A New Clue for the Late Eocene Freshwater Ecosystem of Central China Evidenced by New Fossils of Trapa L. and Hemitrapa Miki (Lythraceae)
Abstract
Simple Summary
Abstract
1. Introduction
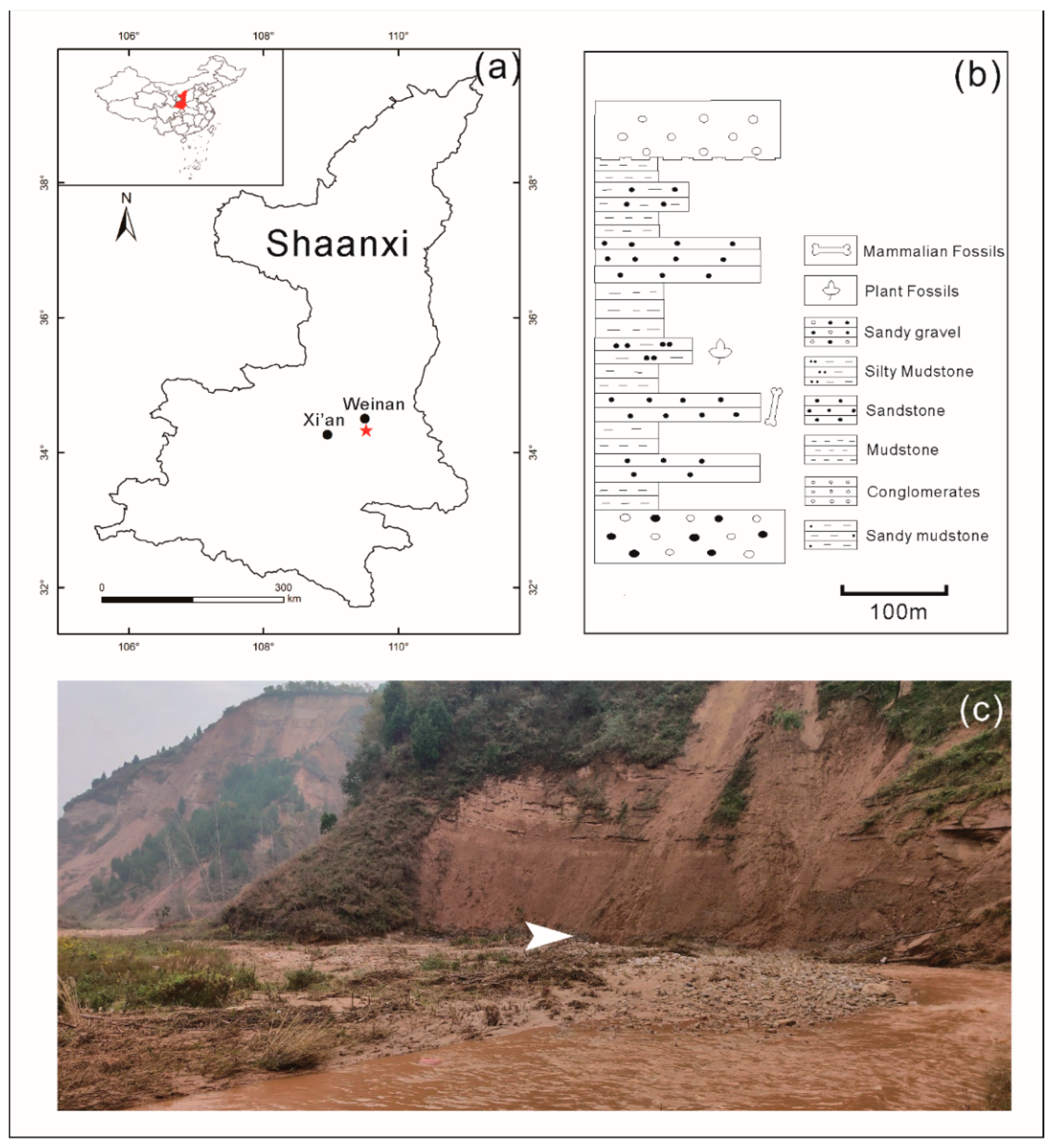
2. Geological Background
3. Material and Methods
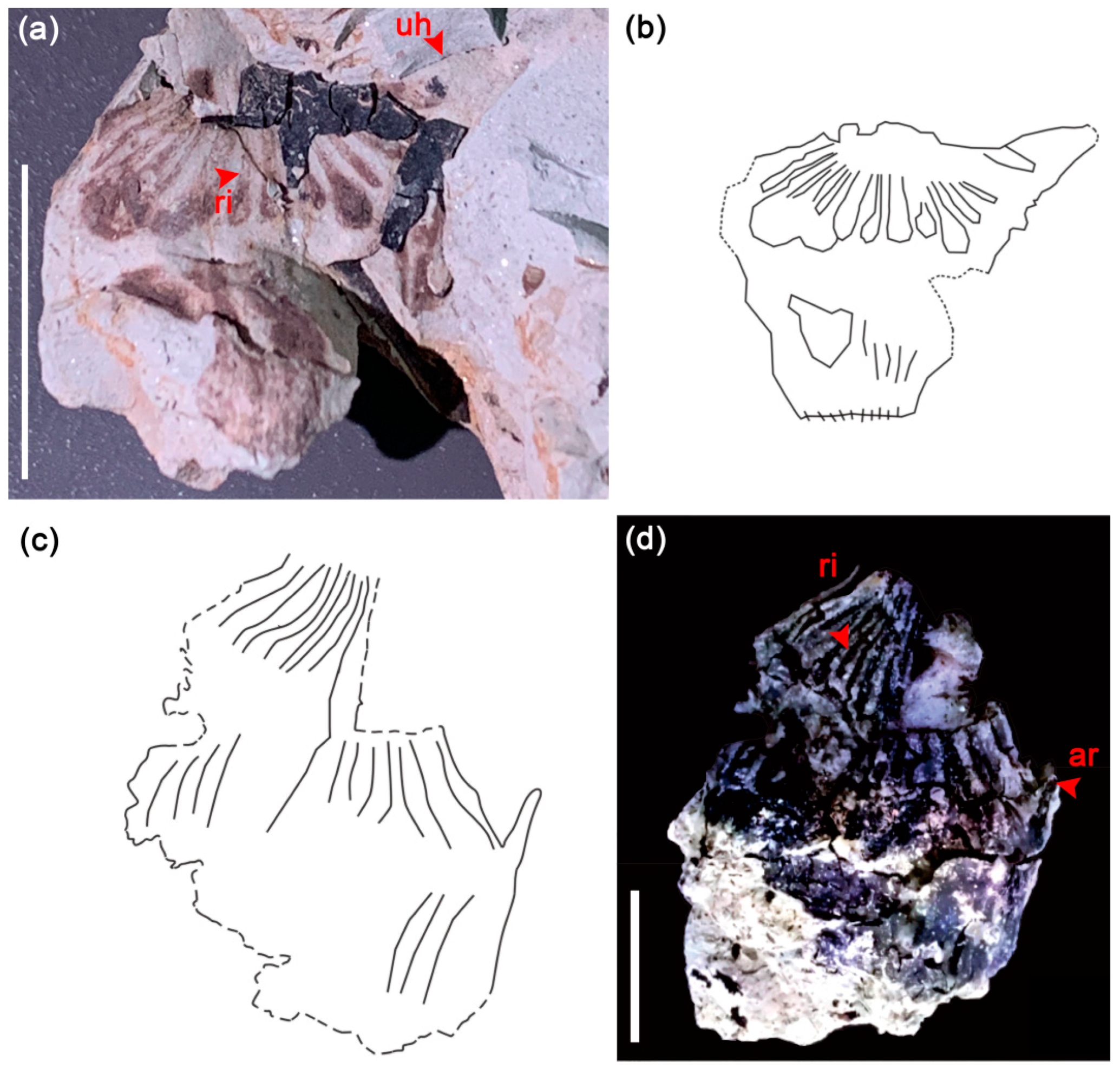
4. Systematics
5. Discussion
