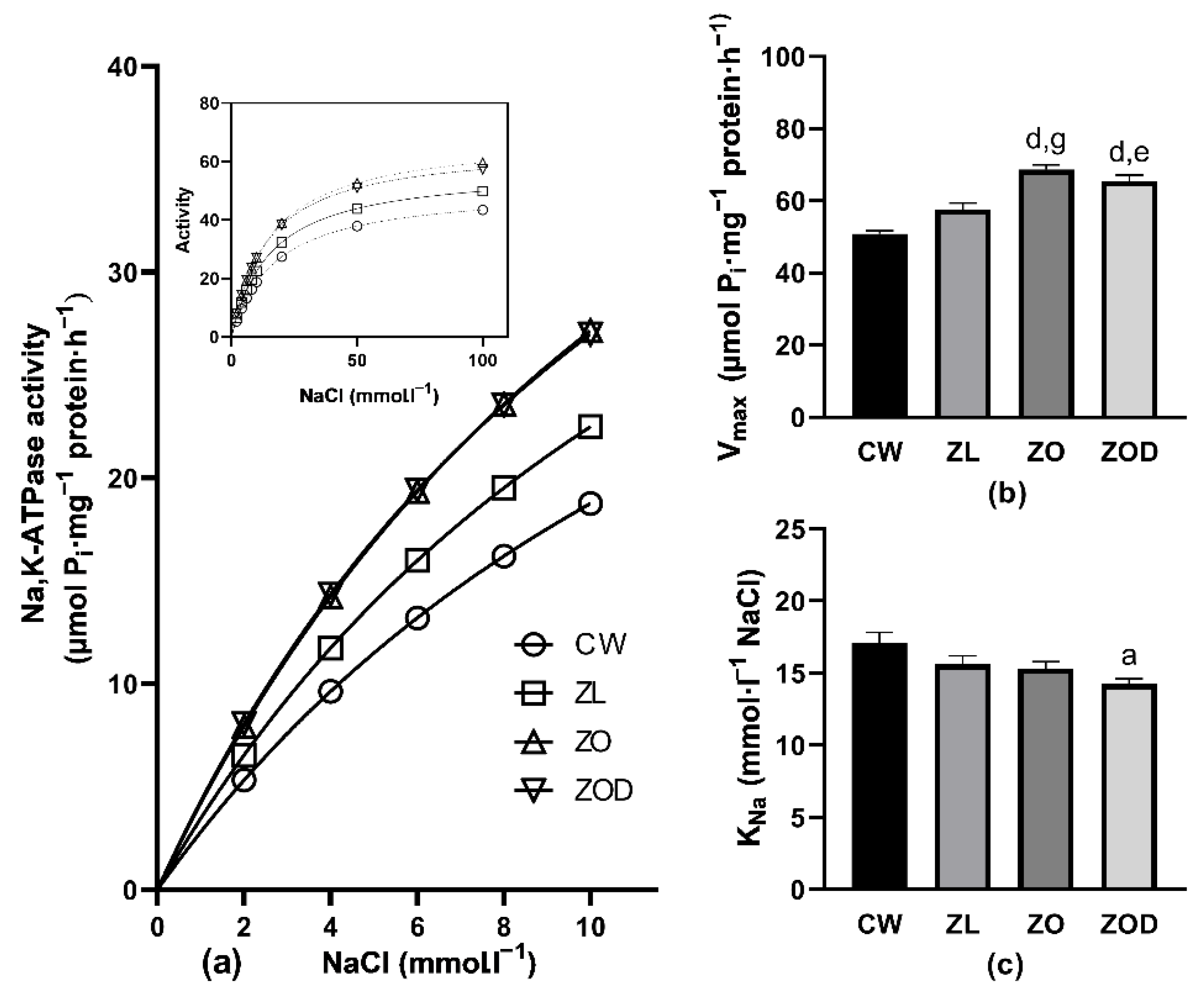
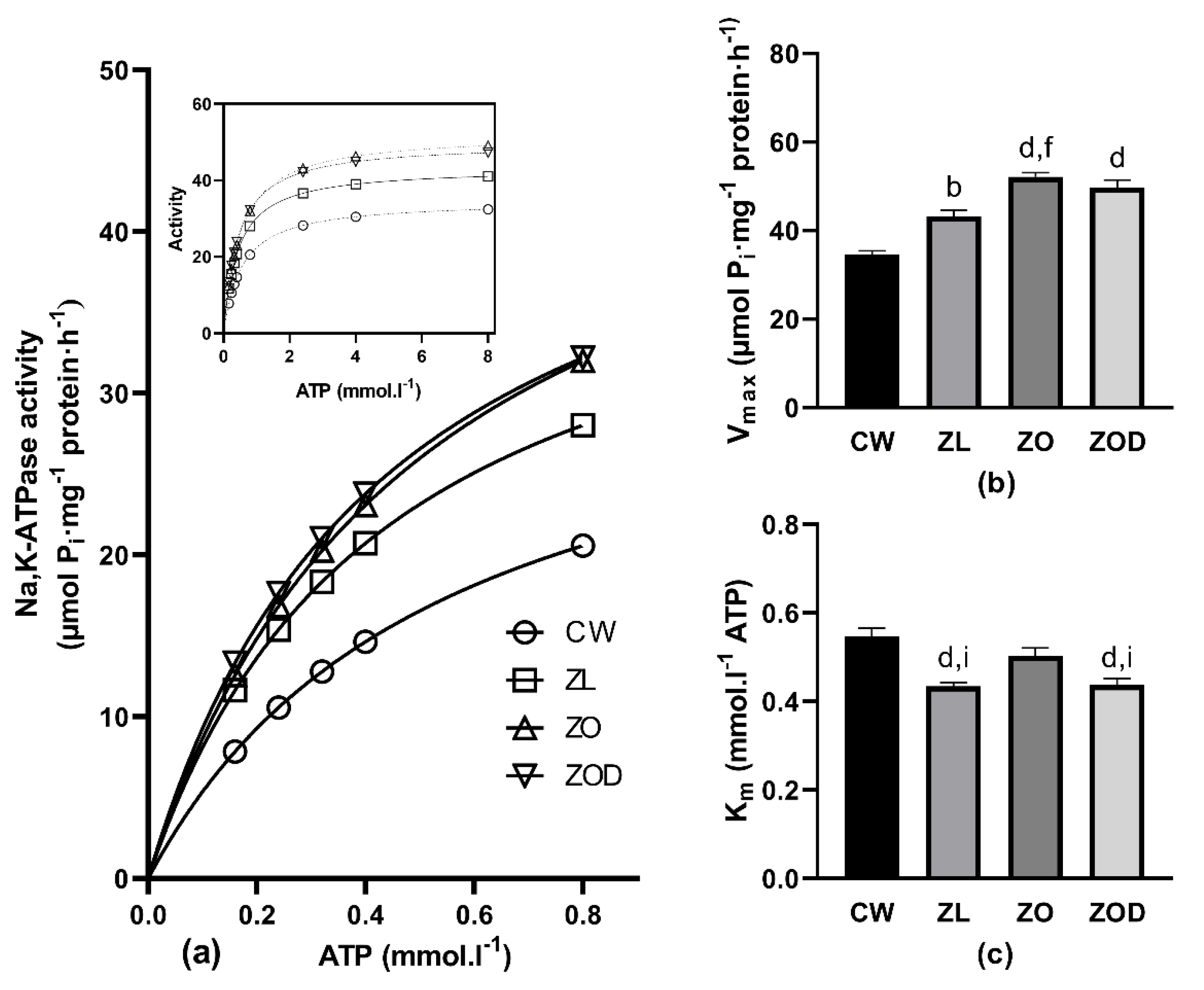
3.3. Na,K-ATPase
4. Discussion
4.1. Basic Biometric and Biochemical Parameters
4.2. Parameters of Oxidative and Carbonyl Stress
4.3. Kinetic Measurements of Na,K-ATPase Enzyme
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bashir, S.O. Concomitant administration of resveratrol and insulin protects against diabetes mellitus type-1-induced renal damage and impaired function via an antioxidant-mediated mechanism and up-regulation of Na+/K+-ATPase. Arch. Physiol. Biochem. 2019, 125, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zeng, J.; Dong, Y.; Xu, K.Y. Concurrent impairment of (Na++K+)-ATPase activity in multi-organ of type-1 diabetic NOD mice. J. Diabetes Complicat. 2013, 27, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Nordquist, L.; Shimada, K.; Ishii, T.; Furuya, D.T.; Kamikawa, A.; Kimura, K. Proinsulin C-peptide prevents type-1 diabetes-induced decrease of renal Na+-K+-ATPase alpha1-subunit in rats. Diabetes Metab. Res. Rev. 2010, 26, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Vrbjar, N.; Strelková, S.; Javorková, V.; Vlkovicová, J.; Mézesová, L.; Stefek, M.; Kysel’ová, Z.; Gajdosíková, A. Effect of the pyridoindole antioxidant stobadine on ATP-utilisation by renal Na,K-ATPase in rats with streptozotocin-induced diabetes. Gen. Physiol. Biophys. 2007, 26, 207–213. [Google Scholar] [PubMed]
- Vrbjar, N.; Strelková, S.; Stefek, M.; Kyselová, Z.; Gajdosíková, A. Effect of the pyridoindole antioxidant stobadine on sodium handling of renal Na,K-ATPase in rats with streptozotocin-induced diabetes. Acta Diabetol. 2004, 41, 172–178. [Google Scholar] [CrossRef]
- Tsimaratos, M.; Coste, T.C.; Djemli-Shipkolye, A.; Vague, P.; Pieroni, G.; Raccah, D. Gamma-linolenic acid restores renal medullary thick ascending limb Na+,K+-ATPase activity in diabetic rats. J. Nutr. 2001, 131, 3160–3165. [Google Scholar] [CrossRef][Green Version]
- Hussain, T.; Beheray, S.A.; Lokhandwala, M.F. Defective dopamine receptor function in proximal tubules of obese zucker rats. Hypertension 1999, 34, 1091–1096. [Google Scholar] [CrossRef]
