High Ecophysiological Plasticity of Desmarestia aculeata (Phaeophyceae) Present in an Arctic Fjord under Varying Salinity and Irradiance Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Algal Material
2.2. Experimental Set-Up
2.3. Physiological Parameters
2.4. Biochemical Parameters
2.5. Statistical Analysis
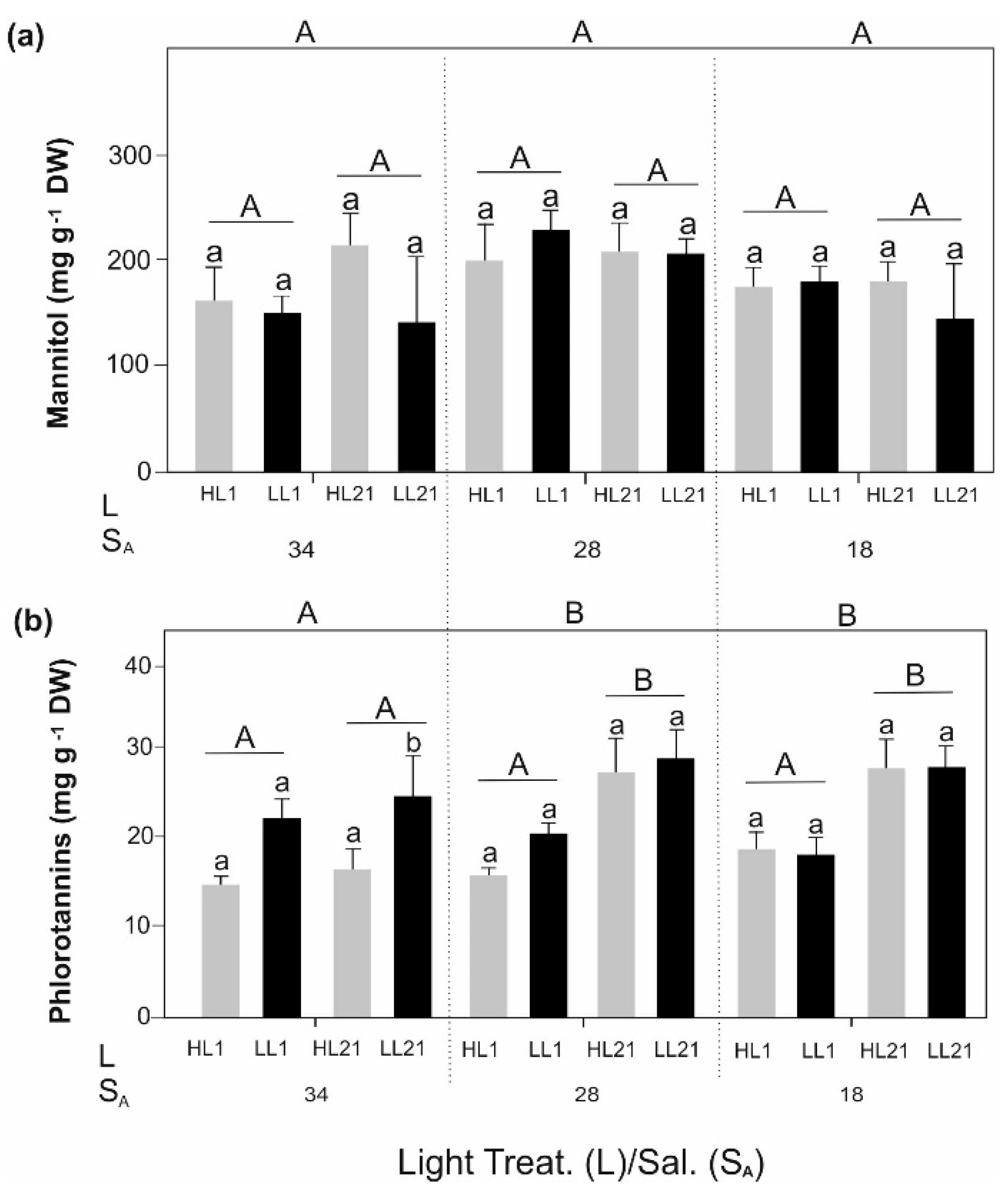
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malavenda, S. Species diversity of macroalgae in Gronfjorden, Spitsbergen, Svalbard. Polar Res. 2021, 40, 3682. [Google Scholar] [CrossRef]
- Blaszczyk, M.; Ignatiuk, D.; Uszczyk, A.; Cielecka-Nowak, K.; Grabiec, M.; Jania, J.; Moskalik, M.; Walczowski, W. Freshwater input to the Arctic fjord Hornsund (Svalbard). Polar Res. 2019, 38, 3506. [Google Scholar] [CrossRef]
- Polyakov, I.V.; Timokhov, L.A.; Alexeev, V.A.; Bacon, S.; Dmitrenko, I.A.; Fortier, L.; Frolov, I.E.; Gascard, J.C.; Hansen, E.; Ivanov, V.V.; et al. Arctic Ocean warming contributes to reduced Polar Ice cap. J. Phys. Oceanogr. 2010, 40, 2743–2756. [Google Scholar] [CrossRef]
- Wiencke, C.; Clayton, M.N.; Goméz, I.; Iken, K.; Lüder, U.H.; Amsler, C.D.; Karsten, U.; Hanelt, D.; Bischof, K.; Dunton, K. Life strategy, ecophysiology and ecology of algae in polar waters. Rev. Environ. Sci. Biotechnol. 2006, 6, 95–126. [Google Scholar] [CrossRef]
- Wiencke, C.; Hop, H. Ecosystem Kongsfjorden: New views after more than a decade of research. Polar Biol. 2016, 39, 1679–1687. [Google Scholar] [CrossRef][Green Version]
- Lippert, H.; Iken, K.; Rachor, E.; Wiencke, C. Macrofauna associated with macroalgae in the Kongsfjorden (Spitsbergen). Polar Biol. 2001, 24, 512–522. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C.M.; Hendriks, I.E.; Meire, L.; Blicher, M.E.; Marbà, N.; Sejr, M.K. Macroalgae contribute to nested mosaics of pH variability in a Subarctic fjord. Biogeosciences 2015, 12, 4895–4911. [Google Scholar] [CrossRef]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Fredriksen, S.; Karsten, U.; Bartsch, I.; Woelfel, J.; Koblowsky, M.; Schumman, R.; Roang, R.; Steneck, R.; Wiktor, J.; Hop, H.; et al. Biodiversity of benthic macro- and microalgae from Svalbard with special focus on Kongsfjorden. In The Ecosystem of Kongsfjorden, Svalbard, Advances in Polar Ecology, 2nd ed.; Hop, H., Wiencke, C., Eds.; Springer: Cham, Switzerland, 2019; pp. 331–372. [Google Scholar]
- Gienapp, P.; Teplitsky, C.; Alho, J.S.; Mills, A.; Merila, J. Climate change and evolution: Disentangling environmental and genetic responses. Mol. Ecol. 2008, 17, 167–178. [Google Scholar] [CrossRef]
