Effect of Mineral Carriers on Biofilm Formation and Nitrogen Removal Activity by an Indigenous Anammox Community from Cold Groundwater Ecosystem Alone and Bioaugmented with Biomass from a “Warm” Anammox Reactor
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Contaminated Groundwater, Containing an Indigenous Anammox Community
2.2. Bioaugmentating Anammox Biomass
2.3. Mineral Carriers
2.4. Experimental Setup
2.5. High-Throughput Sequencing of 16S rRNA Genes
2.6. Analytical Methods
2.7. Microscopy
2.8. Calculations and Correlation Analysis
3. Results
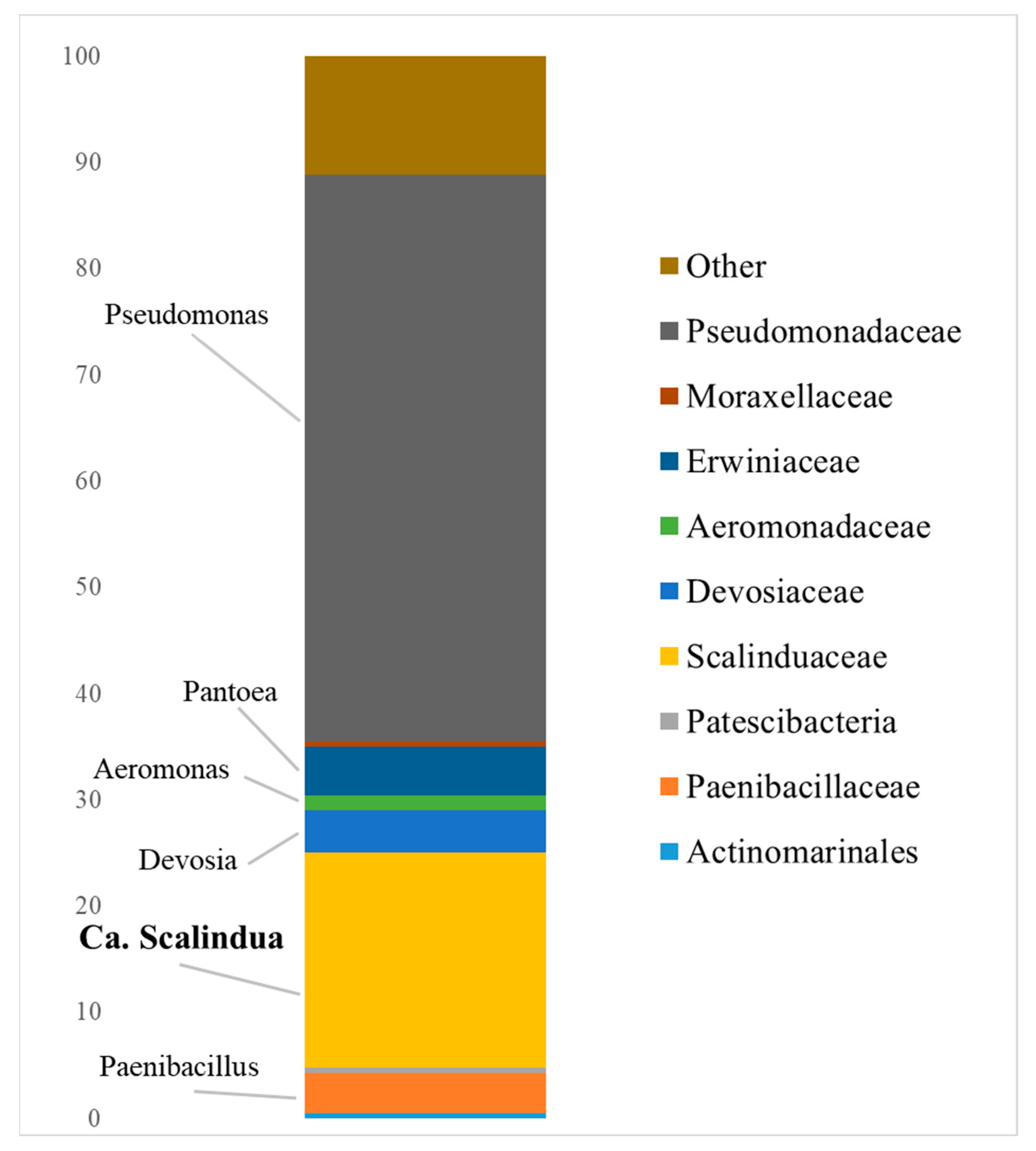