- Slieker, L.J.; Sundell, K.L.; Heath, W.F.; Osborne, H.E.; Bue, J.; Manetta, J.; Sportsman, J.R. Glucose transporter levels in tissues of spontaneously diabetic Zucker fa/fa rat (ZDF/drt) and viable yellow mouse (Avy/a). Diabetes 1992, 41, 187–193. [Google Scholar] [CrossRef]
- Wang, X.; DuBois, D.C.; Sukumaran, S.; Ayyar, V.; Jusko, W.J.; Almon, R.R. Variability in Zucker diabetic fatty rats: Differences in disease progression in hyperglycemic and normoglycemic animals. Diabetes Metab. Syndr. Obes. 2014, 7, 531–541. [Google Scholar] [CrossRef]
- Kollarova, M.; Chomova, M.; Radosinska, D.; Tothova, L.; Shawkatova, I.; Radosinska, J. ZDF fa/fa rats show increasing heterogeneity in main parameters during ageing as confirmed by biometrics, oxidative stress markers and MMP activity. Exp. Physiol. 2022. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.A.; Fazili, F.R.; Marwaha, A.; Lokhandwala, M.F. Mitogen-activated protein kinase upregulation reduces renal D1 receptor affinity and G-protein coupling in obese rats. Kidney Int. 2007, 71, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Winiarska, K.; Focht, D.; Sierakowski, B.; Lewandowski, K.; Orlowska, M.; Usarek, M. NADPH oxidase inhibitor, apocynin, improves renal glutathione status in Zucker diabetic fatty rats: A comparison with melatonin. Chem. Biol. Interact. 2014, 218, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hye Khan, M.A.; Hwang, S.H.; Sharma, A.; Corbett, J.A.; Hammock, B.D.; Imig, J.D. A dual COX-2/sEH inhibitor improves the metabolic profile and reduces kidney injury in Zucker diabetic fatty rat. Prostaglandins Other Lipid Mediat. 2016, 125, 40–47. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; An, W.; Zhang, F.; Niu, M.; Liu, Y.; Shi, R. Nebivolol ameliorated kidney damage in Zucker diabetic fatty rats by regulation of oxidative stress/NO pathway: Comparison with captopril. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1135–1148. [Google Scholar] [CrossRef]
- Banday, A.A.; Marwaha, A.; Tallam, L.S.; Lokhandwala, M.F. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes 2005, 54, 2219–2226. [Google Scholar] [CrossRef]
