Integrative Analysis of Liver Metabolomics and Transcriptomics Reveals Oxidative Stress in Piglets with Intrauterine Growth Restriction
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Widely Targeted Metabolomics and Analysis
2.3. Bioinformatic Analysis of Transcriptome
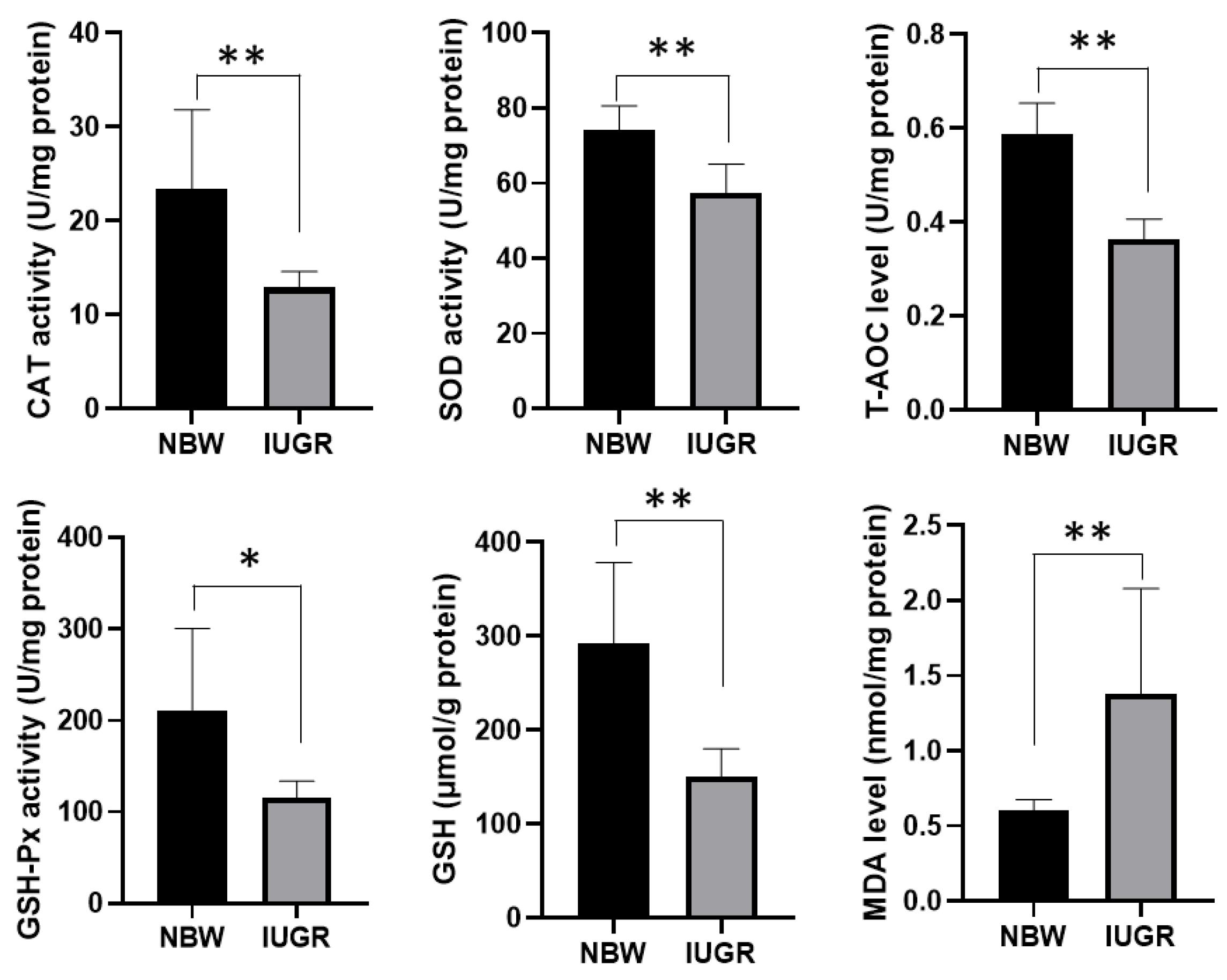
2.4. Detection of Oxidative Stress Biomarkers in Liver
2.5. Transmission Electron Microscopy Analysis
2.6. Liver Morphological Evaluation
2.7. RNA Isolation and Real-Time qPCR
2.8. Statistical Analysis
3. Results
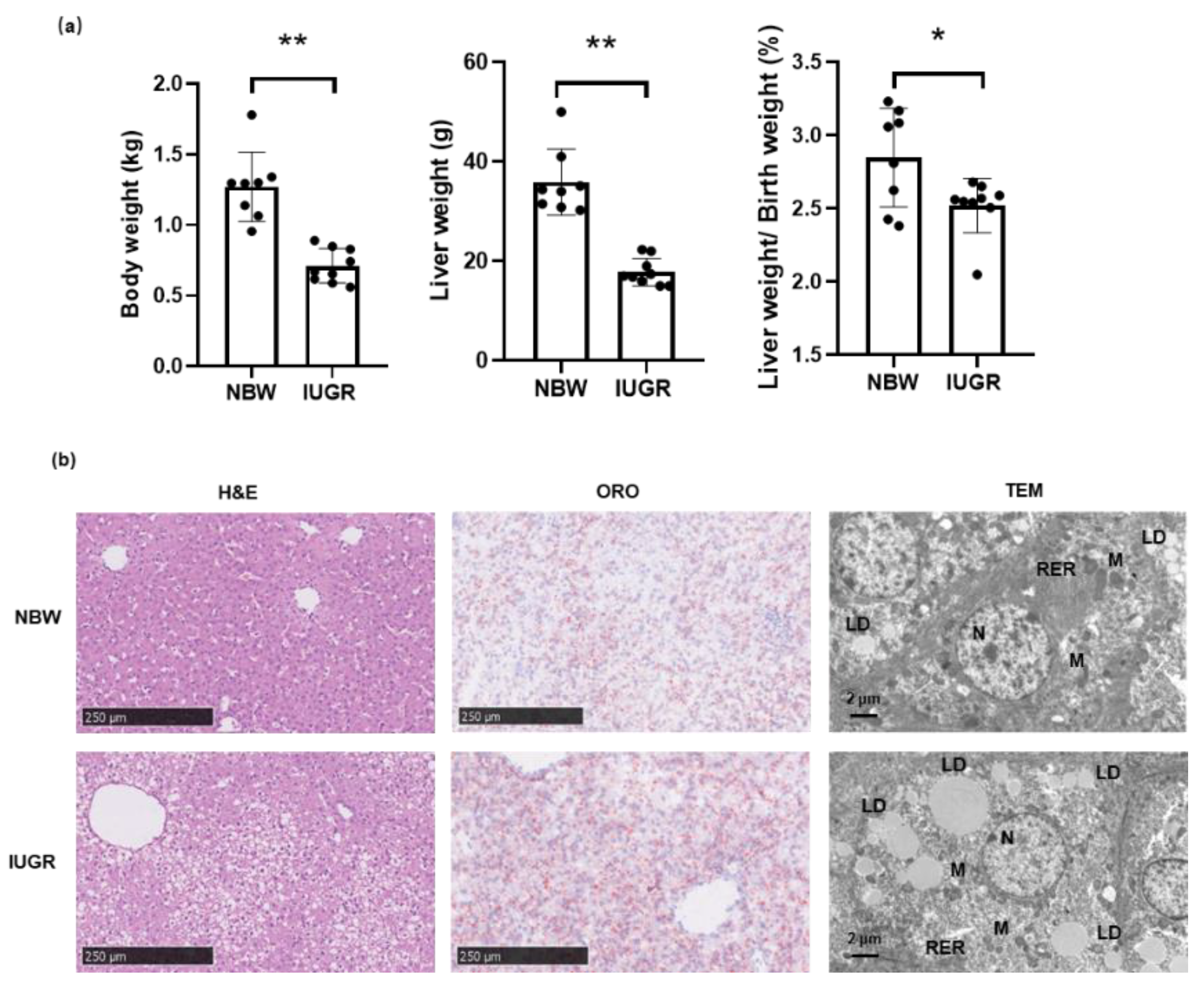
3.1. Phenotypic Traits of Piglets in IUGR and NBW Groups
3.2. Liver Cellular Stress in the Liver of Piglets
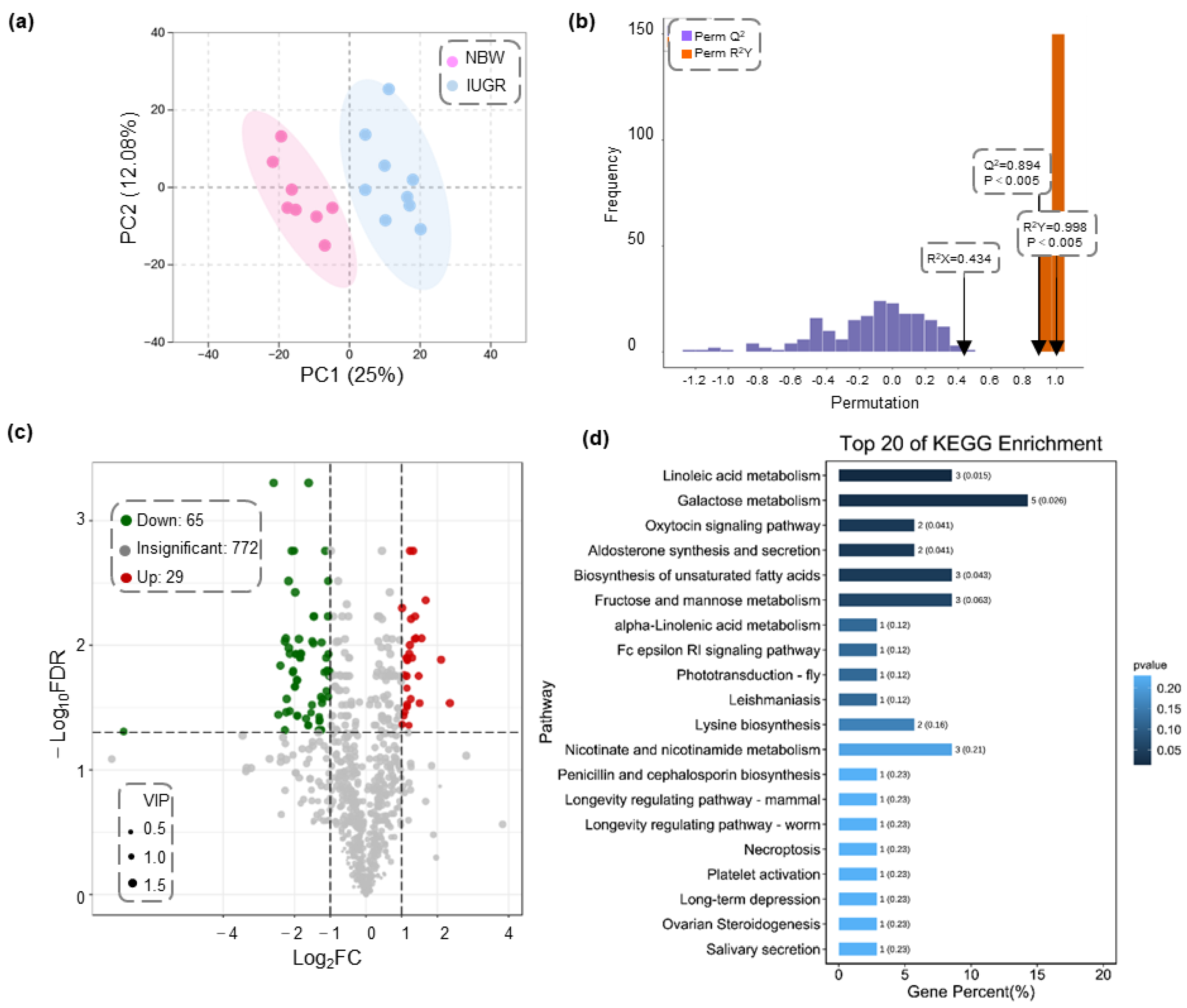
3.3. Liver Metabolic Profiles in IUGR and NBW Piglets
3.4. Liver Transcriptomic Analysis of IUGR and NBW Piglets
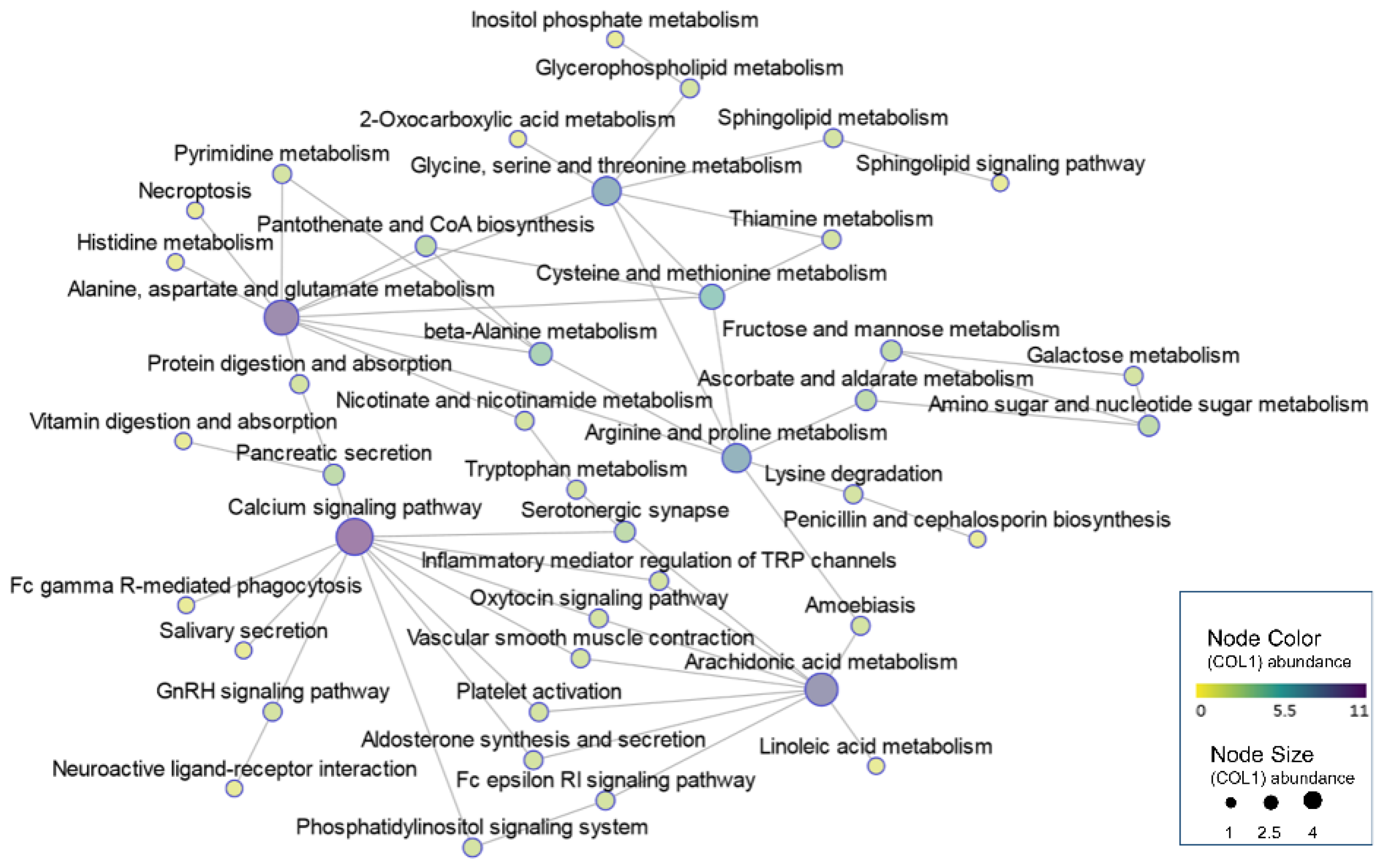
3.5. Integrative Analysis of Differential Transcriptomic and Metabolomic Profiles between IUGR and NBW Piglets
3.6. The Validation of DEGs Expression Involved in the Integrated Pathways between IUGR and NBW Piglets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riddersholm, K.V.; Bahnsen, I.; Bruun, T.S.; de Knegt, L.V.; Amdi, C. Identifying Risk Factors for Low Piglet Birth Weight, High within-Litter Variation and Occurrence of Intrauterine Growth-Restricted Piglets in Hyperprolific Sows. Animals 2021, 11, 2731. [Google Scholar] [CrossRef] [PubMed]
- Amdi, C.; Klarlund, M.V.; Hales, J.; Thymann, T.; Hansen, C.F. Intrauterine growth-restricted piglets have similar gastric emptying rates but lower rectal temperatures and altered blood values when compared with normal-weight piglets at birth1,2. J. Anim. Sci. 2016, 94, 4583–4590. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, Y.; Feng, C.; Lin, G.; Wu, G.; Li, D.; Wang, J. Innate differences and colostrum-induced alterations of jejunal mucosal proteins in piglets with intra-uterine growth restriction. Br. J. Nutr. 2018, 119, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Oke, S.; Hardy, D. The Role of Cellular Stress in Intrauterine Growth Restriction and Postnatal Dysmetabolism. Int. J. Mol. Sci. 2021, 22, 6986. [Google Scholar] [CrossRef]
