Nested Real-Time PCR Assessment of Vertical Transmission of Sandalwood Spike Phytoplasma (‘Ca. Phytoplasma asteris’)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Sandalwood Seeds and Seedlings
2.2. DNA Extraction, Nested End-Point PCR, and Sequencing
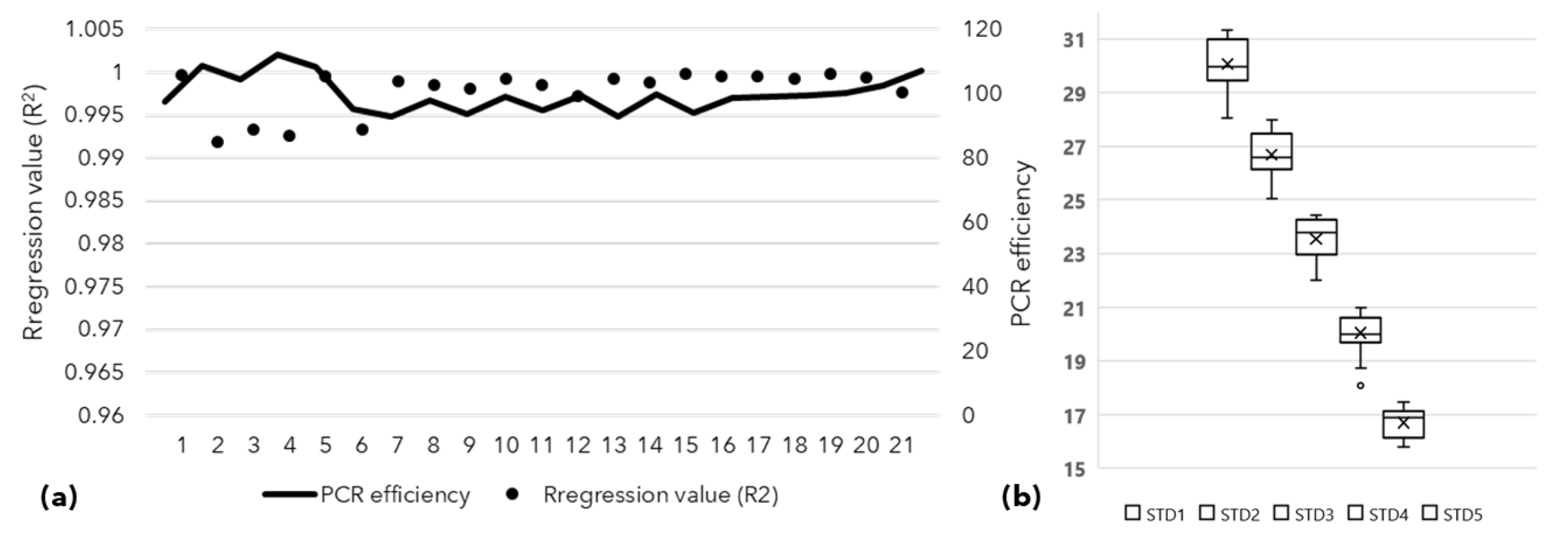
2.3. Assessment of Vertical Transmission of SSD Phytoplasma Using qPCR
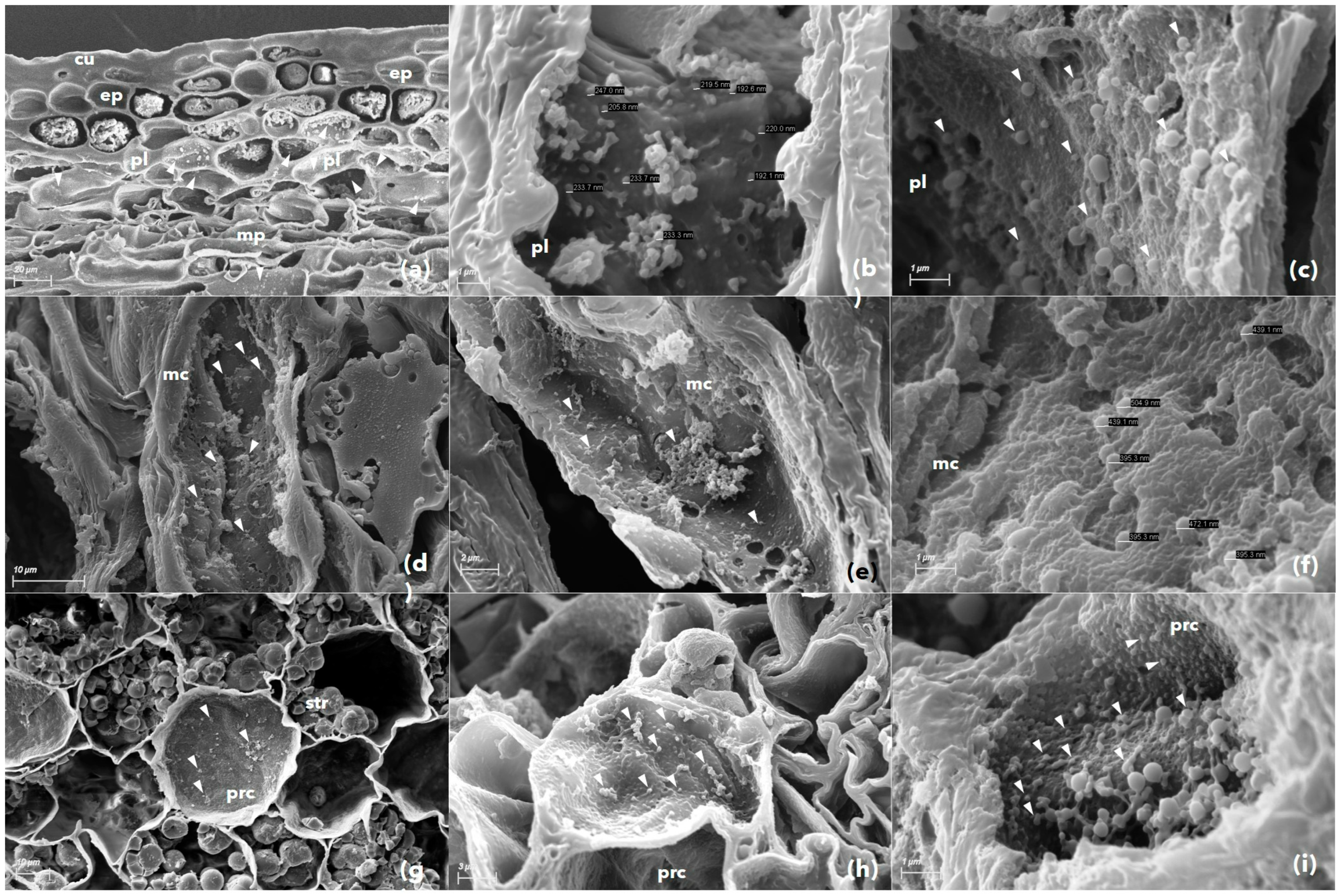
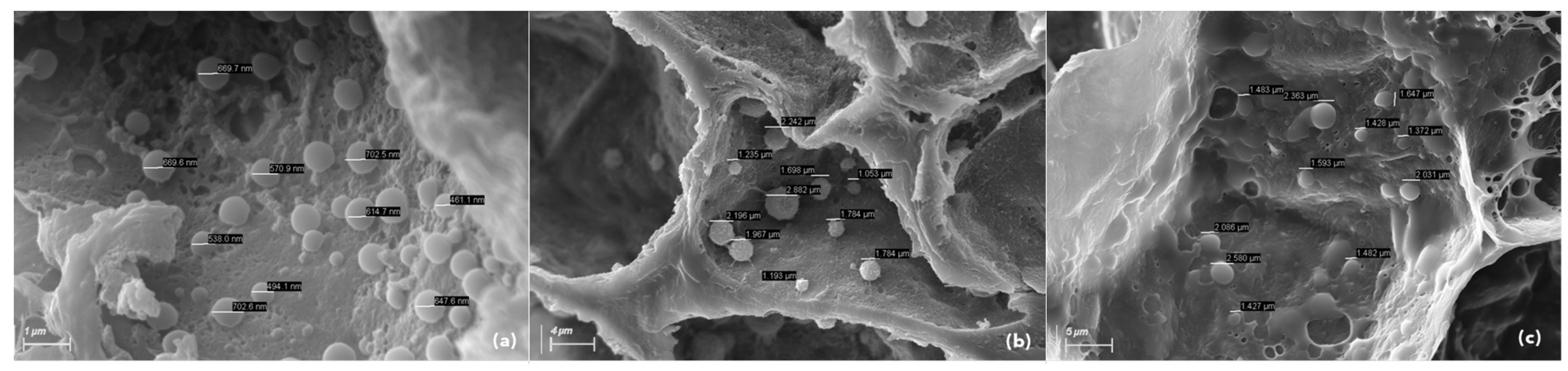
2.4. Assessment of Phytoplasma Presence by Scanning Electron Microscopy
2.5. Culture-Dependent Screening of Endophytic Microbes
3. Results
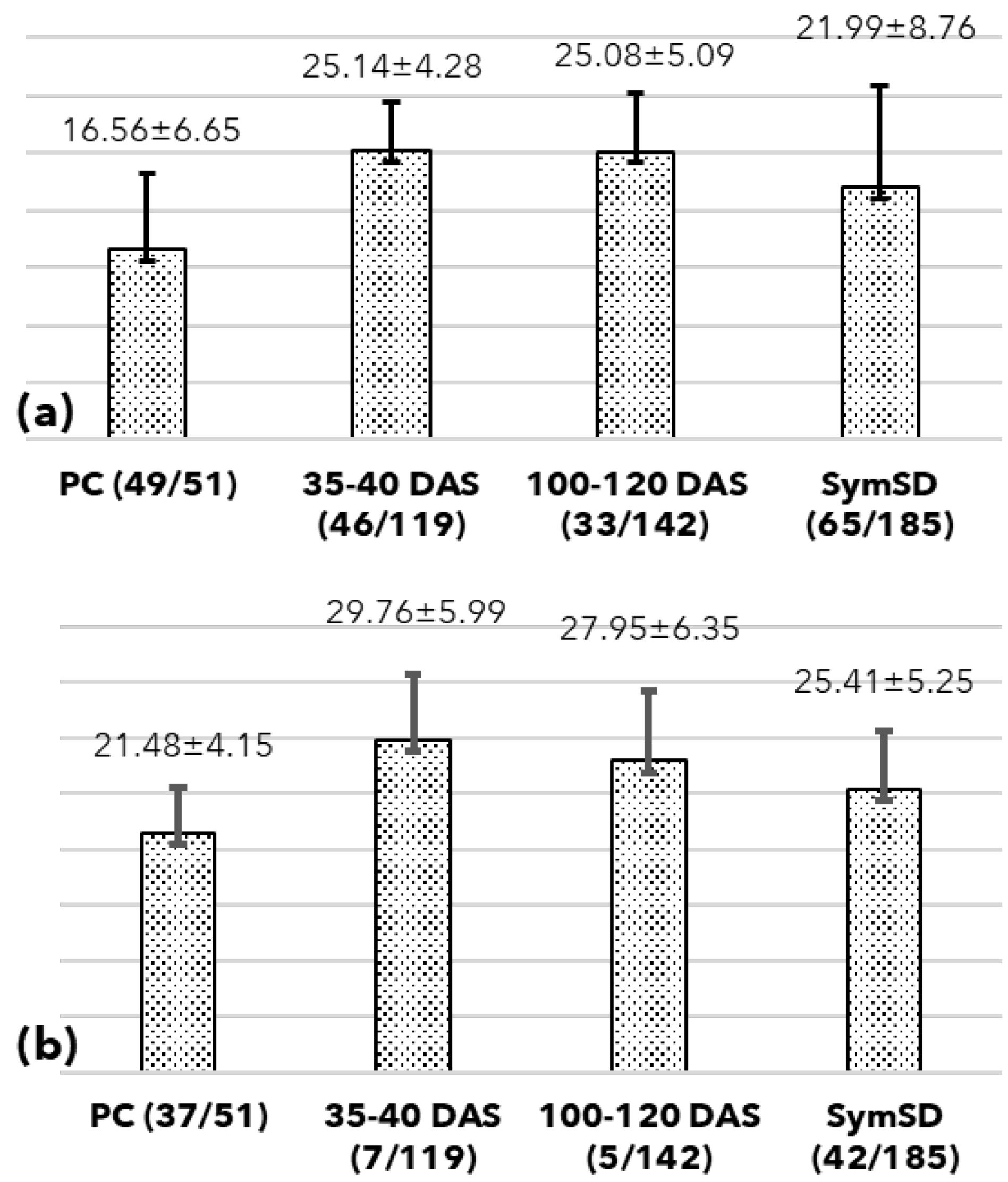
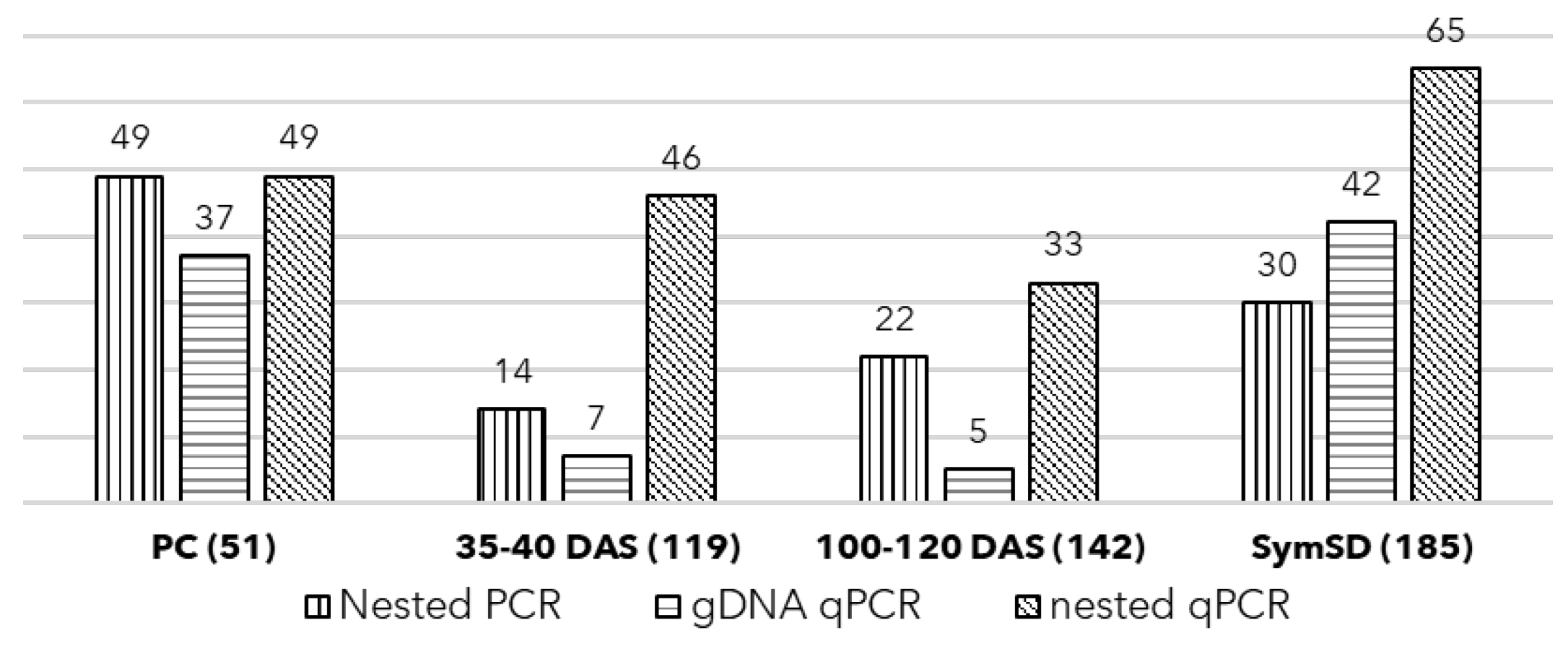
3.1. Detection of Phytoplasma in Symptomatic Plant and Seeds
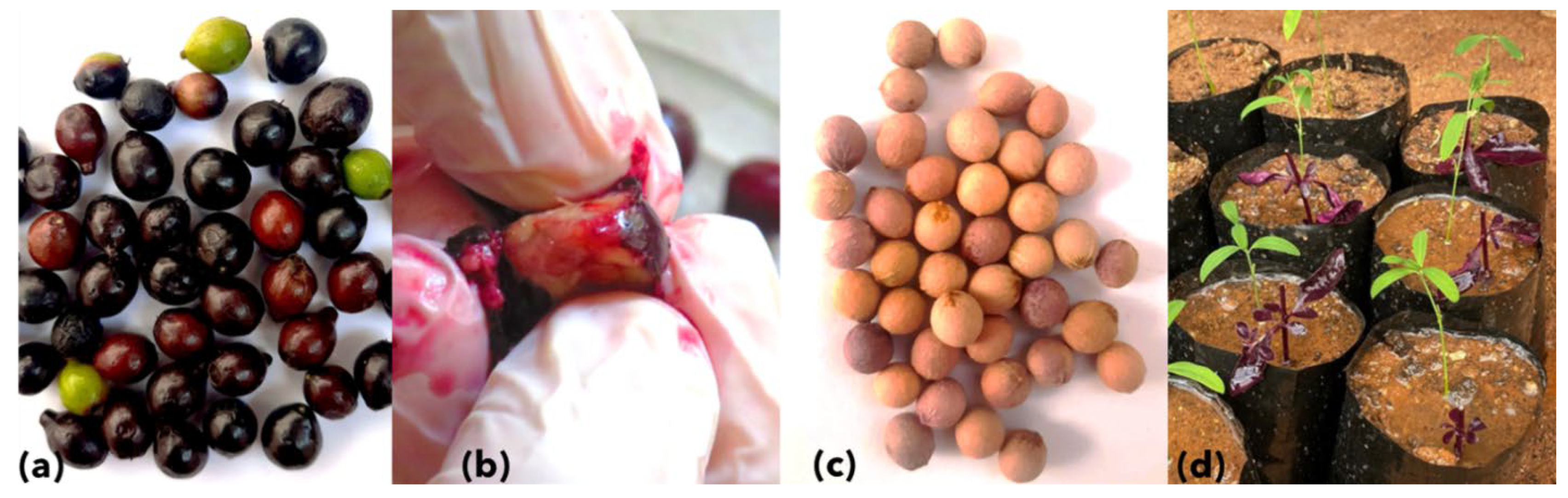
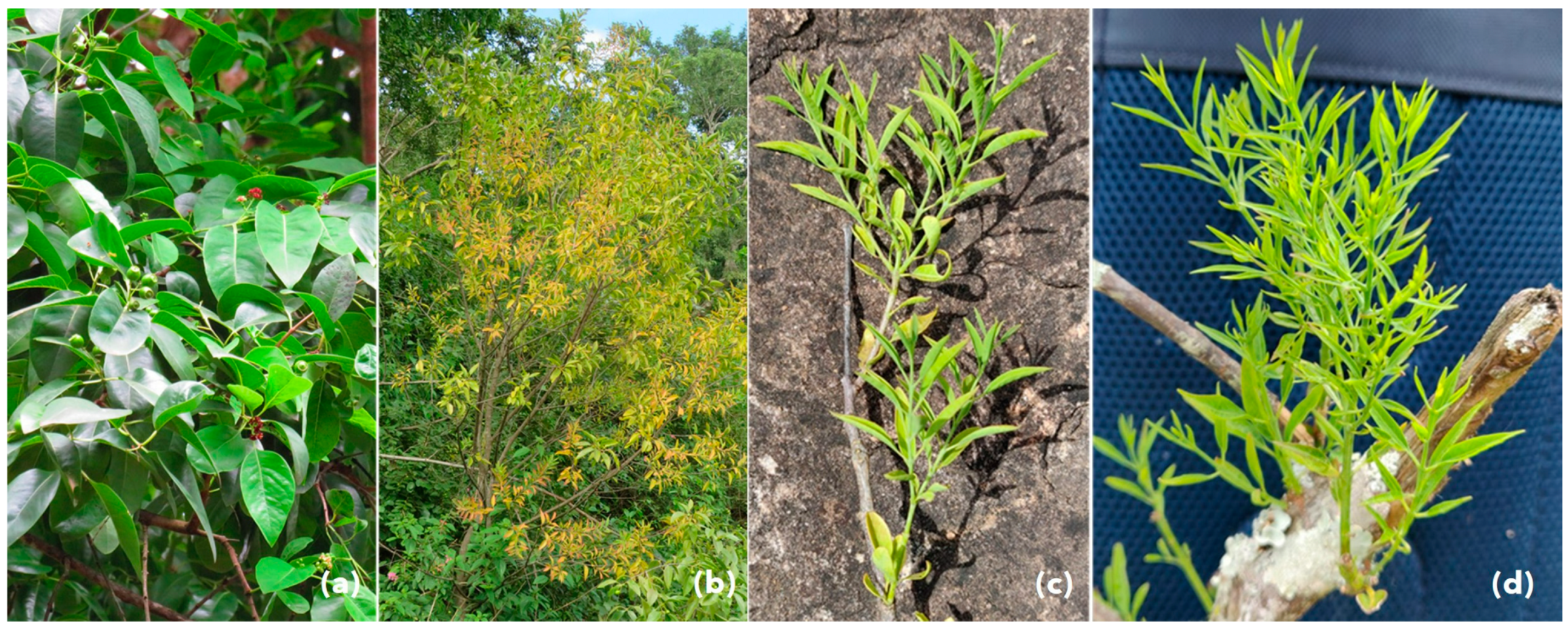
3.2. Seed Germination, Symptoms, and Phytoplasma Detection
3.3. Scanning Electron Microscopy
3.4. Bacterial and Fungal Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gowda, H.S.V.; Patil, K.B.; Anil Kumar, B.H. Natural Sandalwood Industry-Present Scenario and Future Prospects. In Proceedings of the National Seminar on Conservation, Improvement, Cultivation and Management of Sandal, Bangalore, India, 12–13 December 2008; pp. 196–203. [Google Scholar]