- Shah, P.T.; Martin, R.; Yan, Y.; Shapiro, J.I.; Liu, J. Carbonylation Modification Regulates Na/K-ATPase Signaling and Salt Sensitivity: A Review and a Hypothesis. Front. Physiol. 2016, 7, 256. [Google Scholar] [CrossRef]
- Dobrota, D.; Matejovicova, M.; Kurella, E.G.; Boldyrev, A.A. Na/K-ATPase under Oxidative Stress: Molecular Mechanisms of Injury. Cell. Mol. Neurobiol. 1999, 19, 141–149. [Google Scholar] [CrossRef]
- Jasenovec, T.; Radosinska, D.; Kollarova, M.; Balis, P.; Dayar, E.; Bernatova, I.; Zorad, S.; Vrbjar, N.; Cacanyova, S.; Radosinska, J. Angiotensin System Modulations in Spontaneously Hypertensive Rats and Consequences on Erythrocyte Properties; Action of MLN-4760 and Zofenopril. Biomedicines 2021, 9, 1902. [Google Scholar] [CrossRef]
- Jorgensen, P.L.; Skou, J.C.; Solomonson, L.P. Purification and characterization of (Na+ + K+)-ATPase. II. Preparation by zonal centrifugation of highly active (Na+ + K+)-ATPase from the outer medulla of rabbit kidneys. Biochim. Biophys. Acta 1971, 233, 381–394. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Taussky, H.H.; Shorr, E. A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 1953, 202, 675–685. [Google Scholar] [CrossRef]
- Li, Y.; Qi, Y.; Kim, M.S.; Xu, K.Z.; Huang, T.H.; Rong, X.; Murray, M.; Yamahara, J. Increased renal collagen cross-linking and lipid accumulation in nephropathy of Zucker diabetic fatty rats. Diabetes Metab. Res. Rev. 2008, 24, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Jasenovec, T.; Radosinska, D.; Kollarova, M.; Balis, P.; Ferenczyova, K.; Kalocayova, B.; Bartekova, M.; Tothova, L.; Radosinska, J. Beneficial Effect of Quercetin on Erythrocyte Properties in Type 2 Diabetic Rats. Molecules 2021, 26, 4868. [Google Scholar] [CrossRef] [PubMed]
- Kovalčíková, A.G.; Tichá, Ľ.; Šebeková, K.; Celec, P.; Čagalová, A.; Sogutlu, F.; Podracká, Ľ. Oxidative status in plasma, urine and saliva of girls with anorexia nervosa and healthy controls: A cross-sectional study. J. Eat. Disord. 2021, 9, 54. [Google Scholar] [CrossRef]