- Schaum, C.E.; Collins, S. Plasticity predicts evolution in a marine alga. Proc. R. Soc. B 2014, 281, 20141486. [Google Scholar] [CrossRef]
- Aguilera, J.; Bischof, K.; Karsten, U.; Hanelt, D.; Wiencke, C. Seasonal variation in ecophysiological patterns in macroalgae from an Arctic fjord. II. Pigment accumulation and biochemical defence systems against high light stress. Mar. Biol. 2002, 140, 1087–1095. [Google Scholar] [CrossRef]
- Bischof, K.; Hanelt, D.; Aguilera, J.; Karsten, U.; Vögele, B.; Sawall, T.; Wiencke, C. Seasonal variation in ecophysiological patterns in macroalgae from an Arctic fjord. I. Sensitivity of photosynthesis to ultraviolet radiation. Mar. Biol. 2002, 140, 1097–1106. [Google Scholar] [CrossRef]
- Mathieson, A.C.; Dawes, C.J. Seaweeds of the Northwest Atlantic; University of Massachusetts Press: Amherst, MA, USA, 2017. [Google Scholar]
- Wiencke, C.; Vögele, B.; Kovaltchouk, N.A.; Hop, H. Species composition and zonation of marine benthic macroalgae at Hansneset in Kongsfjorden, Svalbard. Ber. Polarforsch. Meeresforsch. 2004, 492, 55–62. [Google Scholar]
- Nielsen, R.; Lundsteen, S. Denmark’s Sea Algae, Brown Algae (Phaeophyceae) and Green Algae (Chlorophyta), Scientia Danica; The Royal Danish Academy of Sciences and Letters: Copenhagen, Denmark, 2019; pp. 1–476. [Google Scholar]
- Conway, E. Aspects of algal ecology. Br. Phycol. Bull. 1967, 3, 161–173. [Google Scholar] [CrossRef]
- Kain, J.; Jones, S. Algal recolonization of some cleared subtidal areas. J. Ecol. 1975, 63, 739–765. [Google Scholar] [CrossRef]
- Kitching, J.A. Studies in sublittoral ecology, III. Laminaria forest on the west coast of Scotland; A study of zonation in relation to wave action and illumination. Biol. Bull. 1941, 80, 324–337. [Google Scholar] [CrossRef]
- Pehlke, C.; Bartsch, I. Changes in depth distribution and biomass of sublittoral seaweeds at Helgoland (North Sea) between 1970 and 2005. Clim. Res. 2008, 37, 135–147. [Google Scholar] [CrossRef]
- Iñiguez, C.; Carmona, R.; Lorenzo, M.R.; Niell, F.X.; Wiencke, C.; Gordillo, F.J.L. Increased CO2 modifies the carbon balance and the photosynthetic yield of two common Arctic brown seaweeds: Desmarestia aculeata and Alaria esculenta. Polar Biol. 2015, 39, 1979–1991. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Aguilera, J.; Jiménez, C. The response of nutrient assimilation and biochemical composition of Arctic seaweeds to a nutrient input in summer. J. Exp. Bot. 2006, 57, 2661–2671. [Google Scholar] [CrossRef]
- López-Figueroa, F. Control by light quality of Chlorophyll synthesis in the brown alga Desmarestia aculeata. Z. Für Nat. C 1991, 46, 542–548. [Google Scholar] [CrossRef]