3.1. Composition of the Indigenous Anammox Community
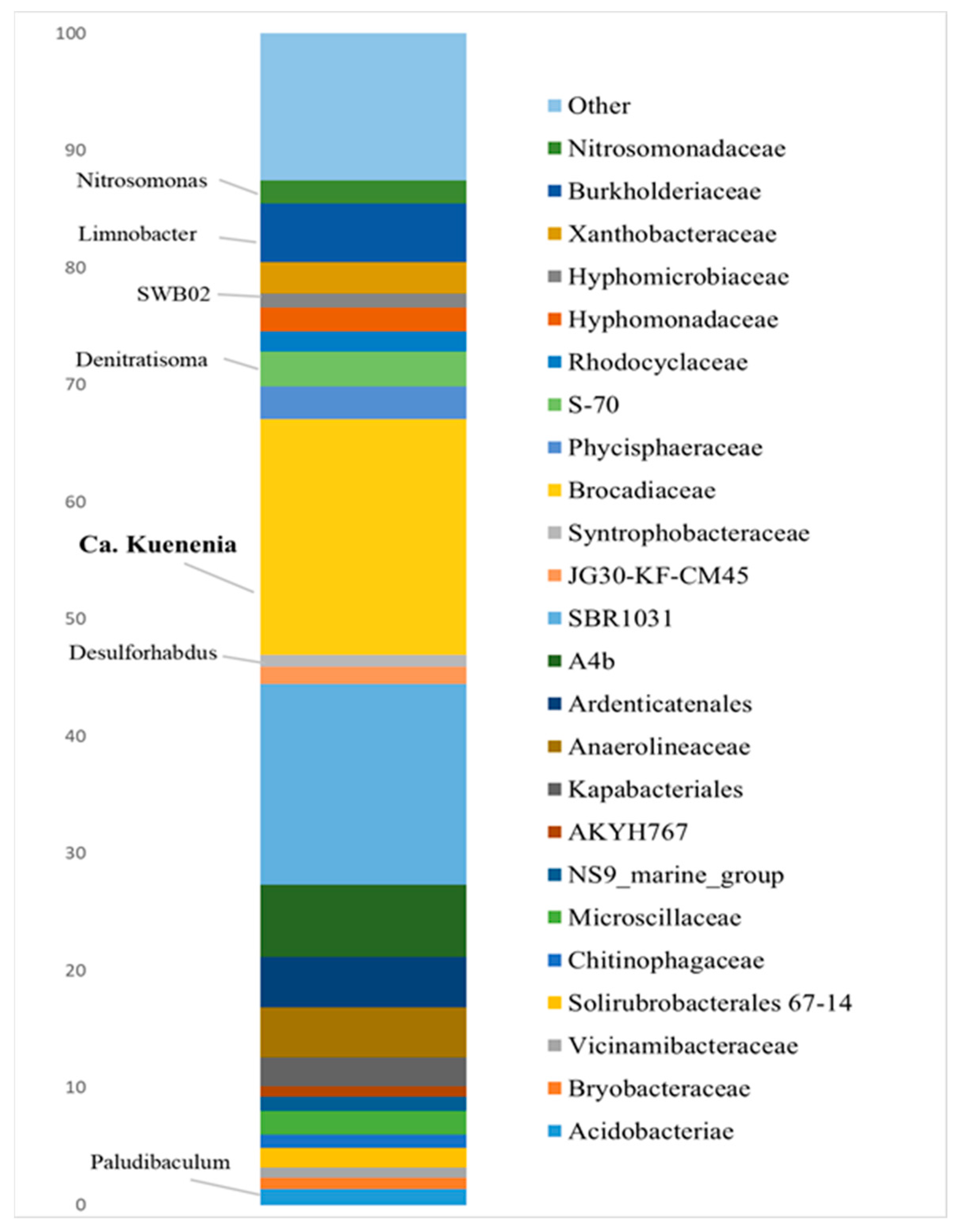
3.2. Bioaugmenting Anammox Community
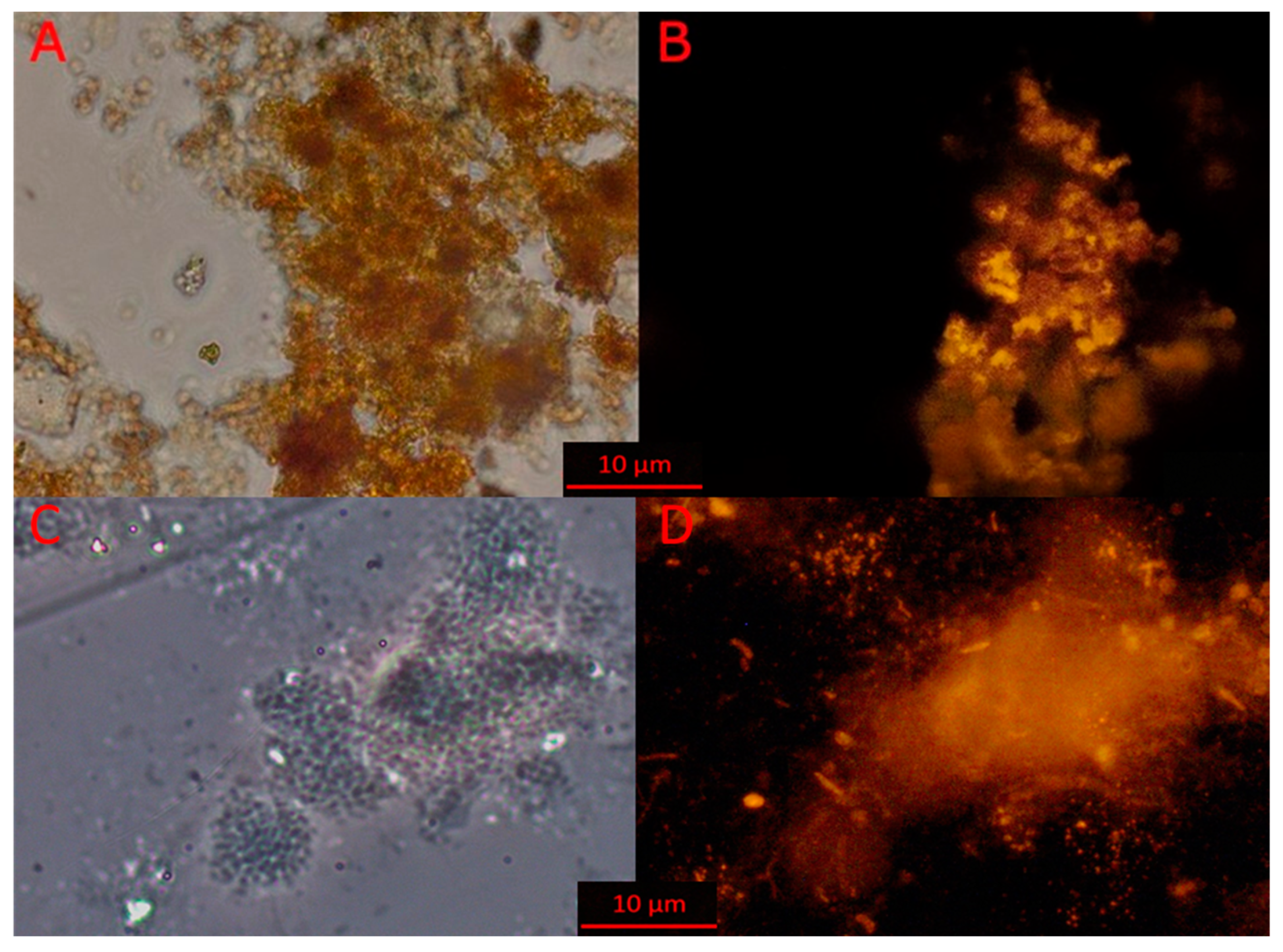
3.3. Microscopy of Indigenous and Bioaugmentation Anammox Community
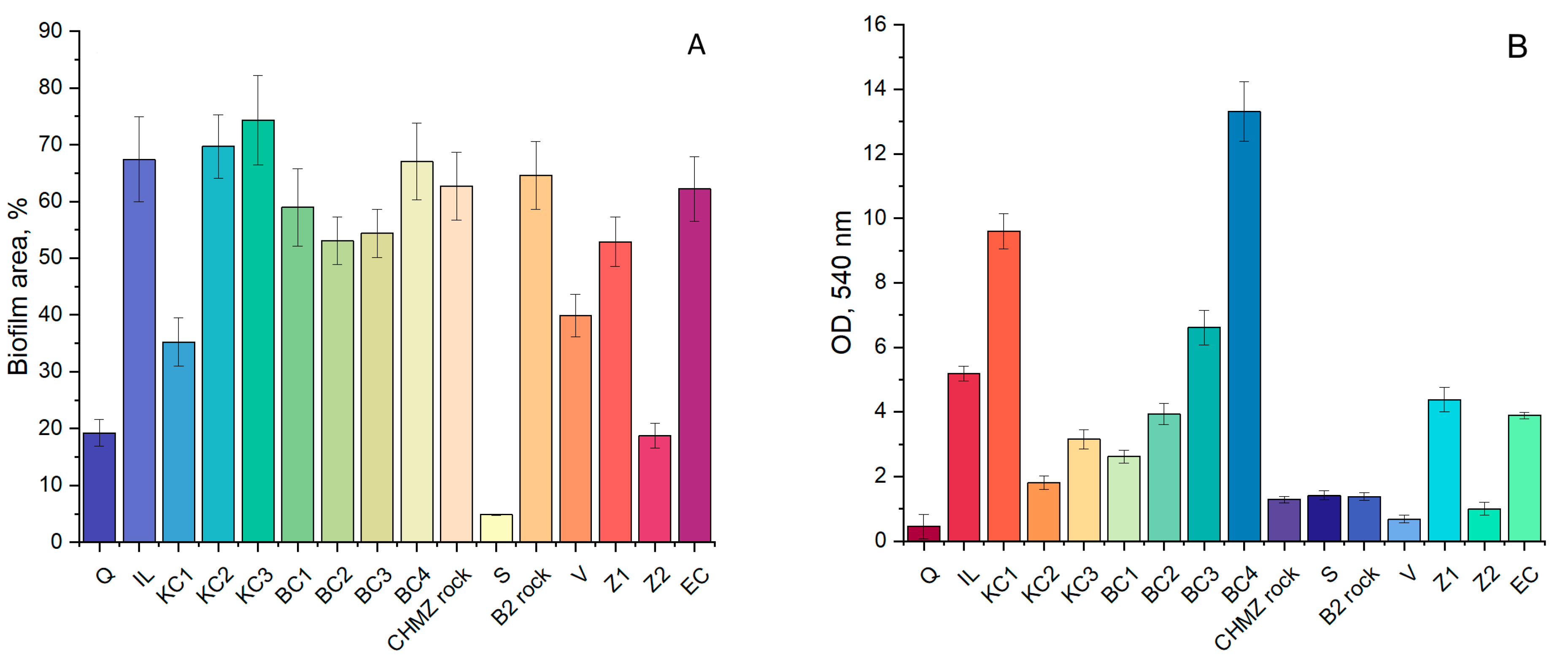
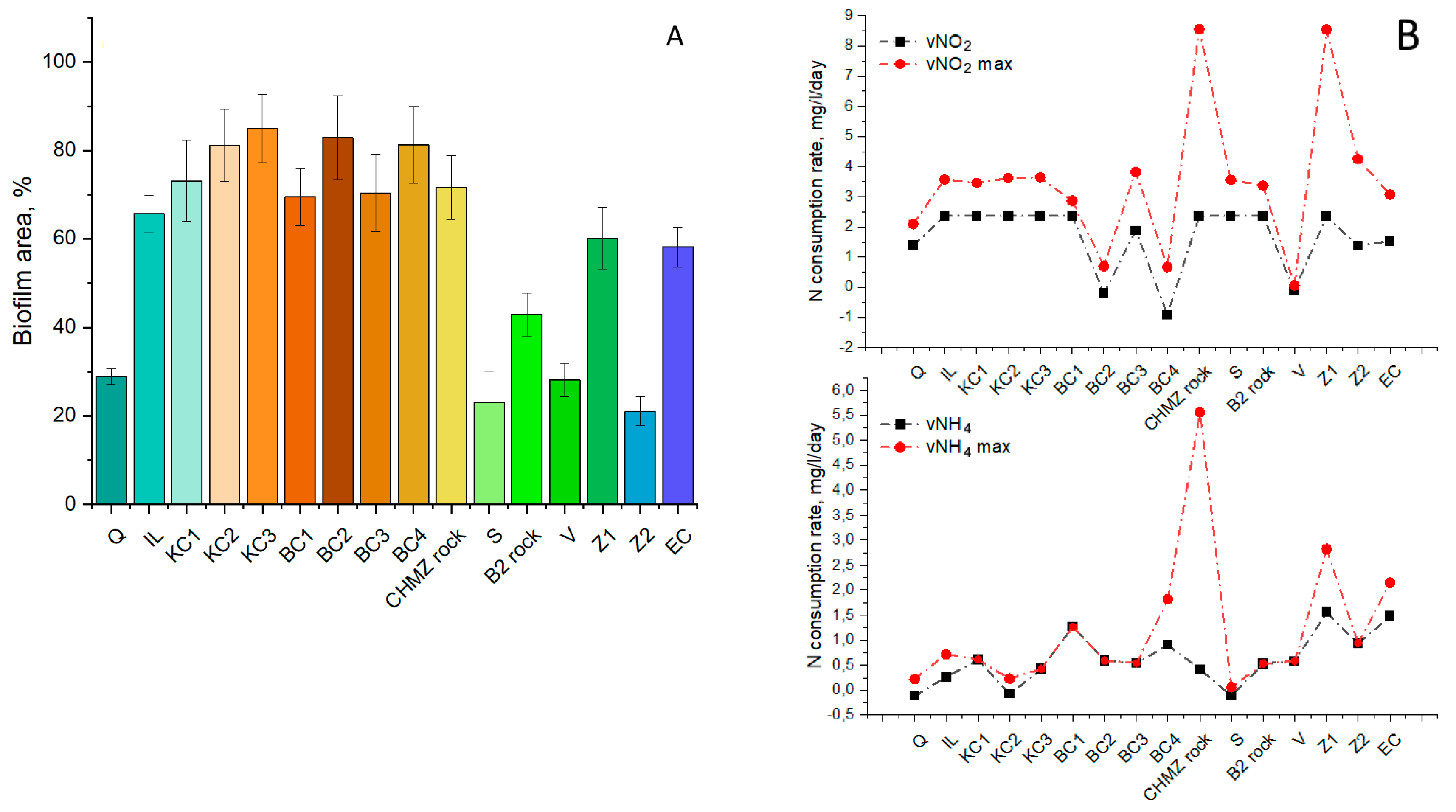
3.4. Effect of Mineral Carriers on the Performance of the Indigenous Anammox Community
3.5. Effect of Bioaugmentation with a “Warm” Anammox Community
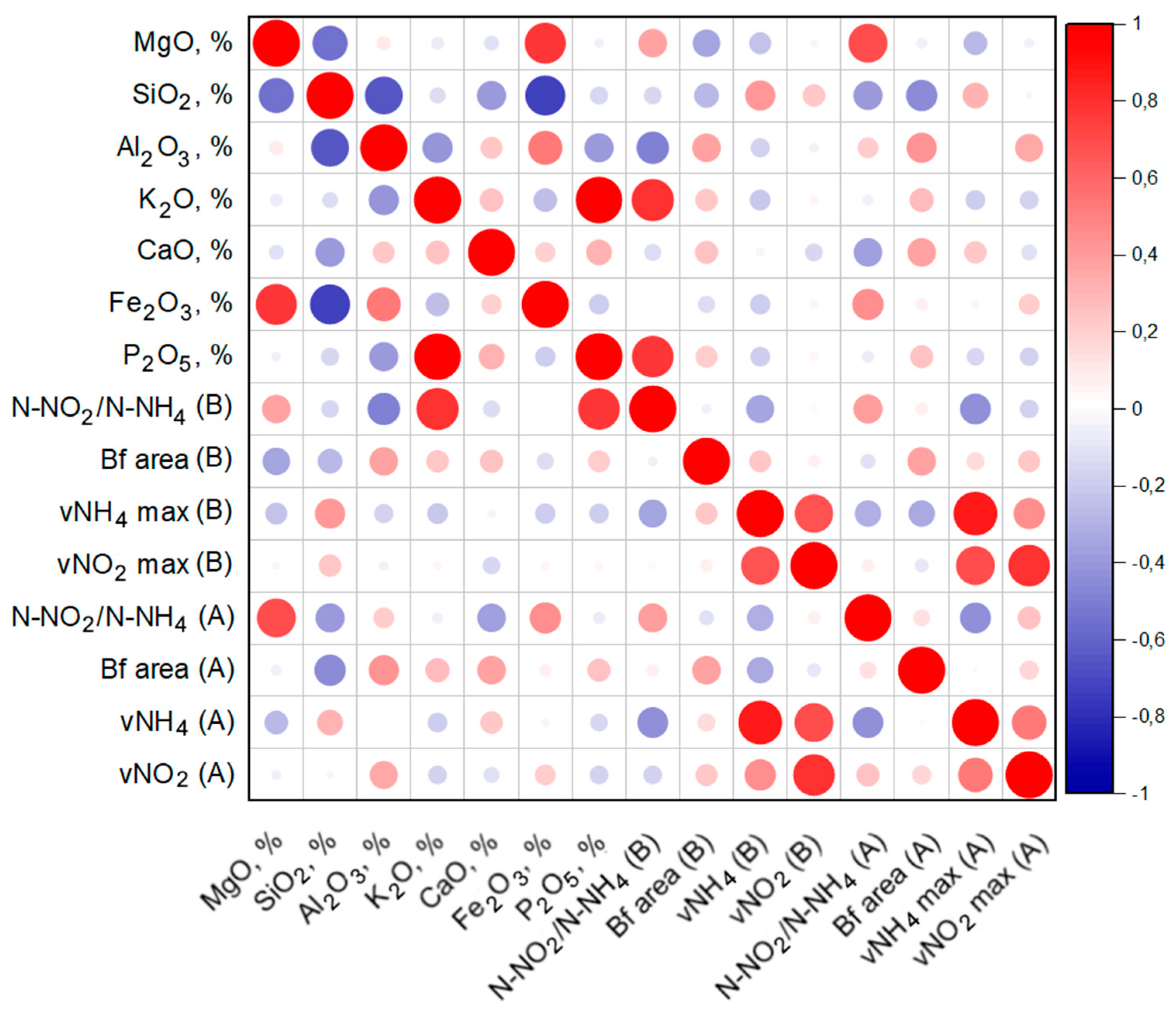
4. Discussion
5. Conclusions
- Quartz and kaolin clays of the Kantatskoye and Kamalinskoye deposits, bentonite clay of the Berezovskoye deposit, and zeolite of the Kholinskoye deposit can be used as components of a permeable barrier in the removal of ammonium and nitrite nitrogen in situ in the anammox process using an indigenous cold anammox community dominated by Ca. Scalindua.
- It can be assumed that the activity of the anammox process is determined by a number of factors, including the presence of the necessary trace elements in the material, the features of the surface morphology, as well as competition with other groups of organisms that can develop on materials at a faster rate.