- Cottrell, C. Indian sandalwood’s heartwood of history: A global sketch from 3000 BCE to 2020. In Indian Sandalwood; Springer: Berlin/Heidelberg, Germany, 2022; pp. 3–26. [Google Scholar] [CrossRef]
- da Silva, J.A.T. Sandalwood Spike Disease: A Brief Synthesis. Environ. Exp. Biol. 2016, 14, 199–204. [Google Scholar] [CrossRef]
- Sundararaj, R.; Kirdat, K.; Mondal, S.; Reddy, M.K.; Thorat, V.; Rishi, R.; Yadav, A. A Threat to Sandalwood Cultivation in the Naturalised Marayoor Sandalwood Reserve (Kerala, India) through Single and Mixed Phytoplasma Infections. Phytopathogenic Mollicutes 2020, 10, 89–95. [Google Scholar] [CrossRef]
- Rashkow, E.D. Indian sandalwood: A history of overexploitation and endangerment. In Indian Sandalwood; Springer: Berlin/Heidelberg, Germany, 2022; pp. 27–43. [Google Scholar] [CrossRef]
- Arunkumar, A.N.; Dhyani, A.; Joshi, G. Santalum album. In IUCN Red List Threatened Species; IUCN: Gland, Switzerland, 2019. [Google Scholar] [CrossRef]
- Thomas, S.; Balasundaran, M. In Situ Detection of Phytoplasma in Spike-Disease-Affected Sandal Using DAPI strain. Curr. Sci. 1998, 74, 989–993. [Google Scholar]
- Rao, G.; Madhupriya, V.T.; Manimekalai, R.; Tiwari, A.; Yadav, A. A Century Progress of Research on Phytoplasma Diseases in India. Phytopathogenic Mollicutes 2017, 7, 1–38. [Google Scholar] [CrossRef]