- Peterside, I.E.; Selak, M.A.; Simmons, R.A. Impaired oxidative phosphorylation in hepatic mitochondria in growth-retarded rats. Am. J. Physiol. Metab. 2003, 285, E1258–E1266. [Google Scholar] [CrossRef]
- Limesand, S.W.; Jensen, J.; Hutton, J.C.; Hay, W.W., Jr. Diminished beta-cell replication contributes to reduced beta-cell mass in fetal sheep with intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1297–R1305. [Google Scholar] [CrossRef]
- Su, W.; Xu, W.; Zhang, H.; Ying, Z.; Zhou, L.; Zhang, L.; Wang, T. Effects of dietary leucine supplementation on the hepatic mitochondrial biogenesis and energy metabolism in normal birth weight and intrauterine growth-retarded weanling piglets. Nutr. Res. Pract. 2017, 11, 121–129. [Google Scholar] [CrossRef]
- Feng, C.; Bai, K.; Wang, A.; Ge, X.; Zhao, Y.; Zhang, L.; Wang, T. Effects of dimethylglycine sodium salt supplementation on growth performance, hepatic antioxidant capacity, and mitochondria-related gene expression in weanling piglets born with low birth weight1. J. Anim. Sci. 2018, 96, 3791–3803. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Hou, X.; Zhang, L.; Wang, T. Medium-chain TAG improve energy metabolism and mitochondrial biogenesis in the liver of intra-uterine growth-retarded and normal-birth-weight weanling piglets. Br. J. Nutr. 2016, 115, 1521–1530. [Google Scholar] [CrossRef]
- Li, W.; Li, B.; Lv, J.; Dong, L.; Zhang, L.; Wang, T. Choline supplementation improves the lipid metabolism of intrauterine-growth-restricted pigs. Asian-Australas. J. Anim. Sci. 2018, 31, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, L.; Wang, L.; Liu, X.; Hou, X.; Zhao, F.; Yan, H.; Wang, L. Liver transcriptome profiling and functional analysis of intrauterine growth restriction (IUGR) piglets reveals a genetic correction and sexual-dimorphic gene expression during postnatal development. BMC Genom. 