- Bioaugmentation with a “warm” anammox community with a predominance of Ca. Kuenenia resulted in improved nitrogen removal on most carriers, including bentonite clay of the Dinozavrovoye deposit, loamy rock, and zeolite-containing tripoli, in addition to carriers that perform best with the indigenous anammox community. To further enhance the anammox process in a cold, nitrate-rich underground habitat, research is needed on the feasibility of coupled partial denitrification–anammox and the adaptation of a “warm” anammox community to low temperatures and hazardous compounds contained in polluted groundwater.
- Biofouling of loamy aquifers is due to their polymineral composition. For example, potassium feldspars and clays can serve as a source of potassium, while clays, amorphous iron oxides, and ferruginous minerals, such as siderite, can serve as a source of iron. It is important to note that biofouling by bacteria carrying out the anammox process on the ChMZ rock was quite successful, which suggests that under optimal conditions, the anammox process is possible in situ in the polluted aquifer.
- It can be assumed that when polymineral rocks are overgrown with anammox bacteria, competition with more rapidly developing bacteria can take place. Thus, for biofouling by anammox bacteria, it is not necessary to use a mineral carrier with a high area, such as expanded clay. However, it is necessary to select a carrier that will contain the elements necessary for anammox bacteria, while not providing advantages for the rapid development of bacteria of other groups.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Strous, M.; Heijnen, J.; Kuenen, J.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Botchkova, E.A.; Litti, Y.V.; Kuznetsov, B.B.; Nozhevnikova, A.N. Microbial biofilms formed in a laboratory-scale anammox bioreactor with flexible brush carrier. J. Biomater. Nanobiotechnol. 2014, 5, 44392. [Google Scholar] [CrossRef]
- Zhang, J.; Miao, Y.; Zhang, Q.; Sun, Y.; Wu, L.; Peng, Y. Mechanism of stable sewage nitrogen removal in a partial nitrification-anammox biofilm system at low temperatures: Microbial community and EPS analysis. Bioresour. Technol. 2020, 297, 122459. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Schopf, A.; Amaral-Stewart, B.; Christensson, M.; Morgan-Sagastume, F.; St-Pierre, J.-P.; Vincent, S.; Mercier, E.; Zhang, X.; Delatolla, R. Carrier surface modification for enhanced attachment and growth of anammox biofilm. Sci. Total Environ. 2022, 811, 151317. [Google Scholar] [CrossRef]
- Safonov, A.; Popova, N.; Andrushenko, N.; Boldyrev, K.; Yushin, N.; Zinicovscaia, I. Investigation of materials for reactive permeable barrier in removing cadmium and chromium (VI) from aquifer near a solid domestic waste landfll. Environ. Sci. Pollut. Res. 2021, 28, 4645–4659. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.; Vishnyakova, A.; Artemiev, G.; Sitanskaia, A.; Litti, Y.; Safonov, A. Biofilms of anammox bacteria on mineral carriers to establish a subterranean permeable barrier. Int. J. Environ. Sci. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Cao, Y.; van Loosdrecht, M.; Daigger, G.T. Mainstream partial nitritation–anammox in municipal wastewater treatment: Status, bottlenecks, and further studies. Appl. Microbiol. Biotechnol. 2017, 101, 1365–1383. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; Kleerebezem, R.; Van Loosdrecht, M.C.M. Effect of temperature change on anammox activity. Biotechnol. Bioeng. 2015, 112, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Kouba, V.; Bachmannová, C.; Podzimek, T.; Lipovova, P.; van Loosdrecht, M.C.M. Physiology of anammox adaptation to low temperatures and promising biomarkers: A review. Bioresour. Technol. 2022, 394, 126847. [Google Scholar] [CrossRef]
- Chen, H.; Jin, R.C. Summary of the preservation techniques and the evolution of the anammox bacteria characteristics during preservation. Appl. Microbiol. Biotechnol. 2017, 101, 4349–4362. [Google Scholar] [CrossRef]
- Sobotka, D.; Zhai, J.; Makinia, J. Generalized temperature dependence model for anammox process kinetics. Sci. Total Environ. 2021, 775, 145760. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; Kleerebezem, R.; Abelleira-Pereira, J.M.; Abbas, B.; Van Loosdrecht, M.C.M. Faster through training: The anammox case. Water Res. 2015, 81, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Dosta, J.; Fernández, I.; Vázquez-Padín, J.R.; Mosquera-Corral, A.; Campos, J.L.; Mata-Alvarez, J.; Méndez, R. Short-and long-term effects of temperature on the Anammox process. J. Hazard. Mater. 2008, 154, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Canion, A.; Kostka, J.E.; Gihring, T.M.; Huettel, M.; van Beusekom, J.E.E.; Gao, H.; Lavik, G.; Kuypers, M.M.M. Temperature response of denitrification and anammox reveals the adaptation of microbial communities to in situ temperatures in permeable marine sediments that span 50 in latitude. Biogeosciences 2014, 11, 309–320. [Google Scholar] [CrossRef]
- Rysgaard, S.; Glud, R.N.; Risgaard-Petersen, N.; Dalsgaard, T. Denitrification and anammox activity in Arctic marine sediments. Limnol. Oceanogr. 2004, 49, 1493–1502. [Google Scholar] [CrossRef]
- Thamdrup, B.; Dalsgaard, T. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl. Environ. Microbiol. 2022, 68, 1312–1318. [Google Scholar] [CrossRef]
- Tribelli, P.M.; López, N.I. Reporting key features in cold-adapted bacteria. Life 2018, 8, 8. [Google Scholar] [CrossRef]
- Yang, E.; Chena, J.; Jiang, Z.; Deng, Z.; Tu, Z.; Wang, H.; Wu, S.; Kong, Z.; Hendrik Sanjaya, E.; Chen, H. Insights into rapidly recover-ing the autotrophic nitrogen removal performance of single-stage partial nitritation-anammox systems: Reconstructing granular sludge and its functional microbes synergy. Bioresour. Technol. 2022, 361, 127750. [Google Scholar] [CrossRef]
- Wang, H.; Yu, G.; He, W.; Du, C.; Deng, Z.; Wang, D.; Yang, M.; Yang, E.; Zhou, Y.; Sanjaya, E.H.; et al. Enhancing autotrophic ni-trogen removal with a novel dissolved oxygen-differentiated airlift internal circulation reactor: Long-term operational perfor-mance and microbial characteristics. J. Environ. Manag. 2021, 296, 113271. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.; Yang, E.; Tu, Z.; Liang, J.; Dai, X.; Chen, H. Revealing the effect of biofilm formation in partial nitrita-tion-anammox systems: Start-up, performance stability, and recovery. Bioresour. Technol. 2022, 357, 127379. [Google Scholar] [CrossRef]