- Marambio, J.; Bischof, K. Differential acclimation responses to irradiance and temperature in two co-occurring seaweed species in Arctic fjords. Polar Res. 2021, 40, 5702. [Google Scholar] [CrossRef]
- Lüning, K. Temperature tolerance and biogeography of seaweeds: The marine algal flora of Helgoland (North Sea) as an example. Helgol. Mar. Res. 1984, 28, 305–317. [Google Scholar] [CrossRef]
- Diehl, N.; Karsten, U.; Bischof, K. Impacts of combined temperature and salinity stress on the endemic Arctic brown seaweed Laminaria solidungula J. Agardh. Polar Biol. 2020, 43, 647–656. [Google Scholar] [CrossRef]
- Karsten, U. Salinity tolerance of Arctic kelps from Spitsbergen. Phycol. Res. 2007, 55, 257–262. [Google Scholar] [CrossRef]
- Marambio, J.; Rosenfeld, S.; Bischof, K. Hyposalinity affects diurnal photoacclimation patterns in the rhodophyte Palmaria palmata under mimicked Arctic summer conditions. J. Photochem. Photobiol. 2022, 11, 100124. [Google Scholar] [CrossRef]
- Provasoli, L. Media and prospects for the cultivation of marine algae. In Cultures and Collections of Algae, Proceedings of the Japanese Conference Hakone, Hakone, Japan, 12–15 September 1966; Watanabe, A., Hattori, A., Eds.; Japanese Society of Plant Physiologists: Tokyo, Japan, 1968; pp. 63–75. [Google Scholar]
- Tatewaki, M. Formation of a crustaceous sporophyte with unilocular sporangia in Scytosiphon lomentaria. Phycologia 1966, 6, 62–66. [Google Scholar] [CrossRef]
- Bendtsen, J.; Mortensen, J.; Rysgaard, S. Seasonal surface layer dynamics and sensitivity to runoff in a high Arctic fjord (Young Sound/Tyrolerfjord, 74° N). J. Geophys. Res.-Oceans 2014, 119, 6461–6478. [Google Scholar] [CrossRef]
- Bartsch, I.; Paar, M.; Fredriksen, S.; Schwanitz, M.; Daniel, C.; Hop, H.; Wiencke, C. Changes in kelp forest biomass and depth distribution in Kongsfjorden, Svalbard, between 1996–1998 and 2012–2014 reflect Arctic warming. Polar Biol. 2016, 39, 2021–2036. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Koch, K.; Thiel, M.; Tellier, F.; Hagen, W.; Graeve, M.; Tala, F.; Laesecke, P.; Bischof, K. Species separation within the Lessonia nigrescens complex (Phaeophyceae, Laminariales) is mirrored by ecophysiological traits. Bot. Mar. 2015, 58, 81–92. [Google Scholar] [CrossRef]
- Wright, S.W.; Jeffrey, S.W.; Mantoura, R.F.C.; Llewellyn, C.A.; Bjørnland, T.; Repeta, D.; Welschmeyer, N. Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton. Mar. Ecol. Prog. Ser. 1991, 77, 183–196. [Google Scholar] [CrossRef]