2020, 21, 701. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Sun, G.Y.; Zhang, M.; Yuan, H.J.; Zhu, S.; Jiao, G.Z.; Luo, M.J.; Tan, J.H. Mechanisms for the species difference between mouse and pig oocytes in their sensitivity to glucorticoids. Biol. Reprod. 2017, 96, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Putri, S.P.; Nakayama, Y.; Matsuda, F.; Uchikata, T.; Kobayashi, S.; Matsubara, A.; Fukusaki, E. Current metabolomics: Practical applications. J. Biosci. Bioeng. 2013, 115, 579–589. [Google Scholar] [CrossRef]
- Wang, X.; Lin, G.; Liu, C.; Feng, C.; Zhou, H.; Wang, T.; Li, D.; Wu, G.; Wang, J. Temporal proteomic analysis reveals defects in small-intestinal development of porcine fetuses with intrauterine growth restriction. J. Nutr. Biochem. 2014, 25, 785–795. [Google Scholar] [CrossRef]
- Hansen, C.F.; Hales, J.; Amdi, C.; Moustsen, V.A. Intrauterine growth-restricted piglets defined by their head shape have impaired survival and growth during the suckling period. Anim. Prod. Sci. 2019, 59, 1056. [Google Scholar] [CrossRef]
- Tang, Y.; Pan, Y.; Chen, Y.; Kong, X.; Chen, J.; Zhang, H.; Tang, G.; Wu, J.; Sun, X. Metabolomic Profiling of Aqueous Humor and Plasma in Primary Open Angle Glaucoma Patients Points towards Novel Diagnostic and Therapeutic Strategy. Front. Pharmacol. 2021, 12, 621146. [Google Scholar] [CrossRef]
- Riva, G.; Villanova, M.; Cima, L.; Ghimenton, C.; Bronzoni, C.; Colombari, R.; Crestani, M.; Sina, S.; Brunelli, M.; D’Errico, A.; et al. Oil Red O Is a Useful Tool to Assess Donor Liver Steatosis on Frozen Sections during Transplantation. Transplant. Proc. 2018, 50, 3539–3543. [Google Scholar] [CrossRef]