5.1. Morphological Comparison
5.1.1. Leaf
5.1.2. Fruit
5.1.3. Root
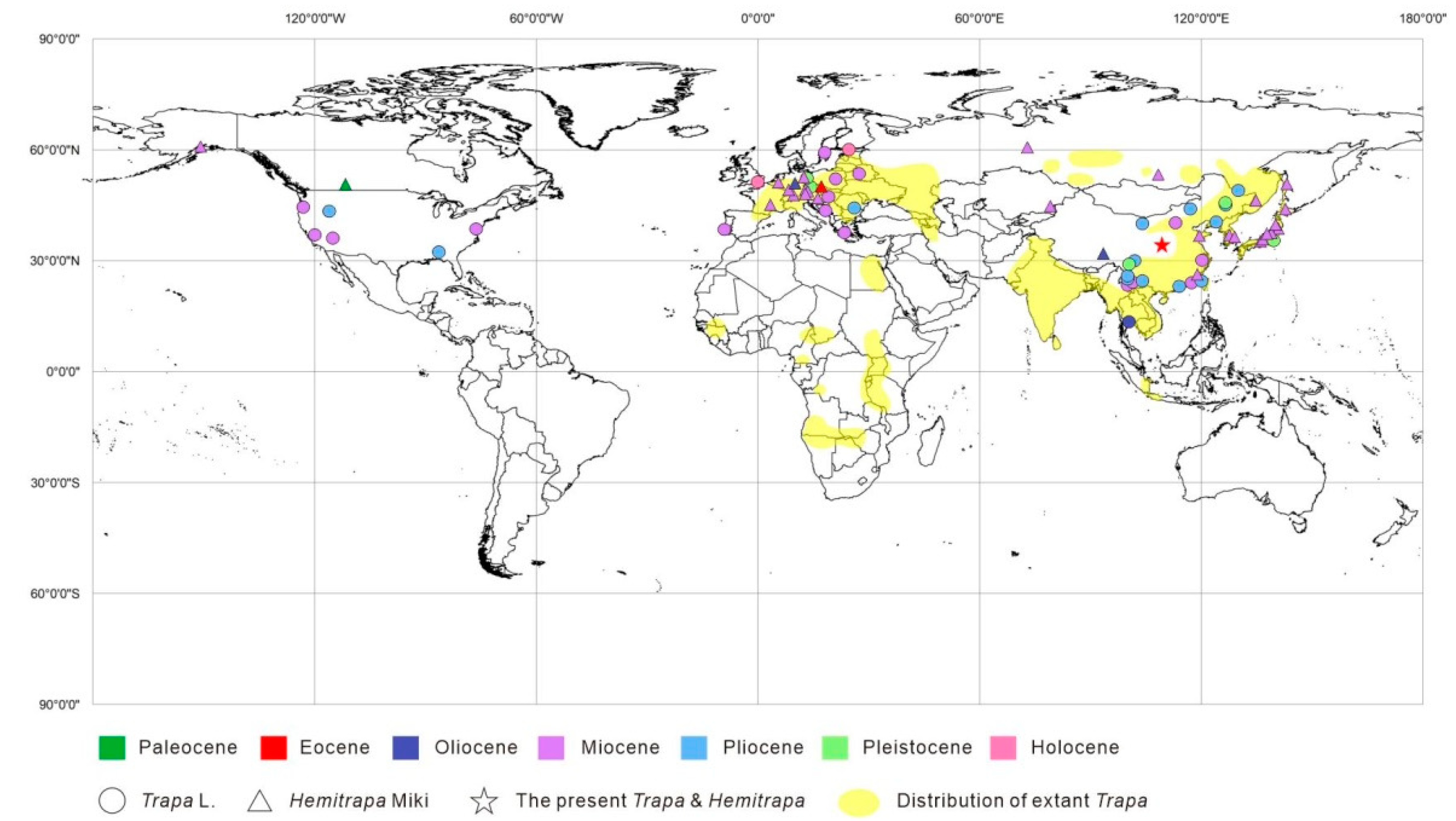
5.2. Systematic Affinity
5.3. Paleoecology
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- POWO. Plants of the World Online. Available online: http://www.plantsoftheworldonline.org (accessed on 20 August 2022).
- Graham, S.A.; Hall, J.; Sytsma, K.; Shi, S.-H. Phylogenetic Analysis of the Lythraceae Based on Four Gene Regions and Morphology. Int. J. Plant Sci. 2005, 166, 995–1017. [Google Scholar] [CrossRef]
- Graham, S.A. Fossil Records in the Lythraceae. Bot. Rev. 2013, 79, 48–145. [Google Scholar] [CrossRef]
- IPNI. International Plant Names Index. Available online: https://www.ipni.org/ (accessed on 23 May 2022).