- Karsten, U.; Thomas, D.N.; Weykam, G.; Daniel, C.; Kirst, G.O. A simple and rapid method for extraction and separation of low molecular weight carbohydrates from macroalgae using high-performance liquid chromatography. Plant Physiol. Biochem. 1991, 29, 373–378. [Google Scholar]
- Cruces, E.; Huovinen, P.; Gómez, I. Phlorotannin and antioxidant responses upon short-term exposure to UV radiation and elevated temperature in three south pacific kelps. Photochem. Photobiol. 2012, 88, 58–66. [Google Scholar] [CrossRef]
- Van Pelt, W.J.; Kohler, J. Modelling the long-term mass balance and firn evolution of glaciers around Kongsfjorden, Svalbard. J. Glaciol. 2015, 61, 731–744. [Google Scholar] [CrossRef]
- Małecki, J. Accelerating retreat and high-elevation thinning of glaciers in central Spitsbergen. Cryosphere 2016, 10, 1317–1329. [Google Scholar] [CrossRef]
- Fredersdorf, J.; Müller, R.; Becker, S.; Wiencke, C.; Bischof, K. Interactive effects of radiation, temperature and salinity on different life history stages of the Arctic kelp Alaria esculenta (Phaeophyceae). Oecologia 2009, 160, 483–492. [Google Scholar] [CrossRef]
- Hanelt, D.; Huppertz, K.; Nultsch, W. Daily course of photosynthesis and photoinhibition in marine macroalgae investigated in the laboratory and field. Mar. Ecol. Progr. Ser. 1993, 97, 31–37. [Google Scholar] [CrossRef]
- Hanelt, D.; Jaramillo, M.J.; Nultsch, W.; Senger, S.; Westermeier, R. Photoinhibition as a regulative mechanism of photosynthesis in marine algae of Antarctica. Ser. Cient. INACH 1994, 44, 76–77. [Google Scholar]
- Wiencke, C.; Gómez, I.; Dunton, K. Phenology and seasonal physiological performance of polar seaweeds. Bot. Mar. 2009, 52, 585–592. [Google Scholar] [CrossRef]
- Hanelt, D.; Melchersmann, B.; Wiencke, C.; Nultsch, W. Effects of high light stress on photosynthesis of polar macroalgae in relation to depth distribution. Mar. Ecol. Progr. Ser. 1997, 149, 255–266. [Google Scholar] [CrossRef]
- Hallerud, C.B. Pigment Composition of Macroalgae from a Norwegian Kelp Forest. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2014. [Google Scholar]
- Sagert, S.; Forster, R.; Feuerpfeil, P.; Schibert, H. Daily course of photosynthesis and photoinhibition in Chondrus crispus (Rhodophyta) from different shore levels. Eur. J. Phycol. 1997, 32, 363–371. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Monteiro, C.; Heinrich, S.; Bartsch, I.; Valentin, K.; Harms, L.; Glöckner, G.; Corre, E.; Bischof, K. Responses of the kelp Saccharina latissima (Phaeophyceae) to the warming Arctic: From physiology to transcriptomics. Physiol. Plantarum. 2020, 168, 5–26. [Google Scholar] [CrossRef]