- Du, J.; Zhang, X.; Han, J.; Man, K.; Zhang, Y.; Chu, E.S.; Nan, Y.; Yuemin, N. Pro-Inflammatory CXCR3 Impairs Mitochondrial Function in Experimental Non-Alcoholic Steatohepatitis. Theranostics 2017, 7, 4192–4203. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, C.; Tao, L.; Hao, J.; Tan, K.; Miao, K.; Yu, Y.; Sui, L.; Wu, Z.; Tian, J.; et al. High-resolution profiles of gene expression and DNA methylation highlight mitochondrial modifications during early embryonic development. J. Reprod. Dev. 2017, 63, 247–261. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Martyn, C.N.; Osmond, C.; Hales, C.N.; Fall, C.H. Growth in utero and serum cholesterol concentrations in adult life. BMJ 1993, 307, 1524–1527. [Google Scholar] [CrossRef]
- Shen, L.; Gan, M.; Zhang, S.; Ma, J.; Tang, G.; Jiang, Y.; Li, M.; Wang, J.; Li, X.; Che, L.; et al. Transcriptome Analyses Reveal Adult Metabolic Syndrome with Intrauterine Growth Restriction in Pig Models. Front. Genet. 2018, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Jebasingh, F.; Thomas, N. Barker Hypothesis and Hypertension. Front. Public Health 2022, 9, 767545. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Massart, J.; Robin, M.A.; Bonnet, F.; Fromenty, B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Li, D.; Yin, Y.; Wang, X.; Li, P.; Dangott, L.J.; Hu, W.; Wu, G. Intrauterine Growth Restriction Affects the Proteomes of the Small Intestine, Liver, and Skeletal Muscle in Newborn Pigs. J. Nutr. 2008, 138, 60–66. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Gasser, A.; Bruhn, S.; Guse, A.H. Second Messenger Function of Nicotinic Acid Adenine Dinucleotide Phosphate Revealed by an Improved Enzymatic Cycling Assay. J. Biol. Chem. 2006, 281, 16906–16913. [Google Scholar] [CrossRef]
- Koch-Nolte, F.; Kernstock, S.; Mueller-Dieckmann, C.; Weiss, M.S.; Haag, F. Mammalian ADP-ribosyltransferases and ADP-ribosylhydrolases. Front. Biosci. 2008, 13, 6716–6729. [Google Scholar] [CrossRef]
- Ding, C.; Leow, M.K.S.; Magkos, F. Oxytocin in metabolic homeostasis: Implications for obesity and diabetes management. Obes. Rev. 2018, 20, 22–40. [Google Scholar] [CrossRef]