- Verdcourt, B. FSA Contributions 10: Trapaceae. Bothalia 1998, 28, 11–14. [Google Scholar] [CrossRef]
- Chen, C.-J.; Ding, B.-Y.; Funston, A.M. Trapaceae. Flora of China. Missouri Botanical Garden. St. Louis; Science Press: Beijing, China, 2007; pp. 290–291. [Google Scholar]
- Miki, S. On the Remains of the Water Plants in Asia. Jpn. J. Limnol. Rikusuigaku Zasshi. 1938, 8, 410–416. [Google Scholar] [CrossRef]
- Miki, S. Floral Remains in Kinki and Adjacent Districts since the Pliocene with Description of 8 New Species. Mineral. Geol. 1948, 9, 105–144. [Google Scholar]
- Miki, S. Trapa of Japan with Special Reference to Its Remains., Series D. J. Inst. Polytech. 1952, 3, 1–29. [Google Scholar]
- Baranov, V.I. Etapy razvitija flory i rastitel’nosti SSSR v tretichnom periode. Chast’3. Itogi izuchenija iskopaemykh tretichnykh lor i Problema Reliktov v Sovremennoj Rastitel’nosti SSSR [Stages of the Development of the Flora and Vegetation of the USSR in the Tertiary Period. Part 3. Summary of the Study of the Fossil Tertiary Floras and the Problem of Relics in Modern Vegetation of the USSR]. Uchenye Zap. Kazan. Gos. Univ. 1954, 4, 111–122. [Google Scholar]
- Vassiljev, V.N. Novye Iskopaemye Vidy Roda Trapa L. [New Fossil Species of the Genus Trapa L.]. Bot. Zhurnal. 1952, 37, 226–230. [Google Scholar]
- Vassiljev, V.N. Iskopaemyj Vodjanoj Orekh Iz Yuzhnoj Yakutii. In Sbornik Pamjati Afrikana Nikolaevicha Krishtofovicha; Izdateľstvo Akademii Nauk SSSR: Moscow, Russia; Leningrad, Russia, 1957; pp. 327–330. [Google Scholar]
- Budantsev, L. The Water Chestnuts (Trapa and Hemitrapa) from the Tertiary Deposits of the Southeastern Baikal Coast. Bot. J. 1960, 45, 139–144. [Google Scholar]
- Dorofeev, P.I. Tretichnye Flory Zapadnoi Sibiri (Tertiary Floras of Western Siberia); Izdatel’stvo Akademii Nauk SSSR: Moscow, Russia, 1963; p. 343. [Google Scholar]
- Wójcicki, J.J.; Zastawniak, E. The Trapaceae Family in the Tertiary of Europe—Preliminary Results. Bot. Guideb. 2003, 26, 153–185. [Google Scholar]
- Kovar-Eder, J.; Wójcicki, J.J.; Zetter, R. Trapaceae from the Late Miocene of Austria and the European Context. Acta Paleobot. 2005, 45, 165–186. [Google Scholar]
- Wójcicki, J.J.; Velitzelos, D. Trapa Kvacekii (Trapaceae), a Remarkable New Fossil Species from the Late Miocene of Greece. Acta Palaeobot. 2007, 47, 419. [Google Scholar]
- Berry, E.W. Two new Tertiary species of Trapa. Torreya 1914, 14, 105–108. [Google Scholar]
- Dorf, E. A Late Tertiary Flora from Southwestern Idaho; Carnegie Institution of Washington Publication: Washington, CA, USA, 1936; p. 476. [Google Scholar]
- Wójcicki, J.J.; Song, S.; Wang, Y. Fossil Trapa L. of China. 1. a new locality from the Miocene of the Liang he coal mine, west Yunnan. Acta Palaeobot. 1999, 11, 5–14. [Google Scholar]
- Dong, J.-L. The Miocene Plant Fossils, Paleoatmospheric CO2 and Plant-Insect Interations from Fujian Province. Doctorate Thesis, Lanzhou University, Lanzhou, China, 2019. [Google Scholar]
- Miki, S. On the Change of Flora in Eastern Asia since Tertiary Period (1) The Clay or Lignite Beds Flora in Japan with Special Reference to the Pinus Trifolia Beds in Central Hondo. Jap. J. Bot. 1941, 11, 237–303. [Google Scholar]