- Contaldo, N.; Satta, E.; Zambon, Y.; Paltrinieri, S.; Bertaccini, A. Development and Evaluation of Different Complex Media for Phytoplasma Isolation and Growth. J. Microbiol. Methods 2016, 127, 105–110. [Google Scholar] [CrossRef]
- Contaldo, N.; Bertaccini, A. Phytoplasma Cultivation. In Phytoplasmas: Plant Pathogenic Bacteria—III; Springer: Singapore, 2019; ISBN 978-981-13-9632-8. [Google Scholar] [CrossRef]
- Contaldo, N.; Bertaccini, A.; Paltonieri, S.M.; Windsor, H.; Windsor, G.D. Axenic Culture of Plant Pathogenic Phytoplasmas. Phytopathol. Mediterr. 2012, 51, 607–617. [Google Scholar] [CrossRef]
- Ashwini, B.N.; Madhu Kiran, G.V.N.S.; Padmaja, A.S.; Nagaraju, N. Diseases, diagnosis and their management of indian sandalwood. In Indian Sandalwood; Springer: Berlin/Heidelberg, Germany, 2022; pp. 269–279. [Google Scholar] [CrossRef]
- Kiran, K.; Ramachandran, S.; Soma, M.; Reddy, M.K.; Vipool, T.; Amit, Y. Novel Aster Yellows Phytoplasma Subgroup Associated with Sandalwood Spike Disease in Kerala, India. Phytopathogenic Mollicutes 2019, 9, 33–34. [Google Scholar] [CrossRef]
- Khan, J.A.; Singh, S.K.; Ahmad, J. Characterisation and Phylogeny of a Phytoplasma Inducing Sandal Spike Disease in Sandal (Santalum album). Ann. Appl. Biol. 2008, 153, 365–372. [Google Scholar] [CrossRef]