- Madeira, C.L.; de Araújo, J.C. Inhibition of anammox activity by municipal and industrial wastewater pollutants: A review. Sci. Total Environ. 2021, 799, 149449. [Google Scholar] [CrossRef] [PubMed]
- Pimenov, N.V.; Nikolaev, Y.A.; Dorofeev, A.G.; Grachev, V.A.; Kallistova, A.Y.; Kanapatskii, T.A.; Litti, Y.V.; Gruzdev, E.V.; Begmatov, S.A.; Ravin, N.V.; et al. Introduction of Exogenous Activated Sludge as a Way to Enhance the Efficiency of Nitrogen Removal in the Anammox Process. Microbiology 2022, 91, 356–363. [Google Scholar] [CrossRef]
- Pimenov, N.V.; Nikolaev, Y.A.; Dorofeev, A.G.; Grachev, V.A.; Kallistova, A.Y.; Mironov, V.V.; Vanteeva, A.V.; Grigor’Eva, N.V.; Berestovskaya, Y.Y.; Gruzdev, E.V.; et al. Bioaugmentation of Anammox Activated Sludge with a Nitrifying Bacterial Community as a Way to Increase the Nitrogen Removal Efficiency. Microbiology 2022, 91, 133–142. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Q.; Wang, S.; Li, B.; Wang, Z.; Zhang, S.; Zhang, M.; Peng, Y. Characterization of EPS compositions and microbial community in an Anammox SBBR system treating landfill leachate. Bioresour. Technol. 2018, 249, 108–116. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Wu, P.; Ma, L.; Chen, J.; Wang, C.; Li, X.; Liu, W.; Xu, L. Hydroxylamine metabolism in mainstream denitrifying ammonium oxidation (DEAMOX) process: Achieving fast start-up and robust operation with bio-augmentation assistance under ambient temperature. J. Hazard. Mater. 2022, 421, 126736. [Google Scholar] [CrossRef]
- Wett, B.; Podmirseg, S.M.; Gómez-Brandón, M.; Hell, M.; Nyhuis, G.; Bott, C.; Murthy, S. Expanding DEMON sidestream deammonification technology towards mainstream application. Water Environ. Res. 2015, 87, 2084–2089. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, L.; Li, B.; Zhang, Q.; Wang, S.; Peng, Y. Enhancing ammonium oxidizing bacteria activity was key to single-stage partial nitrification-anammox system treating low-strength sewage under intermittent aeration condition. Bioresour. Technol. 2017, 231, 36–44. [Google Scholar] [CrossRef]
- Jin, R.C.; Zhang, Q.Q.; Zhang, Z.Z.; Liu, J.H.; Yang, B.E.; Guo, L.X.; Wang, H.Z. Bio-augmentation for mitigating the impact of transient oxytetracycline shock on anaerobic ammonium oxidation (ANAMMOX) performance. Bioresour. Technol. 2014, 163, 244–253. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Yang, G.F.; Sun, K.K.; Tian, G.M.; Jin, R.C. Insights into the effects of bio-augmentation on the granule-based anammox process under continuous oxytetracycline stress: Performance and microflora structure. Chem. Eng. J. 2018, 348, 503–513. [Google Scholar] [CrossRef]
- Zhu, W.; Van Tendeloo, M.; Alloul, A.; Vlaeminck, S.E. Towards mainstream partial nitritation/anammox in four seasons: Feasibility of bioaugmentation with stored summer sludge for winter anammox assistance. Bioresour. Technol. 2022, 347, 126619. [Google Scholar] [CrossRef]
- El Fantroussi, S.; Agathos, S.N. Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr. Opin. Microbiol. 2005, 8, 268–275. [Google Scholar] [CrossRef]
- Li, F.; Deng, D.; Zeng, L.; Abrams, S.; Li, M. Sequential anaerobic and aerobic bioaugmentation for commingled groundwater contamination of trichloroethene and 1, 4-dioxane. Sci. Total Environ. 2021, 774, 145118. [Google Scholar] [CrossRef]
- Michalsen, M.M.; King, A.S.; Istok, J.D.; Crocker, F.H.; Fuller, M.E.; Kucharzyk, K.H.; Gander, M.J. Spatially-distinct redox conditions and degradation rates following field-scale bioaugmentation for RDX-contaminated groundwater remediation. J. Hazard. Mater. 2020, 387, 121529. [Google Scholar] [CrossRef]
- Michalsen, M.M.; King, A.S.; Rule, R.A.; Fuller, M.E.; Hatzinger, P.B.; Condee, C.W.; Crocker, F.H.; Indest, K.J.; Jung, C.M.; Istok, J.D. Evaluation of biostimulation and bioaugmentation to stimulate hexahydro-1,3,5-trinitro-1,3,5,-triazine degradation in an aerobic groundwater aquifer. Environ. Sci. Technol. 2016, 50, 7625–7632. [Google Scholar] [CrossRef]
- Poi, G.; Shahsavari, E.; Aburto-Medina, A.; Mok, P.C.; Ball, A.S. Large scale treatment of total petroleum-hydrocarbon contaminated groundwater using bioaugmentation. J. Environ. Manag. 2018, 214, 157–163. [Google Scholar] [CrossRef]
- Demenev, A.; Maksimovich, N.; Khmurchik, V.; Rogovskiy, G.; Rogovskiy, A.; Baryshnikov, A. Field Test of In Situ Groundwater Treatment Applying Oxygen Diffusion and Bioaugmentation Methods in an Area with Sustained Total Petroleum Hydrocarbon (TPH) Contaminant Flow. Water 2022, 14, 192. [Google Scholar] [CrossRef]
- Dominika, G.; Joanna, M.; Jacek, M. Sulfate reducing ammonium oxidation (SULFAMMOX) process under anaerobic conditions. Environ. Technol. Innov. 2021, 22, 101416. [Google Scholar] [CrossRef]
- Safonov, A.V.; Andryushchenko, N.D.; Ivanov, P.V.; Boldyrev, K.A.; Babich, T.L.; German, K.E.; Zakharova, E.V. Biogenic factors of radionuclide immobilization on sandy rocks of upper aquifers. Radiochemistry 2019, 61, 99–108. [Google Scholar] [CrossRef]
- Opdyke, S.M.; Evans, J.C. Slag-Cement-Bentonite Slurry Walls. J. Geotech. Geoenviron. Eng. 2005, 131, 673–681. [Google Scholar] [CrossRef]
- Abramova, E.; Popova, N.; Artemiev, G.; Zharkova, V.; Zakharova, E.; Safonov, A. Characteristics and rates of microbial processes in clays of different mineral and elemental composition in relation to safety prediction for ESB clay materials. Appl. Sci. 2022, 12, 1843. [Google Scholar] [CrossRef]
- Gohl, D.M.; MacLean, A.; Hauge, A.; Becker, A.; Walek, D.; Beckman, K.B. An optimized protocol for high-throughput amplicon-based microbiome profiling. Protoc. Exch. 2016, 1–15. [Google Scholar] [CrossRef]
- Hugerth, L.W.; Wefer, H.A.; Lundin, S.; Jakobsson, H.E.; Lindberg, M.; Rodin, S.; Engstrand, L.; Andersson, A.F. DegePrime, a program for degenerate primer design for broad-taxonomic-range PCR in microbial ecology studies. Appl. Environ. Microbiol. 2014, 80, 5116–5123. [Google Scholar] [CrossRef]
- Merkel, A.Y.; Tarnovetskii, I.Y.; Podosokorskaya, O.A.; Toshchakov, S.V. Analysis of 16S rRNA Primer Systems for Profiling of Thermophilic Microbial Communities. Microbiology 2019, 88, 671–680. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. Dada2: High-resolution sample inference from illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 55, 143–169. [Google Scholar] [CrossRef]