- Groisillier, A.; Shao, Z.; Miche, G.; Goulitquer, S.; Bonin, P.; Krahulec, S.; Nidetzky, B.; Duan, D.; Boyen, C.; Tonon, T. Mannitol metabolism in brown algae involves a new phosphatase family. J. Exp. Bot. 2013, 65, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Conde, A.; Silva, P.; Agasse, A.; Conde, C.; Geró, H. Mannitol transport and mannitol dehydrogenase activities are coordinated in Olea europaea under salt and osmotic stresses. Plant Cell Physiol. 2011, 52, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Dittami, S.M.; Gravot, A.; Renault, D.; Goulitquer, S.; Eggert, A.; Bouchereau, A.; Boyen, C.; Tonon, T. Integrative analysis of metabolite and transcript abundance during the short-term response to abiotic stress in the brown alga Ectocarpus siliculosus. Plant Cell Environ. 2011, 34, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef]
- Monteiro, C.; Li, J.; Diehl, N.; Collén, J.; Heinrich, S.; Bischof, K.; Bartsch, I. Modulation of physiological performance by temperature and salinity in the sugar kelp Saccharina latissima. Phycol. Res. 2021, 69, 48–57. [Google Scholar] [CrossRef]
- Springer, K.; Cornelius, L.; Lütz-Meindl, U.; Wendt, A.; Bischof, K. Hyposaline conditions affect UV susceptibility in the Arctic kelp Alaria esculenta (Phaeophyceae). Phycologia 2017, 56, 675–685. [Google Scholar] [CrossRef]
- Schoenwaelder, M.E.A. The occurrence and cellular significance of physodes in brown algae. Phycologia 2002, 41, 125–139. [Google Scholar] [CrossRef]
- Amsler, C.D.; Fairhead, V.A. Defensive and sensory chemical ecology of brown algae. Adv. Bot. Res. 2006, 43, 1–91. [Google Scholar] [CrossRef]
- Falkenberg, L.; Connell, S.; Russell, B. Herbivory mediates the expansion of an algal habitat under nutrient and CO2 enrichment. Mar. Ecol. Progr. Ser. 2014, 497, 87–92. [Google Scholar] [CrossRef]
- Ragan, M.A.; Jensen, A. Quantitative studies on brown algal phenols. II. Seasonal variation in polyphenol content of Ascophyllum nodosum (L.) Le Jol. and Fucus vesiculosus (L.). J. Exp. Mar. Biol. Ecol. 1978, 34, 245–258. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Dring, M.J.; Savidge, G. Nitrate and phosphate uptake characteristics of three species of brown algae cultured at low salinity. Mar. Ecol. Progr. Ser. 2002, 234, 111–118. [Google Scholar] [CrossRef]
- Atkinson, M.J.; Smith, S.V. C: N: P ratios of benthic marine plants. Limnol. Oceanogr. 1983, 28, 568–574. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Alexieva, V.; Ivanov, S.; Sergiev, I.; Karanov, E. Interaction between stresses. Bulg. J. Plant Physiol. 2003, 29, 1–17. [Google Scholar]