- Tanai, T. Miocene Sakipenpetsu Flora from Ashibetsu Area, Central Hokkaido, Japan. Nat. Sci. Mus. Tokyo 1971, 4, 127–172. [Google Scholar]
- Akhmetiev, M.A. Some Miocene Plants of the Far East, having Stratigraphic Importance. Proc. USSR Acad. Sci. Geol. Ser. 1978, 6, 67–72. [Google Scholar]
- Gregor, H. Fruktifikationen der Gattung Hemitrapa Miki (Trapellaceae) in den Ablagerungen der Oberen Süßwasser-Molasse Bayerns (mit Bemerkungen zu den fossilen Vorkommen Eurasiens). Feddes Reper. 1982, 93, 351–362. [Google Scholar] [CrossRef]
- Mai, D.H.; Walther, H. Die Oligozänen und Untermiozänen Floren NW-Sachsens und des Bitterfelder Raumes. Abh. Staatl. Mus. Min. Geol. Dresd. 1991, 38, 1–230. [Google Scholar]
- Mai, H.D. Die mittelmiozänen und obermiozänen Floren aus der Meuroer und Raunoer Folge in der Lausitz. Teil II: Dicotyledonen. Palaeontogr. Abt. B 2001, 25, 35–174. [Google Scholar] [CrossRef]
- Kovar-Eder, J.; Schwarz, J.; Wójcicki, J.J. The Predominantly Aquatic Flora from Pellendorf, Lower Austria, Late Miocene, Pannonian—A Systematic Study. Acta Paleobot. 2002, 42, 125–151. [Google Scholar]
- Yabe, A. Early Miocene terrestrial climate inferred from plant megafossil assemblages of the Joban and Soma areas, northeast Honshu, Japan. Bull. Geol. Surv. 2008, 59, 397–413. [Google Scholar]
- Dawson, J.W. Note on the Plants, Collected by Mr. G.M. Dawson, from the Lignite Tertiary Deposits, near the Forty-ninth P-arallel. In Report on the Geology and Resources of the Region in the Vicinity of the Forty-Ninth Parallel from the Lake of the Woods to the Rocky Mountains; Dawson, G.M., Ed.; Dawson Brothers: Montreal, QC, USA, 1875; pp. 327–331. [Google Scholar]
- Wang, Q. Fruits of Hemitrapa (Trapaceae) from the Miocene of Eastern China, Their Correlation with Sporotrapoidites Erdtmanii Pollen and Paleobiogeographic Implications. J. Paleontol. 2012, 86, 156–166. [Google Scholar] [CrossRef]
- Su, T.; Li, S.-F.; Tang, H.; Huang, Y.-J.; Li, S.-H.; Deng, C.-L.; Zhou, Z.-K. Hemitrapa Miki (Lythraceae) from the Earliest Oligocene of Southeastern Qinghai-Tibetan Plateau and Its Phytogeographic Implications. Rev. Palaeobot. Palynol. 2018, 257, 57–63. [Google Scholar] [CrossRef]
- Song, Z.-C.; Li, W.-B.; He, C.-Q. Cretaceous and Palaeogene palynofloras and distribution of organic rocks in China. Sci. China Ser. B-Chem. Biol. Agric. Med. Earth Sci. 1983, 26, 538–549. [Google Scholar] [CrossRef]
- Guo, S.-X. Preliminary Interpretation of Tertiary Climate by Using Megafossil Floras in China. Palaeontol. Cathayana 1985, 2, 169–176. [Google Scholar]
- Sun, X.-J.; Wang, P.-X. How Old Is the Asian Monsoon System?—Palaeobotanical Records from China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 222, 181–222. [Google Scholar] [CrossRef]
- Quan, C.; Liu, Y.-S.C.; Utescher, T. Eocene Monsoon Prevalence over China: A Paleobotanical Perspective. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 365–366, 302–311. [Google Scholar] [CrossRef]
- Li, Z.-C.; Li, W.-H.; Li, Y.-X.; Li, Y.-H.; Han, W. Cenozoic stratigraphy and Paleoenvironments in the Weihe area, Shaanxi province. J. Stratigr. 2016, 40, 168–178. [Google Scholar] [CrossRef]