- Botchkova, E.A.; Litti, Y.V.; Novikov, A.A.; Grouzdev, D.S.; Bochkareva, E.S.; Beskorovayny, A.V.; Kuznetsov, B.B.; Nozhevnikova, A.N. Description of “Candidatus Jettenia ecosi” sp. nov., a new species of anammox bacteria. Microbiology 2018, 87, 766–776. [Google Scholar] [CrossRef]
- Vishnyakova, A.V.; Litti, Y.V.; Botchkova, E.A.; Ermoshin, A.A.; Nozhevnikova, A.N. Changes in Relative Abundance of Microbial Groups Involved in Nitrogen Removal in the Anammox–Partial Nitrification Reactor System at Increase in Ammonium Nitrogen and COD Load. Microbiology 2020, 89, 205–211. [Google Scholar] [CrossRef]
- Rajta, A.; Bhatia, R.; Setia, H.; Pathania, P. Role of heterotrophic aerobic denitrifying bacteria in nitrate removal from wastewater. J. Appl. Microbiol. 2020, 128, 1261–1278. [Google Scholar] [CrossRef]
- Arisah, F.M.; Amir, A.F.; Ramli, N.; Ariffin, H.; Maeda, T.; Hassan, M.A.; Yusoff, M.Z.M. Bacterial Resistance against Heavy Metals in Pseudomonas aeruginosa RW9 Involving Hexavalent Chromium Removal. Sustainability 2021, 13, 9797. [Google Scholar] [CrossRef]
- Fazli, M.; Almblad, H.; Rybtke, M.L.; Givskov, M.; Eberl, L.; Tolker-Nielsen, T. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ. Microbiol. 2014, 16, 1961–1981. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, J.D. Dominance of Candidatus Scalindua species in anammox community revealed in soils with different duration of rice paddy cultivation in Northeast China. Appl. Microbiol. Biotechnol. 2013, 97, 1785–1798. [Google Scholar] [CrossRef] [PubMed]
- Lincy, J.; Manohar, C.S. 16S rRNA and hydrazine gene-based profiling of the Candidatus Scalindua community from the Arabian Sea hypoxic sediment. Curr. Sci. 2021, 120, 684–693. [Google Scholar] [CrossRef]
- Awata, T.; Oshiki, M.; Kindaichi, T.; Ozaki, N.; Ohashi, A.; Okabe, S. Physiological Characterization of an Anaerobic Ammonium-Oxidizing Bacterium Belonging to the “Candidatus Scalindua” Group. Appl. Environ. Microbiol. 2013, 79, 4145–4148. [Google Scholar] [CrossRef]
- Francis, C.A.; Obraztsova, A.Y.; Tebo, B.M. Dissimilatory metal reduction by the facultative anaerobe Pantoea agglomerans SP1. Appl. Environ. Microbiol. 2000, 66, 543. [Google Scholar] [CrossRef]
- Wu, Q.; Du, J.; Zhuang, G.; Jing, C. Bacillus sp. SXB and Pantoea sp. IMH, aerobic As(V)-reducing bacteria isolated from arsenic-contaminated soil. J. Appl. Microbiol. 2013, 114, 713–721. [Google Scholar] [CrossRef]
- Ahmed, B.; Cao, B.; McLean, J.S.; Ica, T.; Dohnalkova, A.; Istanbullu, O.; Paksoy, A.; Fredrickson, J.K.; Beyenal, H. Fe(III) reduction and U(VI) immobilization by Paenibacillus sp. strain 300A, isolated from Hanford 300A subsurface sediments. Appl. Environ. Microbiol. 2012, 78, 8001–8009. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef]
- Xie, J.-B.; Du, Z.; Bai, L.; Tian, C.; Zhang, Y.; Xie, J.-Y.; Wang, T.; Liu, X.; Chen, X.; Cheng, Q.; et al. Comparative genomic analysis of N2-fixing and non-N2-fixing Paenibacillus spp.: Organization, evolution and expression of the nitrogen fixation genes. PLoS Genet. 2014, 10, 1004231. [Google Scholar] [CrossRef]
- Xie, J.; Shi, H.; Du, Z.; Wang, T.; Liu, X.; Chen, S. Comparative genomic and functional analysis reveal conservation of plant growth promoting traits in Paenibacillus polymyxa and its closely related species. Sci. Rep. 2016, 6, 21329. [Google Scholar] [CrossRef] [PubMed]
- Vanparys, B.; Heylen, K.; Lebbe, L.; Vos, P.D. Devosia limi sp. nov., isolated from a nitrifying inoculums. Int. J. Syst. Evol. Microbiol. 2005, 55, 1997–2000. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Liu, B.; Braker, G. Isolation, genetic and functional characterization of novel soil nirK-type denitrifiers. Syst. Appl. Microbiol. 2010, 33, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.A.; Jung, S.J.; Phung, N.T.; Lee, J.; Chang, I.S.; Kim, B.H.; Yi, H.; Chun, J. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol. Lett. 2003, 223, 129–134. [Google Scholar] [CrossRef]
- Chen, M.; Wang, W.; Feng, Y.; Zhu, X.; Zhou, H.; Tan, Z.; Li, X. Impact resistance of different factors on ammonia removal by heterotrophic nitrification–aerobic denitrification bacterium Aeromonas sp. HN-02. Bioresour. Technol. 2014, 167, 456–461. [Google Scholar] [CrossRef]
- Mardanov, A.V.; Beletsky, A.V.; Ravin, N.V.; Botchkova, E.A.; Litti, Y.V.; Nozhevnikova, A.N. Genome of a Novel Bacterium “Candidatus Jettenia ecosi” reconstructed from the metagenome of an anammox bioreactor. Front. Microbiol. 2019, 10, 2442. [Google Scholar] [CrossRef]
- Kindaichi, T.; Yuri, S.; Ozaki, N.; Ohashi, A. Ecophysiological role and function of uncultured Chloroflexi in an anammox reactor. Water Sci. Technol. 2012, 66, 2556–2561. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y.; Guo, Y.; Zhao, M.; Wang, S. Illumina MiSeq sequencing reveals the key microorganisms involved in partial nitritation followed by simultaneous sludge fermentation, denitrification and anammox process. Bioresour. Technol. 2016, 207, 118–125. [Google Scholar] [CrossRef]
- Lawson, C.E.; Wu, S.; Bhattacharjee, A.S.; Hamilton, J.J.; Mcmahon, K.D.; Goel, R.; Noguera, D.R. Metabolic network analysis reveals microbial community interactions in anammox granules. Nat. Commun. 2017, 8, 15416. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, Q.; Wang, S.; Ji, J.; Zhang, Y.; Yang, M.; Hojo, T.; Li, Y.-Y. Upgrading of the symbiosis of Nitrosomanas and anammox bacteria in a novel single-stage partial nitritation–anammox system: Nitrogen removal potential and Microbial characterization. Bioresour. Technol. 2017, 244, 463–472. [Google Scholar] [CrossRef]
- Akaboci, T.R.; Gich, F.; Ruscalleda, M.; Balaguer, M.D.; Colprim, J. Assessment of operational conditions towards mainstream partial nitritation-anammox stability at moderate to low temperature: Reactor performance and bacterial community. Chem. Eng. J. 2018, 350, 192–200. [Google Scholar] [CrossRef]