| SA | Days | L | rETRmax | Significance | α | Significance | Ek | Significance | Fv/Fm | Significance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (rel. Units) | L | Days | SA | (µmol Photons m−2s−1)−1 | L | Days | SA | (µmol Photons m−2s−1) | L | Days | SA | (rel. Units) | L | Days | SA | |||
| 34 | 1 | HL | 31.360 (±2.05) | a | a | ab | 0.202 (±0.01) | a | a | a | 156.473 (±22.28) | a | a | a | 0.618 (±0.01) | a | a | a |
| LL | 20.183 (±2.78) | b | 0.213 (±0.01) | a | 94.597 (±8.38) | b | 0.427 (±0.10) | b | ||||||||||
| 21 | HL | 13.130 (±1.77) | a | b | 0.193 (±0.02) | a | a | 69.221 (±17.52) | a | b | 0.399 (±0.10) | a | a | |||||
| LL | 12.893 (±1.25) | a | 0.229 (±0.02) | a | 56.430 (±6.35) | a | 0.532 (±0.02) | a | ||||||||||
| 28 | 1 | HL | 18.557 (±3.12) | a | a | a | 0.235 (±0.03) | a | a | a | 79.370 (±12.64) | a | a | b | 0.613 (±0.06) | a | a | a |
| LL | 20.656 (±2.12) | a | 0.256 (±0.02) | a | 80.903 (±7.68) | a | 0.590 (±0.06) | a | ||||||||||
| 21 | HL | 13.046 (±0.52) | a | b | 0.202 (±0.02) | a | b | 64.996 (±7.43) | a | a | 0.511 (±0.03) | a | a | |||||
| LL | 14.239 (±2.14) | a | 0.187 (±0.02) | a | 76.627 (±14.93) | a | 0.394 (±0.04) | b | ||||||||||
| 18 | 1 | HL | 22.529 (±3.42) | a | a | b | 0.233 (±0.02) | a | a | a | 97.819 (±22.09) | a | a | a | 0.498 (±0.11) | a | a | a |
| LL | 21.794 (±5.33) | a | 0.201 (±0.04) | a | 110.722 (±29.33) | a | 0.359 (±0.05) | a | ||||||||||
| 21 | HL | 20.863 (±4.95) | a | a | 0.173 (±0.02) | a | b | 120.807 (±24.36) | a | a | 0.512 (±0.03) | a | a | |||||
| LL | 16.210 (±3.97) | a | 0.178 (±0.02) | a | 90.114 (±13.74) | a | 0.532 (±0.03) | a | ||||||||||
| SA | Days | L | Chl a | Significance | Chl c2 | Significance | β-Car | Significance | Fucox | Significance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (µg g−1 DW) | L | Days | SA | (µg g−1 DW) | L | Days | SA | (µg g−1 DW) | L | Days | SA | (µg g−1 DW) | LL | Days | SA | |||
| 34 | 1 | HL | 478.73 (±61.74) | a | a | a | 60.60 (±15.42) | a | a | ab | 21.03 (±4.32) | a | a | a | 178.83 (±6.48) | a | a | a |
| LL | 450.60 (±44.34) | a | 51.07 (±5.52) | a | 17.07 (±3.76) | a | 192.60 (±6.24) | a | ||||||||||
| 21 | HL | 447.53 (±51.20) | a | a | 58.10 (±9.40) | a | a | 18.10 (±2.07) | a | a | 203.63 (±27.75) | a | a | |||||
| LL | 328.70 (±41.66) | a | 87.73 (± 3.25) | a | 12.03 (±1.68) | a | 140.93 (±28.62) | a | ||||||||||
| 28 | 1 | HL | 526.90 (±144.48) | a | a | a | 85.60 (±11.82) | a | a | a | 19.57 (±5.86) | a | a | a | 177.47 (±9.46) | a | a | a |
| LL | 536.83 (±192.22) | a | 62.53 (±4.83) | a | 21.10 (±7.95) | a | 144.10 (±1.73) | a | ||||||||||