- Bickel, C.A.; Verbalis, J.G.; Knepper, M.A.; Ecelbarger, C.A. Increased renal Na-K-ATPase, NCC, and beta-ENaC abundance in obese Zucker rats. Am. J. Physiol. Renal. Physiol. 2001, 281, F639–F648. [Google Scholar] [CrossRef]
- Fakhruddin, S.; Alanazi, W.A.; Alhamami, H.N.; Briski, K.P.; Jackson, K.E. Hyperglycaemia induced by chronic i.p. and oral glucose loading leads to hypertension through increased Na+ retention in proximal tubule. Exp. Physiol. 2018, 103, 236–249. [Google Scholar] [CrossRef]
- Kamran, M.; Peterson, R.G.; Dominguez, J.H. Overexpression of GLUT2 gene in renal proximal tubules of diabetic Zucker rats. J. Am. Soc. Nephrol. 1997, 8, 943–948. [Google Scholar] [CrossRef]
- Zou, M.; Chen, Y.; Zheng, Z.; Sheng, S.; Jia, Y.; Wang, X.; Ren, S.; Yang, Y.; Li, X.; Dong, W.; et al. High-Salt Attenuates the Efficacy of Dapagliflozin in Tubular Protection by Impairing Fatty Acid Metabolism in Diabetic Kidney Disease. Front. Pharmacol. 2021, 12, 741087. [Google Scholar] [CrossRef]
| Group | BW (g) | KW (g) | KW/BW × 103 | Glucose (mmol·L−1) | Insulin (ng·mL−1) |
|---|---|---|---|---|---|
| CW | 494 ± 22 | 1.45 ± 0.04 | 2.78 ± 0.09 | 7.3 ± 0.1 | 7.9 ± 1.5 |
| ZL | 417 ± 8 b | 1.23 ± 0.03 c | 2.97 ± 0.05 | 6.7 ± 0.2 | 1.6 ± 0.08 b |
| ZO | 628 ± 8 d,h | 1.59 ± 0.05 h | 2.54 ± 0.05 f | 8.8 ± 0.2 e | 19.3 ± 2.1 d,h |
| ZOD | 528 ± 17 g,j | 1.77 ± 0.04 d,h,i | 3.36 ± 0.12 d,f,l | 18.3 ± 1.2 d,h,l | 8.7 ± 1.0 f,l |
| Cortex | |||||
|---|---|---|---|---|---|
| Group | GSH/GSSG | AOPP (µmol·g−1) | TBARS (µmol·L−1) | TAC (mmol·L−1) | FRAP (mmol·L−1) |
| CW | 2.9 ± 0.2 | 10.2 ± 0.8 | 2.1 ± 0.3 | 4.1 ± 0.1 | 0.63 ± 0.02 |
| ZL | 3.2 ± 0.2 | 13.2 ± 1.3 | 2.9 ± 0.4 | 4.3 ± 0.1 | 0.62 ± 0.02 |
| ZO | 2.7 ± 0.1 | 10.1 ± 0.3 | 6.2 ± 0.6 d,h | 5.1 ± 0.1 d,h | 0.55 ± 0.03 |
| ZOD | 3.0 ± 0.2 | 11.1 ± 1.1 | 4.7 ± 0.5 b,e | 5.3 ± 0.1 d,h | 0.58 ± 0.02 |
| Medulla | |||||
| Group | GSH/GSSG | AOPP (µmol·g−1) | TBARS (µmol·L−1) | TAC (mmol·L−1) | FRAP (mmol·L−1) |
| CW | 3.8 ± 0.4 | 9.0 ± 0.2 | 9.5 ± 0.9 | 4.3 ± 0.1 | 0.47 ± 0.02 |
| ZL | 6.6 ± 0.7 a | 10.0 ± 0.7 | 6.0 ± 0.6 | 4.4 ± 0.1 | 0.46 ± 0.03 |