- Mkhize, B.C.; Mosili, P.; Ngubane, P.S.; Sibiya, N.H.; Khathi, A. Diet-induced prediabetes: Effects on the activity of the renin–angiotensin–aldosterone system in selected organs. J. Diabetes Investig. 2022, 13, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Fekete, K.; Györei, E.; Lohner, S.; Verduci, E.; Agostoni, C.; Decsi, T. Long-chain polyunsaturated fatty acid status in obesity: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 488–497. [Google Scholar] [CrossRef]
- Chandrasekharan, J.A.; Marginean, A.; Sharma-Walia, N. An insight into the role of arachidonic acid derived lipid mediators in virus associated pathogenesis and malignancies. Prostaglandins Other Lipid Mediat. 2016, 126, 46–54. [Google Scholar] [CrossRef]
- Depner, C.M.; Philbrick, K.A.; Jump, D.B. Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a Ldlr(-/-) mouse model of western diet-induced nonalcoholic steatohepatitis. J. Nutr. 2013, 143, 315–323. [Google Scholar] [CrossRef] [PubMed]
- AlSaleh, A.; Maniou, Z.; Lewis, F.J.; Hall, W.L.; Sanders, T.A.B.; O’Dell, S.D. ELOVL2 gene polymorphisms are associated with increases in plasma eicosapentaenoic and docosahexaenoic acid proportions after fish oil supplement. Genes Nutr. 2013, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Steer, C.D.; Hibbeln, J.R.; Golding, J.; Davey Smith, G. Polyunsaturated fatty acid levels in blood during pregnancy, at birth and at 7 years: Their associations with two common FADS2 polymorphisms. Hum. Mol. Genet. 2012, 21, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Maguolo, A.; Zusi, C.; Giontella, A.; Del Giudice, E.M.; Tagetti, A.; Fava, C.; Morandi, A.; Maffeis, C. Influence of genetic variants in FADS2 and ELOVL2 genes on BMI and PUFAs homeostasis in children and adolescents with obesity. Int. J. Obes. 2020, 45, 56–65. [Google Scholar] [CrossRef]