| 21 | HL | 394.53 (±74.28) | a | a | 53.87(±14.77) | a | a | 11.37 (±2.94) | a | b | 183.17 (±43.07) | a | a | |||||
| LL | 391.57 (±33.67) | a | 70.63 (±3.40) | a | 11.07 (±0.67) | a | 163.97 (±13.98) | a | ||||||||||
| 18 | 1 | HL | 454.47 (±111.25) | a | a | a | 68.80 (±2.51) | a | a | b | 16.03 (±1.44) | a | a | a | 167.43 (±6.82) | a | a | a |
| LL | 405.10 (±12.87) | a | 48.37 (±2.32) | b | 15.17 (±1.22) | a | 165.27 (±2.25) | a | ||||||||||
| 21 | HL | 334.97 (±43.70) | a | a | 39.37 (±5.89) | a | a | 15.10 (±0.50) | a | a | 140.03 (±20.06) | a | a | |||||
| LL | 472.90 (±33.20) | a | 76.77 (±6.62) | b | 11.00 (±3.80) | a | 228.37 (±15.87) | b | ||||||||||
| SA | Days | L | VAZ | Significance | DPS | Significance | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (µg g−1 DW) | L | Days | SA | L | Days | SA | ||||
| 34 | 1 | HL | 0.35 (±0.03) | a | a | a | 1.58 (±0.12) | a | a | a |
| LL | 0.27 (±0.04) | a | 1.95 (±0.28) | a | ||||||
| 21 | HL | 0.26 (±0.02) | a | b | 2.16 (±0.07) | a | b | |||
| LL | 0.21 (±0.03) | a | 2.70 (±0.32) | a | ||||||
| 28 | 1 | HL | 0.40 (±0.06) | a | a | a | 1.48 (±0.26) | a | a | a |
| LL | 0.29 (±0.08) | b | 2.01 (±0.66) | a | ||||||
| 21 | HL | 0.23 (±0.04) | a | b | 2.44 (±0.37) | a | b | |||
| LL | 0.19 (±0.04) | a | 2.82 (±0.41) | a | ||||||
| 18 | 1 | HL | 0.29 (±0.02) | a | a | a | 1.83 (±0.15) | a | a | a |
| LL | 0.24 (±0.02) | b | 2.26 (±0.17) | a | ||||||
| 21 | HL | 0.22 (±0.03) | a | a | 2.52 (±0.31) | a | a | |||
| LL | 0.26 (±0.01) | a | 2.17 (±0.13) | a | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marambio, J.; Diehl, N.; Bischof, K. High Ecophysiological Plasticity of Desmarestia aculeata (Phaeophyceae) Present in an Arctic Fjord under Varying Salinity and Irradiance Conditions. Biology 2022, 11, 1499. https://doi.org/10.3390/biology11101499
Marambio J, Diehl N, Bischof K. High Ecophysiological Plasticity of Desmarestia aculeata (Phaeophyceae) Present in an Arctic Fjord under Varying Salinity and Irradiance Conditions. Biology. 2022; 11(10):1499. https://doi.org/10.3390/biology11101499
Chicago/Turabian StyleMarambio, Johanna, Nora Diehl, and Kai Bischof. 2022. "High Ecophysiological Plasticity of Desmarestia aculeata (Phaeophyceae) Present in an Arctic Fjord under Varying Salinity and Irradiance Conditions" Biology 11, no. 10: 1499. https://doi.org/10.3390/biology11101499
APA StyleMarambio, J., Diehl, N., & Bischof, K. (2022). High Ecophysiological Plasticity of Desmarestia aculeata (Phaeophyceae) Present in an Arctic Fjord under Varying Salinity and Irradiance Conditions. Biology, 11(10), 1499. https://doi.org/10.3390/biology11101499