- Dosta, J.; Vila, J.; Sancho, I.; Basset, N.; Grifoll, M.; Mata-Álvarez, J. Two-step partial nitritation/Anammox process in granulation reactors: Start-up operation and microbial characterization. J. Environ. Manag. 2015, 164, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, A.; Rodriguez-Sanchez, A.; Palazon, B.M.; Garcia-Ruiz, M.-J.; Osorio, F.; van Loosdrecht, M.; Gonzalez–Lopez, J. Microbial community analysis of a full-scale DEMON bioreactor. Bioprocess Biosyst. Eng. 2015, 38, 499–508. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, B.-J. Appropriate Fe (II) Addition Significantly Enhances Anaerobic Ammonium Oxidation (Anammox) Activity through Improving the Bacterial Growth Rate. Sci. Rep. 2015, 5, 8204. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Lv, Z.; Hu, C.; Yang, X.; Li, X. Nitrogen removal through different pathways in an aged refuse bioreactor treating mature landfill leachate. Appl. Microbiol. Biotechnol. 2013, 97, 9225–9234. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Wang, S.; Qiao, X.; Gong, X.; Gong, Q.; Liu, X.; Peng, Y. Recovering partial nitritation in a PN/A system during mainstream wastewater treatment by reviving AOB activity after thoroughly inhibiting AOB and NOB with free nitrous acid. Environ. Int. 2020, 139, 105684. [Google Scholar] [CrossRef]
- Jasmin, C.; Anas, A.; Tharakan, B.; Jaleel, A.; Puthiyaveettil, V.; Narayanane, S.; Lincy, J.; Nair, S. Diversity of sediment-associated Planctomycetes in the Arabian Sea oxygen minimum zone. J. Basic Microbiol. 2017, 57, 1010–1017. [Google Scholar] [CrossRef]
- Toro, E.E.R.-D.; Valenzuela, E.I.; López-Lozano, N.E.; Cortés-Martínez, M.G.; Sánchez-Rodríguez, M.A.; Calvario-Martínez, O.; Sánchez-Carrillo, S.; Cervantes, F.J. Anaerobic ammonium oxidation linked to sulfate and ferric iron reduction fuels nitrogen loss in marine sediments. Biodegradation 2018, 29, 429–442. [Google Scholar] [CrossRef]
- Lotti, T.; Kleerebezem, R.; Lubello, C.; Van Loosdrecht, M.C.M. Physiological and kinetic characterization of a suspended cell anammox culture. Water Res. 2014, 60, 1–14. [Google Scholar] [CrossRef]
- Güven, D.; van de Pas-Schoonen, K.; Schmid, M.C.; Strous, M.; Jetten, M.S.M.; Sözen, S.; Orhon, D.; Schmidt, I. Implementation of the Anammox process for improved nitrogen removal. J. Environ. Sci. Health 2004, 39, 1729–1738. [Google Scholar] [CrossRef]
- Padhy, S.R.; Bhattacharyya, P.; Dash, P.K.; Nayak, S.K.; Baig, M.J.; Swain, P.; Mohapatra, T. Soil Metagenome Revealed Contrasting Anammox Bacterial Diversity in Coastal Mangrove and Rice Ecology. Geomicrobiol. J. 2022, 39, 659–668. [Google Scholar] [CrossRef]
- Tian, R.; Ning, D.; He, Z.; Zhang, P.; Spencer, S.J.; Gao, S.; Shi, W.; Wu, L.; Zhang, Y.; Yang, Y.; et al. Small and mighty: Adaptation of superphylum Patescibacteria to groundwater environment drives their genome simplicity. Microbiome 2020, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Murugan, P.A.; Chinnasamy, H.V.; Singh, A.; Matheshwaran, S. Identification and Genome Analysis of an Arsenic-Metabolizing Strain of Citrobacter youngae IITK SM2 in Middle Indo-Gangetic Plain Groundwater. BioMed Res. Int. 2022, 2022, 6384742. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Xie, J.; Xie, J.; Chang, Y.; Guo, M.; Chen, C.; Zhang, T.C. The effect of carrier addition on Anammox start-up and microbial community: A review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 355–368. [Google Scholar] [CrossRef]
- Tian, X.; Schopf, A.; Amaral-Stewart, B.; Christensson, M.; Morgan-Sagastume, F.; Vincent, S.; Delatolla, R. Anammox attachment and biofilm development on surface-modified carriers with planktonic-and biofilm-based inoculation. Bioresour. Technol. 2020, 317, 124030. [Google Scholar] [CrossRef]
- Kim, Y.; Yu, J.; Jeong, S.; Kim, J.; Park, S.; Bae, H.; Rhee, S.-K.; Unno, T.; Ni, S.-Q.; Lee, T. Differences in the Effects of Calcium and Magnesium Ions on the Anammox Granular Properties to Alleviate Salinity Stress. Appl. Sci. 2021, 12, 19. [Google Scholar] [CrossRef]
- An, P.; Xu, X.; Yang, F.; Li, Z. Comparison of the characteristics of anammox granules of different sizes. Biotechnol. Bioprocess Eng. 2013, 18, 446–454. [Google Scholar] [CrossRef]
- Tang, S.-M.; Xu, Z.-H.; Liu, Y.-L.; Yang, G.-F.; Mu, J.; Jin, R.-C.; Yang, Q.; Zhang, X.-L. Performance, kinetics characteristics and enhancement mechanisms in anammox process under Fe (II) enhanced conditions. Biodegradation 2020, 31, 223–234. [Google Scholar] [CrossRef]
- Ferousi, C.; Lindhoud, S.; Baymann, F.; Kartal, B.; Jetten, M.S.; Reimann, J. Iron assimilation and utilization in anaerobic ammonium oxidizing bacteria. Curr. Opin. Chem. Biol. 2020, 37, 129–136. [Google Scholar] [CrossRef]
- Trigo, C.; Campos, J.L.; Garrido, J.M.; Mendez, R. Start-up of the Anammox process in a membrane bioreactor. J. Biotechnol. 2006, 126, 475–487. [Google Scholar] [CrossRef]
- Adams, M.; Xie, J.; Kabore, A.W.J.; Chang, Y.; Xie, J.; Guo, M.; Chen, C. Research advances in anammox granular sludge: A review. Crit. Rev. Environ. Sci. Technol. 2020, 52, 631–674. [Google Scholar] [CrossRef]
- Hreiz, R.; Latifi, M.A.; Roche, N. Optimal design and operation of activated sludge processes: State-of-the-art. Chem. Eng. J. 2015, 281, 900–920. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Cheng, Y.F.; Bai, Y.H.; Xu, J.J.; Shi, Z.J.; Shen, Y.Y.; Jin, R.C. Evaluating the effects of metal oxide nanoparticles (TiO2, Al2O3, SiO2 and CeO2) on anammox process: Performance, microflora and sludge properties. Bioresour. Technol. 2018, 266, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Mamo, M.; Taylor, R.W.; Shuford, J.W. Ammonium fixation by soil and pure clay minerals. Commun. Soil Sci. Plant Anal. 1993, 24, 1115–1126. [Google Scholar] [CrossRef]
- Ji, J.; Peng, Y.; Wang, B.; Li, X.; Zhang, Q. Synergistic partial-denitrification, anammox, and in-situ fermentation (SPDAF) process for advanced nitrogen removal from domestic and nitrate-containing wastewater. Environ. Sci. Technol. 2020, 54, 3702–3713. [Google Scholar] [CrossRef]
- You, Q.G.; Wang, J.H.; Qi, G.X.; Zhou, Y.M.; Guo, Z.W.; Shen, Y.; Gao, X. Anammox and partial denitrification coupling: A review. RSC Adv. 2020, 10, 12554–12572. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Ji, B.; Liu, Y. Towards mainstream deammonification of municipal wastewater: Partial nitrification-anammox versus partial denitrification-anammox. Sci. Total Environ. 2019, 692, 393–401. [Google Scholar] [CrossRef]
- Du, R.; Peng, Y.; Ji, J.; Shi, L.; Gao, R.; Li, X. Partial denitrification providing nitrite: Opportunities of extending application for anammox. Environ. Int. 2019, 131, 105001. [Google Scholar] [CrossRef]
- Chen, H.; Tu, Z.; Wu, S.; Yu, G.; Du, C.; Wang, H.; Yang, E.; Zhou, L.; Deng, B.; Wang, D.; et al. Recent advances in partial denitrifica-tion-anaerobic ammonium oxidation process for mainstream municipal wastewater treatment. Chemosphere 2021, 278, 130436. [Google Scholar] [CrossRef]