- Gomez Rodriguez, A.; Talamonti, E.; Naudi, A.; Kalinovich, A.V.; Pauter, A.M.; Barja, G.; Bengtsson, T.; Jacobsson, A.; Pamplona, R.; Shabalina, I.G. Elovl2-Ablation Leads to Mitochondrial Membrane Fatty Acid Remodeling and Reduced Efficiency in Mouse Liver Mitochondria. Nutrients 2022, 14, 559. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.Z.; Shen, Y.; Lin, J.; Wang, H.R.; Talukder, M.; Li, J.L. Lycopene Prevents DEHP-Induced Leydig Cell Damage with the Nrf2 Antioxidant Signaling Pathway in Mice. J. Agric. Food Chem. 2019, 68, 2031–2040. [Google Scholar] [CrossRef]
- López-Navarro, M.E.; Jarquín-Martínez, M.; Sánchez-Labastida, L.A.; Ramírez-Rosales, D.; Godínez-Victoria, M.; Quintas-Granados, L.I.; Trujillo-Ferrara, J.G. Decoding Aging: Understanding the Complex Relationship among Aging, Free Radicals, and GSH. Oxidative Med. Cell. Longev. 2020, 2020, 3970860. [Google Scholar] [CrossRef]
- Morellato, A.E.; Umansky, C.; Pontel, L.B. The toxic side of one-carbon metabolism and epigenetics. Redox Biol. 2020, 40, 101850. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.I.; Berry, G.T.; Rubio-Gozalbo, M.E. Galactose metabolism and health. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 422–427. [Google Scholar] [CrossRef]
- Yazici, H.; Canda, E.; Altınok, Y.A.; Ucar, S.K.; Coker, M. Two siblings with galactose mutarotase deficiency: Clinical differences. JIMD Rep. 2021, 63, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Shaeib, F.; Khan, S.N.; Kohan-Ghadr, H.R.; Jeelani, R.; Aldhaheri, S.R.; Gonik, B.; Abu-Soud, H.M. Galactose and its Metabolites Deteriorate Metaphase II Mouse Oocyte Quality and Subsequent Embryo Development by Disrupting the Spindle Structure. Sci. Rep. 2017, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R. d-Galactose-induced accelerated aging model: An overview. Biogerontology 2019, 20, 763–782. [Google Scholar] [CrossRef]
- Chatree, S.; Thongmaen, N.; Tantivejkul, K.; Sitticharoon, C.; Vucenik, I. Role of Inositols and Inositol Phosphates in Energy Metabolism. Molecules 2020, 25, 5079. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Bizzarri, M. Inositols in Insulin Signaling and Glucose Metabolism. Int. J. Endocrinol. 2018, 2018, 1968450. [Google Scholar] [CrossRef]