- Liu, T.-S.; Ding, M.-L.; Gao, F.-Q. Cenozoic stratigraphic sections between Xi’an and Lantian. Chin. J. Geol. 1960, 3, 199–208. [Google Scholar]
- Jia, L.-P.; Zhang, Y.-P.; Huang, W.-B. The Cenozoic Group of Lantian; Shaanxi Province. Institute of Vertebrate Palaeontology and Palaeoanthropology, Chinese Academy of Sciences; Symposium of the Field Conference on the Cenozoic Group of Lantian Shaanxi Province; Science Press: Beijing, China, 1966; pp. 1–31. [Google Scholar]
- Zhang, Y.-P.; Huang, W.-B.; Tang, Y.-J. Cenozoic of the Lantian Region, Shaanxi Province; Science Press: Beijing, China, 1978; pp. 1–60. [Google Scholar]
- Li, Z.-C. The Lithofacies Paleogeography and Paleoenvironmental evolution of the Cenozoic in the Weihe Basin, China. Doctorate Thesis, Northwest University, Xi’an, China, 2017. [Google Scholar]
- Tao, J.-R. A late Eocene florula from the district Weinan of central Shensi. J. Integr. Plant Biol. 1965, 13, 272–282. [Google Scholar]
- Li, H.-M.; Chen, Q.-S. Palibinia from the Eocene of Jiangxi, China—with remarks on the dry climate mechanism of northern hemisphere in Paleogene. Acta Palaeontol. Sin. 2002, 41, 119–129. [Google Scholar]
- Chow, M.-Z. A Lemuroid Primate From The Eocene Of Lantian Shensi. Vertebr. Palasiat. 1964, 8, 257–260+263. [Google Scholar] [CrossRef]
- Li, Q. New materials of Sianodon from Shaanxi, China. Vertebr. Palasiat. 2003, 41, 203–210. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Zhang, H.-Z.; Wang, Y.-C.; Zhao, L.; Wang, H.-L.; Sun, W.-F.; Zhang, H.-Y. Cenozoic depositional sequence in the Weihe basin (central China): A long-term record of Asian monsoon precipitation from the greenhouse to icehouse Earth. Quat. Sci. 2018, 38, 1057–1067. [Google Scholar]
- Harris, J.G.; Harris, M.W. Plant Identification Terminology: An Illustrated Glossary, 2nd ed.; Spring Lake Publishing: Genola, UT, USA, 2001; pp. 198–214. [Google Scholar]
- FOC. Flora of China. Available online: http://www.iplant.cn/foc (accessed on 28 April 2022).
- Aung, A.T.; Del, R.C.; Wang, T.-X.; Liu, J.; Spicer, T.E.V.; Su, T. Fossil Fruits and Pollen Grains of Trapa from the Upper Pliocene of the Sanying Formation (Yunnan, China). Rev. Palaeobot. Palynol. 2021, 293, 104498. [Google Scholar] [CrossRef]
- Lyu, X.-D. Fossil fruit of Trapa L. from the Miocene in Eastern Zhejiang and Its Paleoenvironment. Master’s Thesis, Lanzhou University, Lanzhou, China, 2010. [Google Scholar]
- Goeppert, H.R. Ueber die Braunkohlenflora des nordőstlichen Deutschlands. Z. Dtsch. Geol. Ges. 1855, 4, 484–496. [Google Scholar]
- Newberry, J.S. Geological Report, fossil plants. In Report upon the Colorado River of the West Explored in 1857 and 1858 by Lieutenant Joseph C. Ives; Ives, J.C., Ed.; Corps of Topographical Engineers, Office of Explorations and Surveys; GPO: Washington, DC, USA, 1861; pp. 129–132. [Google Scholar]
- Lesquereux, L. Contributions to the Fossil Flora of the Western Territories. Part 2. The Tertiary Flora; Report of the United States Geological Survey of the Territories; US Government Printing Office: Washington, DC, USA, 1878; Volume 7, p. 366. [Google Scholar]
- Ward, L.F. Types of the Laramie Flora; Bulletin of the United States Geological Survey No. 37; US Government Printing Office: Washington, DC, USA, 1887; pp. 63–64. [Google Scholar]