- Kouba, V.; Darmal, R.; Vejmelkova, D.; Jenicek, P.; Bartacek, J. Cold shocks of Anammox biofilm stimulate nitrogen removal at low temperatures. Biotechnol. Prog. 2018, 34, 277–281. [Google Scholar] [CrossRef]
- Kouba, V.; Gerlein, J.C.; Benakova, A.; Lopez Marin, M.A.; Rysava, E.; Vejmelkova, D.; Bartacek, J. Adaptation of flocculent anammox culture to low temperature by cold shock: Long-term response of the microbial population. Environ. Technol. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kouba, V.; Vejmelkova, D.; Zwolsman, E.; Hurkova, K.; Navratilova, K.; Laureni, M.; Vodickova, P.; Podzimek, T.; Hajslova, J.; Pabst, M.; et al. Adaptation of anammox bacteria to low temperature via gradual acclimation and cold shocks: Distinctions in protein expression, membrane composition and activities. Water Res. 2022, 209, 117822. [Google Scholar] [CrossRef]
- Safonov, A.; Popova, N.; Boldyrev, K.; Lavrinovich, E.; Boeva, N.; Artemiev, G.; Kuzovkina, E.; Emelyanov, A.; Myasnikov, I.; Zakharova, E.; et al. The microbial impact on U, Pu, Np, and Am immobilization on aquifer sandy rocks, collected at the deep LRW injection site. J. Geochem. Explor. 2022, 240, 107052. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Grozdov, D.; Vergel, K.; Popova, N.; Artemiev, G.; Safonov, A. Metal Removal from Nickel-Containing Effluents Using Mineral–Organic Hybrid Adsorbent. Materials 2020, 13, 4462. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Units | Value |
|---|---|---|
| NH4 | mg/L | 58.4 |
| NO2 | mg/L | 28.18 |
| NO3 | mg/L | 7434 |
| HCO3 | mg/L | 244 |
| K | mg/L | 447.6 |
| Na | mg/L | 879.7 |
| Mg | mg/L | 37.6 |
| Ca | mg/L | 1310 |
| Cl | mg/L | 1213 |
| SO4 | mg/L | 1803 |
| Fetot | mg/L | 2.1 |
| Mn | mg/L | 15.7 |
| PO4 | mg/L | 1.1 |
| U | µg/L | 256 |
| Corg | mg/L | 2.5 |
| pH | - | 7.1 |
| Eh | mV | 119 |
| T | °C | 7.5 |
| Abbreviation | The Nature of the Carrier | Sampling Site |
|---|---|---|
| Q | Quartz | Kola Peninsula |
| V | Expanded vermiculite | Provided by PLC “Primver” |
| Z1 | Zeolite (clinoptillolite) | Kholinskoye deposit, Khiloksky district, Trans-Baikal Territory |
| Z2 | Zeolite-containing tripoli | Hotynetskoye deposit, Oryol region |
| EC | Expanded clay | CJSC “Keramzit” Moscow Region, Serpukhov district |
| IL | Illite | Ulyanovsk region |
| KC1 | Kaolin clay | Kantatskiy deposit, Krasnoyarsk Territory |
| KC2 | Kaolin clay | Kamalinskoye deposit, Abakan, Republic of Khakassia |
| KC3 | Kaolin clay | Kompanovskoye deposit, Krasnoyarsk Territory |
| BC1 | Bentonite clay | Dinozavrovoye deposit, Republic of Kazakhstan |
| BC2 | Bentonite clay | Zyryanskoe deposit, Kurgan region |
| BC3 | Bentonite clay | Berezovskoye deposit, Republic of Tatarstan |
| BC4 | Bentonite clay | 10 Hutor deposit, Republic of Khakassia |
| S | River sand | Chernogolovka (Moscow region) |
| B2 rock | Rock of the upper (11 m) water-bearing horizon | JSC “Siberian Chemical Plant” Tomsk region, ZATO Seversk |
| CHMZ rock | Rock of the water-bearing horizon | JSC “Chepetsky Mechanical Plant” Glazov, Republic of Udmurtia |
| Abbreviation | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | MnO | Fe2O3 | P2O5 | Stot. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IL | 0.23 | 1.57 | 12.22 | 68.1 | 2.73 | 0.55 | 0.8 | 0.02 | 4.42 | 0.05 | 0.11 |
| KC1 | 0.68 | 1.11 | 19.71 | 62.0 | 1.3 | 1.81 | 1.01 | 0.04 | 4.54 | 0.08 | <0.02 |
| KC | 0.3 | 0.53 | 21.9 | 61.8 | 2.72 | 0.3 | 0.57 | 0.04 | 2.06 | 0.06 | <0.02 |
| KC3 | 0.1 | 0.98 | 19.45 | 63.2 | 1.57 | 0.68 | 0.96 | 0.02 | 2.29 | 0.05 | <0.02 |
| BC1 | 1.07 | 3.33 | 15.58 | 53.0 | 0.06 | 1.45 | 0.53 | 0.24 | 4.63 | 0.02 | 0.05 |
| BC2 | 0.57 | 1.73 | 16.22 | 49.7 | 0.94 | 3.93 | 0.8 | 0.07 | 5.94 | 0.04 | 0.08 |
| BC3 | 0.36 | 2.46 | 18.54 | 55.8 | 2.34 | 1.27 | 0.89 | 0.05 | 7.77 | 0.14 | 0.33 |
| BC4 | 0.91 | 2.73 | 16.94 | 56.2 | 0.85 | 2.05 | 0.69 | 0.03 | 3.36 | 0.12 | 0.04 |
| S | 0.46 | 0.17 | 2.55 | 94.9 | 0.6 | 0.22 | 0.08 | 0.003 | 0.44 | 0.03 | <0.02 |
| V | 0.56 | 19.8 | 10.5 | 40.3 | 4.5 | 1.01 | 0.48 | 0.245 | 12.5 | 0.22 | 0.28 |
| Z1 | 1.9 | 0.38 | 12.3 | 67.2 | 4 | 1.6 | 0.11 | 0.82 | 1 | 0.02 | <0.02 |
| Z2 | 0.1 | 1.4 | 8.5 | 70.1 | 1.4 | 2.1 | 0.54 | 0.14 | 4.3 | 0.22 | <0.02 |
| EC | 0.86 | 2.2 | 18.8 | 60.1 | 3.8 | 3.4 | 1 | 0.152 | 8.2 | 0.29 | 0.1 |
| B2 rock | 1.96 | 0.72 | 10.23 | 81.1 | 2.09 | 1.55 | 0.52 | 0.044 | 2.25 | 0.07 | <0.02 |
| CHMZ rock | 1.16 | 0.73 | 4.73 | 85.2 | 0.73 | 2.83 | 0.17 | 0.038 | 1.76 | 0.04 | <0.02 |
| Carrier Material | S * | NH4 * | N-NO2/N-NH4 * |
|---|---|---|---|
| Q | 1.5 | 0.8 | 4.7 |
| IL | 1 | 1.5 | 3.4 |
| KC1 | 2.1 | 0.9 | 1.5 |
| KC2 | 1.2 | 2.8 | 12.5 |
| KC3 | 1.1 | 1.6 | 2.1 |
| BC1 | 1.2 | 1.4 | 0.8 |
| BC2 | 1.6 | 0.9 | 0.2 |
| BC3 | 1.3 | 1.3 | 1.3 |
| BC4 | 1.2 | 1.3 | 0.4 |
| CHMZ rock | 1.1 | 0.2 | 1.2 |
| S | 4.7 | 1.1 | 8.4 |
| B2 rock | 0.7 | 7.7 | 1.3 |
| V | 0.7 | 1.2 | 0.1 |
| Z1 | 1.1 | 1 | 0.6 |
| Z2 | 1.1 | 1.1 | 0.6 |
| EC | 0.9 | 2.4 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnyakova, A.; Popova, N.; Artemiev, G.; Botchkova, E.; Litti, Y.; Safonov, A. Effect of Mineral Carriers on Biofilm Formation and Nitrogen Removal Activity by an Indigenous Anammox Community from Cold Groundwater Ecosystem Alone and Bioaugmented with Biomass from a “Warm” Anammox Reactor. Biology 2022, 11, 1421. https://doi.org/10.3390/biology11101421
Vishnyakova A, Popova N, Artemiev G, Botchkova E, Litti Y, Safonov A. Effect of Mineral Carriers on Biofilm Formation and Nitrogen Removal Activity by an Indigenous Anammox Community from Cold Groundwater Ecosystem Alone and Bioaugmented with Biomass from a “Warm” Anammox Reactor. Biology. 2022; 11(10):1421. https://doi.org/10.3390/biology11101421
Chicago/Turabian StyleVishnyakova, Anastasia, Nadezhda Popova, Grigoriy Artemiev, Ekaterina Botchkova, Yuriy Litti, and Alexey Safonov. 2022. "Effect of Mineral Carriers on Biofilm Formation and Nitrogen Removal Activity by an Indigenous Anammox Community from Cold Groundwater Ecosystem Alone and Bioaugmented with Biomass from a “Warm” Anammox Reactor" Biology 11, no. 10: 1421. https://doi.org/10.3390/biology11101421
APA StyleVishnyakova, A., Popova, N., Artemiev, G., Botchkova, E., Litti, Y., & Safonov, A. (2022). Effect of Mineral Carriers on Biofilm Formation and Nitrogen Removal Activity by an Indigenous Anammox Community from Cold Groundwater Ecosystem Alone and Bioaugmented with Biomass from a “Warm” Anammox Reactor. Biology, 11(10), 1421. https://doi.org/10.3390/biology11101421