- Dessi, A.; Fanos, V. Myoinositol: A new marker of intrauterine growth restriction? J. Obstet. Gynaecol. 2013, 33, 776–780. [Google Scholar] [CrossRef]
- Nissen, P.M.; Nebel, C.; Oksbjerg, N.; Bertram, H.C. Metabolomics Reveals Relationship between Plasma Inositols and Birth Weight: Possible Markers for Fetal Programming of Type 2 Diabetes. J. Biomed. Biotechnol. 2010, 2011, 378268. [Google Scholar] [CrossRef]
- Ortmeyer, H.K.; Huang, L.C.; Zhang, L.; Hansen, B.C.; Larner, J. Chiroinositol deficiency and insulin resistance. II. Acute effects of D-chiroinositol administration in streptozotocin-diabetic rats, normal rats given a glucose load, and spontaneously insulin-resistant rhesus monkeys. Endocrinology 1993, 132, 646–651. [Google Scholar] [CrossRef]
- Lefort, G.; Servien, R.; Quesnel, H.; Billon, Y.; Canario, L.; Iannuccelli, N.; Canlet, C.; Paris, A.; Vialaneix, N.; Liaubet, L. The maturity in fetal pigs using a multi-fluid metabolomic approach. Sci. Rep. 2020, 10, 19912. [Google Scholar] [CrossRef] [PubMed]

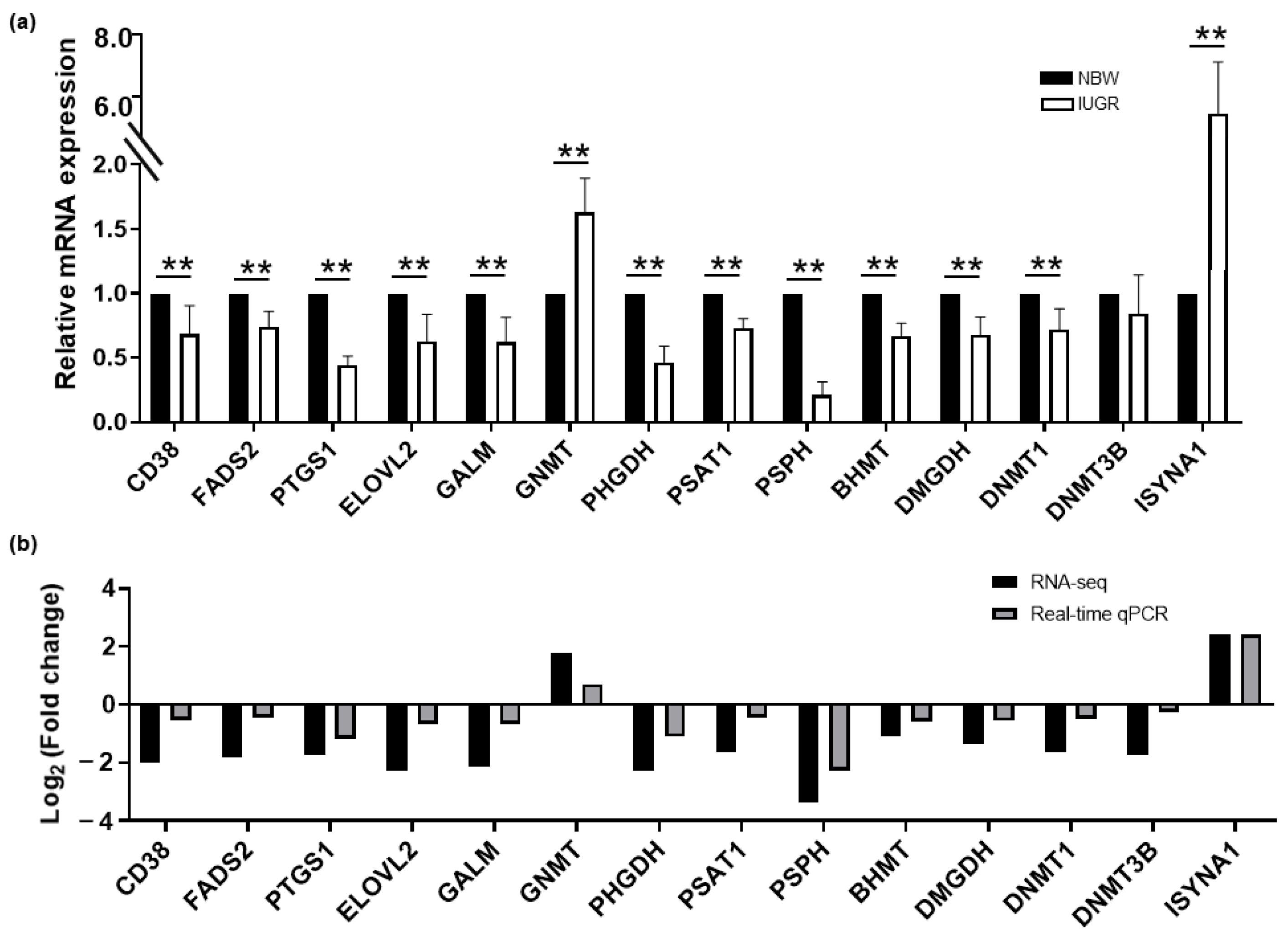
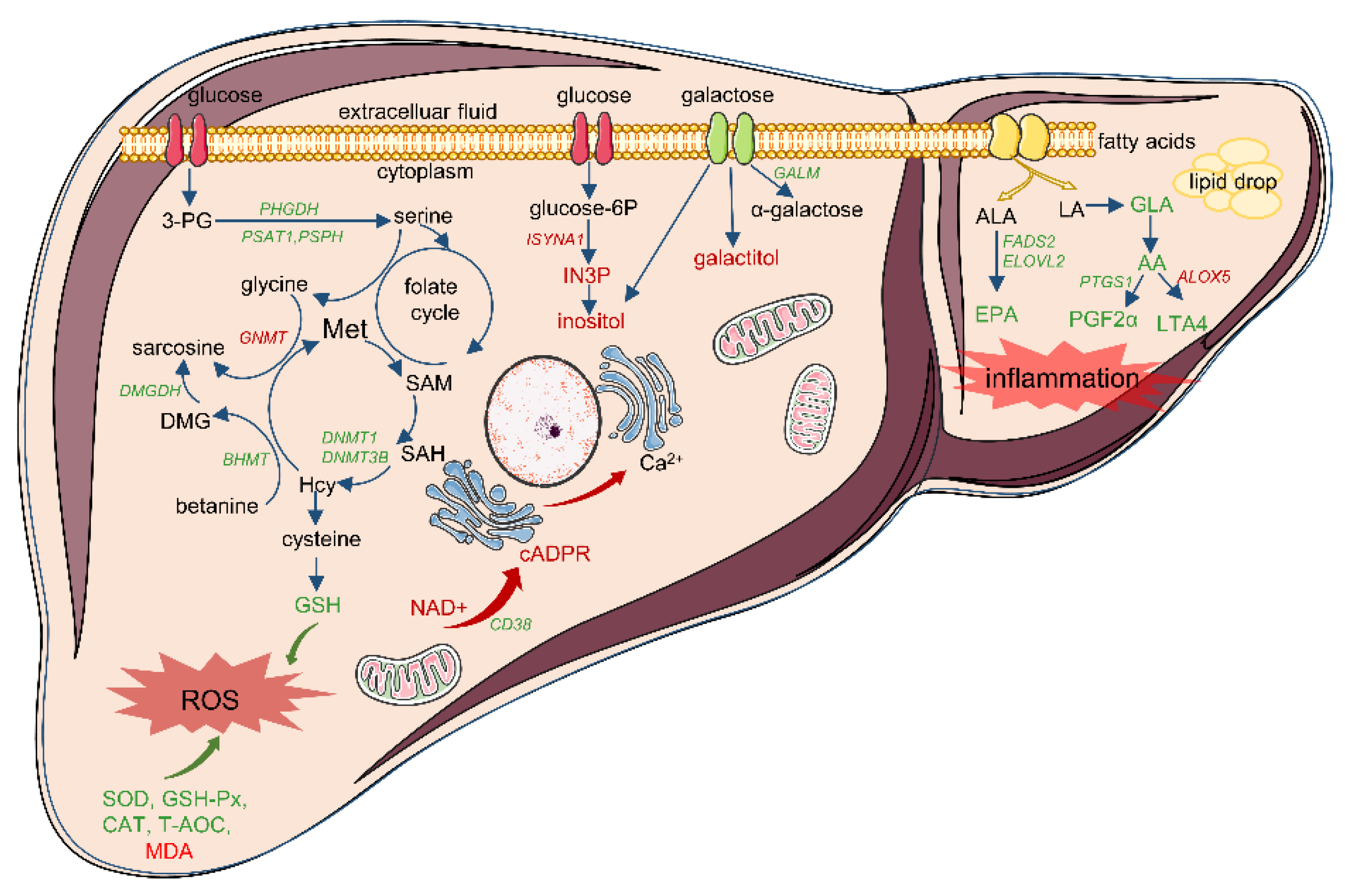
| Physiological Process | Pathway Term | DEGs | DMs |
|---|---|---|---|
| Mitochondrial dysfunction | Nicotinate and nicotinamide metabolism (ko00760); Oxytocin signaling pathway (ko04921); Aldosterone synthesis and secretion (ko04925); Calcium signaling pathway (ko04020) | NT5E, CD38, SIRT4, NNMT, NT5C3A, ENPP3, BST1, NMRK2, LIPE, HSD3B, CDKN1A, CAMK1D, CAMK1, ADCY1, CACNB1, EGFR, CALML4, CACNB3, KCNJ2, PLCE1, PHKA2, ERBB2, GNAL, PLCD1, P2RX3, CXCR4 | NAD+, cADPR, AA, N’-Methyl-2-pyridone-5-carboxamide, 6-hydroxy-3-succinylpyridine |
| Imbalance of fatty acid composition | Biosynthesis of unsaturated fatty acids (ko01040); Linoleic acid metabolism (ko00591); alpha-Linolenic acid metabolism (ko00592); Arachidonic acid metabolism (ko00590) | CYP2C, FADS2, CYP4A24, PTGS1, GGT5, ALOX5, CBR3, AKR1C2, ELOVL2, ACOT HSD17B12 | AA, GLA, EPA, 9,10-DiHOME, 12,13-DiHOME, FFA(18:4) |