- Knowlton, F.H. A catalogue of the Cretaceous and Tertiary Plants of North America; Bulletin of the United States Geological Survey; US Government Printing Office: Washington, DC, USA, 1898; Volume 152, p. 247. [Google Scholar]
- Brown, R.W.; Houldsworth, E. The Fruit of Trapa? Microphylla Lesquereux. J. Wash. Acad. Sci. 1939, 29, 36–39. [Google Scholar]
- Dorf, E. Upper Cretaceous Floras of the Rocky Mountain Region. II: Flora of the Lance Formation at Its Type Locality; Carnegie Institute of Washington Publication: Niobrara County, WY, USA, 1942; p. 494. [Google Scholar]
- Bell, W.A. Uppermost Cretaceous and Paleocene Floras of Western Alberta. Canada Department of Mines and Resources. Geol. Surv. Bull. 1949, 13, 1–231. [Google Scholar]
- Brown, R.W. Paleocene Flora of the Rocky Mountains and Great Plains; Geological Survey Professional Paper; US Government Printing Office: Washington, DC, USA, 1962; Volume 375, pp. 1–119. [Google Scholar]
- Hickey, L.J. On the Nomenclatural Status of the Morphogenera, Quereuxia and Trapago. Taxon 2001, 50, 1119–1124. [Google Scholar] [CrossRef]
- Kvaček, Z. Early Miocene freshwater and swamp ecosystems of the Most Basin (northern Bohemia) with particular reference to the Bílina Mine section. J. Geosci. 2004, 49, 1–40. [Google Scholar]
- Lee, H.-H. Trapa? microphylla Lesq., the first occurrence from the upper Cretaceous formation of China. Acta Palaeontol. Sin. 1959, 7, 35–42. [Google Scholar] [CrossRef]
- Hao, H.; Ferguson, D.K.; Feng, G.-P.; Ablaev, A.; Wang, Y.-F.; Li, C.-S. Early Paleocene Vegetation and Climate in Jiayin, NE China. Clim. Chang. 2010, 99, 547–566. [Google Scholar] [CrossRef]
- Li, J.-D.; Yang, J.; Wang, Y.-F. Aquatic Plants in the Miocene Shanwang Flora. Chin. Bull. Bot. 2000, 17, 261. [Google Scholar]
- Zhao, C.-B. The Discovery of Sporotrapoidites in Fujin Formation in Sanjiang Basin. Acta Pet. Sin. 1992, 13, 9–15. [Google Scholar]
- Wójcicki, J.J.; Kvaček, Z. The Earliest Fossil Record of the Trapaceae in Europe from the Late Eocene Diatomite of Kučlín, North Bohemia. Phytol. Balc. 2003, 9, 165–174. [Google Scholar]
- Wójcicki, J.J.; Zastawniak, E. Trapa Srodoniana, a New Fossil Species from the Pliocene of Bełchatów (Middle Poland). Acta Palaeobot. 1998, 38, 167–174. [Google Scholar]
- Wójcicki, J.J.; Zastawniak, E. Late Miocene Trapa L. (Trapaceae) of Sos´ nica (SW Poland) revisited. Acta Palaeobot. 2002, 42, 29–38. [Google Scholar]
- Wójcicki, J.J. A new species of Trapa (Trapaceae) from Kashmir. Pol. Bot. J. 2001, 46, 133–136. [Google Scholar]
- Wang, X. Numerical Classification of Trapa Fruits and High Resolution Reconstruction of Paleoclimate in Late Miocene Zhejiang Province. Master’s Thesis, Chang’an University, Xi’an, China, 2020. [Google Scholar]
- Li, Y.; Cui, Y.-M.; Gee, C.T.; Liang, X.-Q.; Li, C.-S. Primotrapa gen. nov., an extinct transitional genus bridging the evolutionary gap between Lythraceae and Trapoideae, from the early Miocene of North China. BMC Evol. Biol. 2020, 20, 150. [Google Scholar] [CrossRef]
- Miki, S. Evolution of Trapa from Ancestral Lythrum through Hemitrapa. Proc. Jpn. Acad. 1959, 35, 289–294. [Google Scholar] [CrossRef]