- Khan, J.A.; Srivastava, P.; Singh, S.K. Identification of a ‘Candidatus Phytoplasma asteris’-Related Strain Associated with Spike Disease of Sandal (Santalum album) in India. Plant Pathol. 2006, 55, 572. [Google Scholar] [CrossRef]
- Sen-Sarma, P.K. Insect Vectors of Sandal Spike Disease. Eur. J. For. Pathol. 1982, 12, 297–299. [Google Scholar] [CrossRef]
- Sears, B.B.; Klomparens, K.L. Leaf Tip Cultures of the Evening Primrose Allow Stable, Aseptic Culture of Mycoplasma-like Organism. Can. J. Plant Pathol. 1989, 11, 343–348. [Google Scholar] [CrossRef]
- Siller, W.; Kuhbandner, B.; Marwitz, R.; Petzold, H.; Seemüller, E. Occurrence of Mycoplasma-like Organisms in Parenchyma Cells of Cuscuta Odorata (Ruiz et Pav.). J. Phytopathol. 1987, 119, 147–159. [Google Scholar] [CrossRef]
- Calari, A.; Paltrinieri, S.; Contaldo, N.; Sakalieva, D.; Mori, N.; Duduk, B.; Bertaccini, A. Molecular Evidence of Phytoplasmas in Winter Oilseed Rape, Tomato and Corn Seedlings. Bull. Insectology 2011, 64, S157–S158. [Google Scholar]
- Çağlar, B.K.; Satar, S.; Bertaccini, A.; Elbeaino, T. Detection and Seed Transmission of Bermudagrass Phytoplasma in Maize in Turkey. J. Phytopathol. 2019, 167, 248–255. [Google Scholar] [CrossRef]