| Disruption to sources of one-carbon unit supply | Glycine, serine and threonine metabolism (ko00260); Cysteine and methionine metabolism (ko00270) | GATM, PSAT1, PHGDH, PSPH, DAO, GLYCTK, ALAS2, BHMT, DMGDH, AGXT2, PIPOX, GCAT, GNMT, GCLC, DNMT3B, LDHA, DNMT1, SRM, BCAT1 | N,N-Dimethylglycine, 5′-Deoxy-5′-(Methylthio) Adenosine |
| Abnormal galactose conversion | Galactose metabolism (ko00052); Inositol phosphate metabolism (ko00562); Fructose and mannose metabolism (ko00051) | ISYNA1, PIK3C2G, GANC, GALM, MGAM2, LCT, MGAM, PFKFB4, TKFC, PI4KA, PLCE1, PLCD1, MTMR7 | galactitol, inositol, sorbitol, 3-hydroxyhippuric acid, mannitol, N-acetyl-D-galactosamine, GDP-fucose |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Chen, X.; Zhao, J.; Xue, Z.; Zhang, L.; Zhao, F.; Wang, B.; Wang, L. Integrative Analysis of Liver Metabolomics and Transcriptomics Reveals Oxidative Stress in Piglets with Intrauterine Growth Restriction. Biology 2022, 11, 1430. https://doi.org/10.3390/biology11101430
Gao H, Chen X, Zhao J, Xue Z, Zhang L, Zhao F, Wang B, Wang L. Integrative Analysis of Liver Metabolomics and Transcriptomics Reveals Oxidative Stress in Piglets with Intrauterine Growth Restriction. Biology. 2022; 11(10):1430. https://doi.org/10.3390/biology11101430
Chicago/Turabian StyleGao, Hongmei, Xiaoyou Chen, Junxing Zhao, Zhenhua Xue, Longchao Zhang, Fuping Zhao, Bingyuan Wang, and Lixian Wang. 2022. "Integrative Analysis of Liver Metabolomics and Transcriptomics Reveals Oxidative Stress in Piglets with Intrauterine Growth Restriction" Biology 11, no. 10: 1430. https://doi.org/10.3390/biology11101430
APA StyleGao, H., Chen, X., Zhao, J., Xue, Z., Zhang, L., Zhao, F., Wang, B., & Wang, L. (2022). Integrative Analysis of Liver Metabolomics and Transcriptomics Reveals Oxidative Stress in Piglets with Intrauterine Growth Restriction. Biology, 11(10), 1430. https://doi.org/10.3390/biology11101430