- Mohr, B.A.R.; Gee, C.T. Sporotrapoidites Erdtmanii (Nagy) Nagy, a Trapaceous Pollen Species Pertaining to the Oligocene to Pliocene Genus Hemitrapa Miki. Grana 1990, 29, 285–293. [Google Scholar] [CrossRef][Green Version]
- Mai, D.H. Entwicklung Der Wasser–und Sumpfpflanzen-Gesellschaften Europas von der Kreide bis ins Quartär11)Herrn Prof. Dr. H. Meusel zum 75. Geburtstag gewidmet. Flora 1985, 176, 449–511. [Google Scholar] [CrossRef]
- Li, X.-X.; Zhou, Z.-Y.; Cai, C.-Y.; Sun, G.; Ouyang, S.; Deng, L.-H. Fossil Floras of China through The Geological Ages; Guangdong Science and Technology Press: Guangzhou, China, 1995; ISBN 978-7-5359-1536-8. [Google Scholar]
- Tao, J.-R. The Tertiary vegetation and flora and floristic regions in China. Acta Phytotaxon. Sin. 1992, 30, 25–42. [Google Scholar]
- Tao, J.-R. Floristic Development and Evolution of Late Cretaceous to Cenozoic in China; Science Press: Beijing, China, 2000; ISBN 7-03-007520-X. [Google Scholar]
- Jin, J.-H.; Liao, W.-B.; Wang, B.-S.; Peng, S.-L. Global change in Cenozoic and evolution of flora in China. Guihaia 2003, 23, 217–225. [Google Scholar]
- Quan, C.; Liu, Z.-H.; Utescher, T.; Jin, J.-H.; Shu, J.-W.; Li, Y.-X.; Liu, Y.-S.C. Revisiting the Paleogene Climate Pattern of East Asia: A Synthetic Review. Earth-Sci. Rev. 2014, 139, 213–230. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, H.-Y.; Tang, L.-Y. Cenozoic palynological records and vegetation evolution in the Weihe Basin, Central China. Quat. Sci. 2018, 38, 1083–1093. [Google Scholar] [CrossRef]
- Lyu, H.-Z.; Lu, H.-Y.; Wang, Y.-C.; Zhang, H.-Z.; Wang, Y.; Wang, K.-X.; Lai, W.; Liu, Z.-F.; Li, Y.-L.; Ji, J.-F. East Asian Paleoclimate Change in the Weihe Basin (Central China) since the Middle Eocene Revealed by Clay Mineral Analysis. Sci. China Earth Sci. 2021, 64, 1285–1304. [Google Scholar] [CrossRef]
- Hummel, M.; Kiviat, E. Review of World Literature on Water Chestnut with Implications for Management in North America. J. Aquat. Plant Manag. 2004, 42, 17–28. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Jia, H.; Meng, X.; Ferguson, D.K.; Luo, M.; Liu, P.; Wang, J.; Quan, C. A New Clue for the Late Eocene Freshwater Ecosystem of Central China Evidenced by New Fossils of Trapa L. and Hemitrapa Miki (Lythraceae). Biology 2022, 11, 1442. https://doi.org/10.3390/biology11101442
Han Z, Jia H, Meng X, Ferguson DK, Luo M, Liu P, Wang J, Quan C. A New Clue for the Late Eocene Freshwater Ecosystem of Central China Evidenced by New Fossils of Trapa L. and Hemitrapa Miki (Lythraceae). Biology. 2022; 11(10):1442. https://doi.org/10.3390/biology11101442
Chicago/Turabian StyleHan, Zhuochen, Hui Jia, Xiangning Meng, David K. Ferguson, Mingyue Luo, Ping Liu, Junjie Wang, and Cheng Quan. 2022. "A New Clue for the Late Eocene Freshwater Ecosystem of Central China Evidenced by New Fossils of Trapa L. and Hemitrapa Miki (Lythraceae)" Biology 11, no. 10: 1442. https://doi.org/10.3390/biology11101442
APA StyleHan, Z., Jia, H., Meng, X., Ferguson, D. K., Luo, M., Liu, P., Wang, J., & Quan, C. (2022). A New Clue for the Late Eocene Freshwater Ecosystem of Central China Evidenced by New Fossils of Trapa L. and Hemitrapa Miki (Lythraceae). Biology, 11(10), 1442. https://doi.org/10.3390/biology11101442