- Satta, E.; Carminati, G.; Bertaccini, A. Phytoplasma Presence in Carrot Seedlings. Australas. Plant Dis. Notes 2020, 15, 1–4. [Google Scholar] [CrossRef]
- Nipah, J.O.; Jones, P.; Hodgetts, J.; Dickinson, M. Detection of Phytoplasma DNA in Embryos from Coconut Palms in Ghana, and Kernels from Maize in Peru. Bull. Insectology 2007, 60, 385. [Google Scholar]
- Nayar, R. Damage by mycoplasmas in lndian forests. In Advances in Forestry in India; International Book Distributors: Dehra Dun, India, 1988; Volume II, pp. 127–158. [Google Scholar]
- Doyle, J.J. Isolation of Plant DNA from Fresh Tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Deng, S.; Hiruki, C. Amplification of 16S rRNA Genes from Culturable and Nonculturable Mollicutes. J. Microbiol. Methods 1991, 14, 53–61. [Google Scholar] [CrossRef]
- Schneider, B. Phylogenetic Classification of Plant Pathogenic Mycoplasma-like Organisms or Phytoplasma. Mol. Diagn. Proced. Mycoplasmolgy 1995, 1, 369–380. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Preparation of Single-Stranded Bacteriophage M13 DNA by Precipitation with Polyethylene Glycol. pdb-prot093419. Cold Spring Harb. Protoc. 2017, 11, 941–945. [Google Scholar] [CrossRef]
- Baker, G.C.; Smith, J.J.; Cowan, D.A. Review and Re-Analysis of Domain-Specific 16S Primers. J. Microbiol. Methods 2003, 55, 541–555. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S rRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613. [Google Scholar] [CrossRef]