| ZO | 6.5 ± 1.0 | 9.5 ± 1.4 | 6.7 ± 1.1 | 4.5 ± 0.1 | 0.51 ± 0.07 |
| ZOD | 6.4 ± 0.8 | 8.4 ± 0.3 | 11.3 ± 2.2 e | 4.5 ± 0.1 | 0.57 ± 0.04 |
| Cortex | Medulla | |||
|---|---|---|---|---|
| Group | Fructosamine (µmol·g−1) | AGEs (g·g−1) | Fructosamine (µmol·g−1) | AGEs (g·g−1) |
| CW | 111 ± 5 | 0.15 ± 0.02 | 89 ± 7 | 0.13 ± 0.01 |
| ZL | 122 ± 9 | 0.21 ± 0.02 | 84 ± 5 | 0.17 ± 0.02 |
| ZO | 102 ± 6 | 0.13 ± 0.01 e | 94 ± 6 | 0.15 ± 0.02 |
| ZOD | 115 ± 9 | 0.15 ± 0.01 | 101 ± 8 | 0.14 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrbjar, N.; Jasenovec, T.; Kollarova, M.; Snurikova, D.; Chomova, M.; Radosinska, D.; Shawkatova, I.; Tothova, L.; Radosinska, J. Na,K-ATPase Kinetics and Oxidative Stress in Kidneys of Zucker Diabetic Fatty (fa/fa) Rats Depending on the Diabetes Severity—Comparison with Lean (fa/+) and Wistar Rats. Biology 2022, 11, 1519. https://doi.org/10.3390/biology11101519
Vrbjar N, Jasenovec T, Kollarova M, Snurikova D, Chomova M, Radosinska D, Shawkatova I, Tothova L, Radosinska J. Na,K-ATPase Kinetics and Oxidative Stress in Kidneys of Zucker Diabetic Fatty (fa/fa) Rats Depending on the Diabetes Severity—Comparison with Lean (fa/+) and Wistar Rats. Biology. 2022; 11(10):1519. https://doi.org/10.3390/biology11101519
Chicago/Turabian StyleVrbjar, Norbert, Tomas Jasenovec, Marta Kollarova, Denisa Snurikova, Maria Chomova, Dominika Radosinska, Ivana Shawkatova, Lubomira Tothova, and Jana Radosinska. 2022. "Na,K-ATPase Kinetics and Oxidative Stress in Kidneys of Zucker Diabetic Fatty (fa/fa) Rats Depending on the Diabetes Severity—Comparison with Lean (fa/+) and Wistar Rats" Biology 11, no. 10: 1519. https://doi.org/10.3390/biology11101519
APA StyleVrbjar, N., Jasenovec, T., Kollarova, M., Snurikova, D., Chomova, M., Radosinska, D., Shawkatova, I., Tothova, L., & Radosinska, J. (2022). Na,K-ATPase Kinetics and Oxidative Stress in Kidneys of Zucker Diabetic Fatty (fa/fa) Rats Depending on the Diabetes Severity—Comparison with Lean (fa/+) and Wistar Rats. Biology, 11(10), 1519. https://doi.org/10.3390/biology11101519