- Christensen, N.M.; Nicolaisen, M.; Hansen, M.; Schulz, A. Distribution of Phytoplasmas in Infected Plants as Revealed by Real-Time PCR and Bioimaging. Mol. Plant Microbe Interact. 2004, 17, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M. Scanning electron microscopy: Principle, components and applications. In A Textbook on Fundamentals and Applications of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 81–92. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press, Inc.: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Thomas, S.; Balasundaran, M. Purification of Sandal Spike Phytoplasma for the Production of Polyclonal Antibody. Curr. Sci. 2001, 80, 1489–1494. [Google Scholar]
- Khan, J.A.; Srivastava, P.; Singh, S.K. Efficacy of Nested-PCR for the Detection of Phytoplasma Causing Spike Disease of Sandal. Curr. Sci. 2004, 86, 1530–1533. [Google Scholar]
- Balasundaran, M.; Muralidharan, E.M. Development of Spike Disease Resistant Sandal Seedlings through Biotechnology Involving ELISA Technique and Tissue Culture; Research Report; Kerala Forest Research Institute: Kerala, India, 2004; ISSN 0970-8103. [Google Scholar]
- Arunkumar, A.N.; Joshi, G.; Kumar, S. Marayoor sandalwood reserve—The last bastion of indian sandalwood. In Indian Sandalwood; Springer: Berlin/Heidelberg, Germany, 2022; pp. 93–108. [Google Scholar] [CrossRef]
- Subasinghe, S. Santalum album: Current status, research and future perspectives in Sri Lanka. In Indian Sandalwood; Springer: Berlin/Heidelberg, Germany, 2022; pp. 109–125. [Google Scholar] [CrossRef]
- Khan, A.J.; Botti, S.; Paltrinieri, S.; Al-Subhi, A.M.; Bertaccini, A. Phytoplasmas in Alfalfa Seedlings: Infected or Contaminated Seeds. In Proceedings of the 14th International Organization of Mycoplasmology Conference, Vienna, Austria, 7–12 July 2002; Volume 148. [Google Scholar]
- Satta, E.; Paltrinieri, S.; Bertaccini, A. Phytoplasma transmission by seed. In Phytoplasmas: Plant Pathogenic Bacteria-II; Springer: Berlin/Heidelberg, Germany, 2019; pp. 131–147. [Google Scholar] [CrossRef]
- Bertaccini, A.; Marani, F. Electron Microscopy of Two Viruses and Mycoplasma-like Organisms in Lilies with Deformed Flowers. Phytopathol. Mediterr. 1982, 21, 8–14. [Google Scholar]
- Jiang, H.; Wei, W.; Saiki, T.; Kawakita, H.; Watanabe, K.; Sato, M. Distribution Patterns of Mulberry Dwarf Phytoplasma in Reproductive Organs, Winter Buds, and Roots of Mulberry Trees. J. Gen. Plant Pathol. 2004, 70, 168–173. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirdat, K.; Tiwarekar, B.; Swetha, P.; Padma, S.; Thorat, V.; Manjula, K.N.; Kavya, N.; Sundararaj, R.; Yadav, A. Nested Real-Time PCR Assessment of Vertical Transmission of Sandalwood Spike Phytoplasma (‘Ca. Phytoplasma asteris’). Biology 2022, 11, 1494. https://doi.org/10.3390/biology11101494
Kirdat K, Tiwarekar B, Swetha P, Padma S, Thorat V, Manjula KN, Kavya N, Sundararaj R, Yadav A. Nested Real-Time PCR Assessment of Vertical Transmission of Sandalwood Spike Phytoplasma (‘Ca. Phytoplasma asteris’). Biology. 2022; 11(10):1494. https://doi.org/10.3390/biology11101494
Chicago/Turabian StyleKirdat, Kiran, Bhavesh Tiwarekar, Purushotham Swetha, Sodaliyandi Padma, Vipool Thorat, Kathiruguppe Nagappa Manjula, Narayan Kavya, Ramachandran Sundararaj, and Amit Yadav. 2022. "Nested Real-Time PCR Assessment of Vertical Transmission of Sandalwood Spike Phytoplasma (‘Ca. Phytoplasma asteris’)" Biology 11, no. 10: 1494. https://doi.org/10.3390/biology11101494
APA StyleKirdat, K., Tiwarekar, B., Swetha, P., Padma, S., Thorat, V., Manjula, K. N., Kavya, N., Sundararaj, R., & Yadav, A. (2022). Nested Real-Time PCR Assessment of Vertical Transmission of Sandalwood Spike Phytoplasma (‘Ca. Phytoplasma asteris’). Biology, 11(10), 1494. https://doi.org/10.3390/biology11101494







