Isolation, Propagation and Genotyping of Human Rotaviruses Circulating among Children with Gastroenteritis in Two Egyptian University Hospitals
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
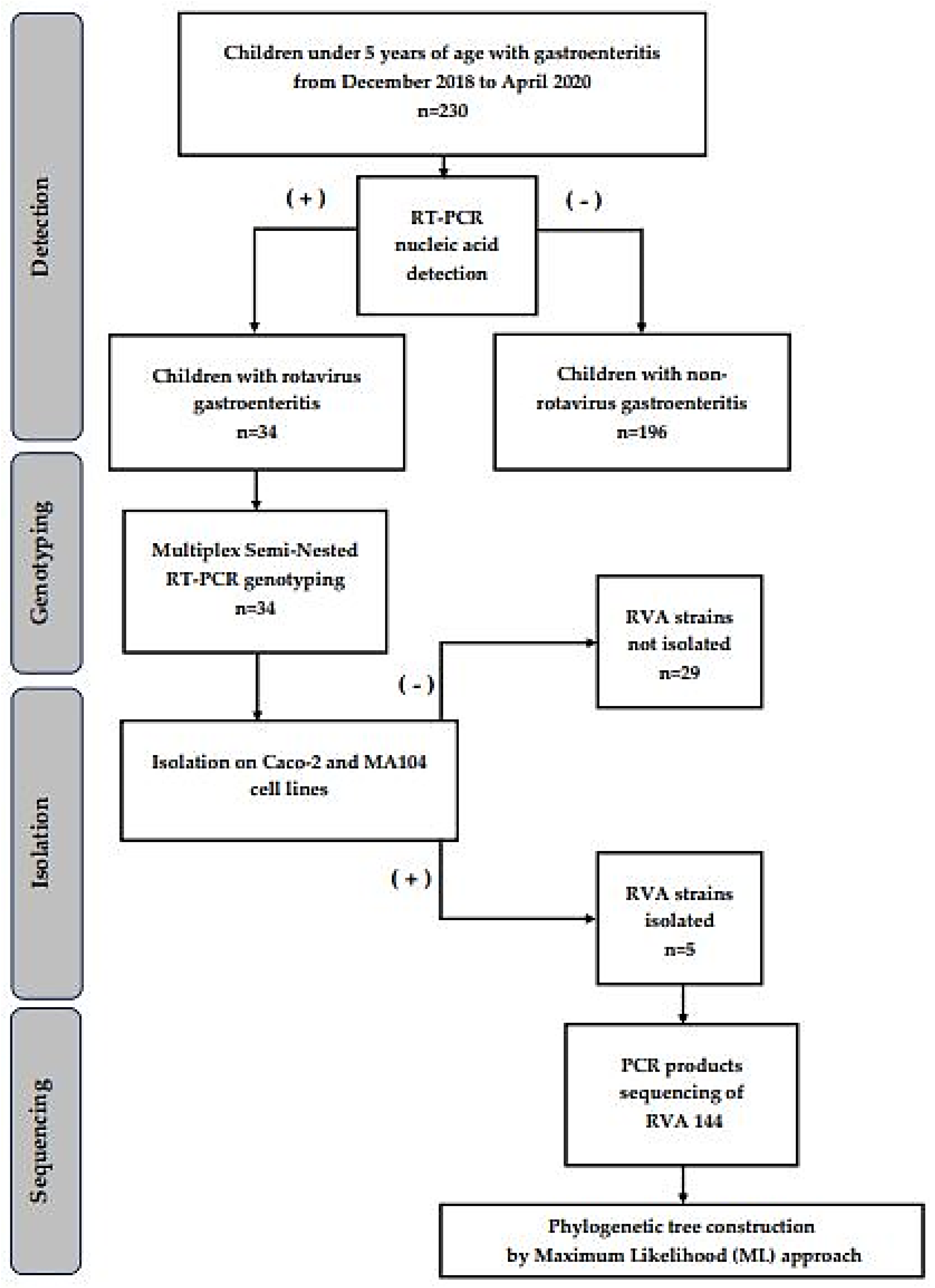
2.1. Specimen Collection and Study Design
2.2. Ethical Approval and Consent
2.3. Demographic Data Collection and Specimens Processing
2.4. Viral RNA Extraction
2.5. Molecular Detection of Rotaviruses Using VP6 Segment
2.6. Cell Lines and Positive Rotavirus Samples Isolation and Propagation
2.7. Molecular Typing of Positive Samples Using VP7 and VP4 Segments
2.8. PCR Products Sequencing
2.9. Nucleotide Sequence Accession Numbers and Phylogenetic Tree Construction
2.10. Statistical Analysis
3. Results
3.1. Virologic Detection of Rotaviruses in Clinical Samples
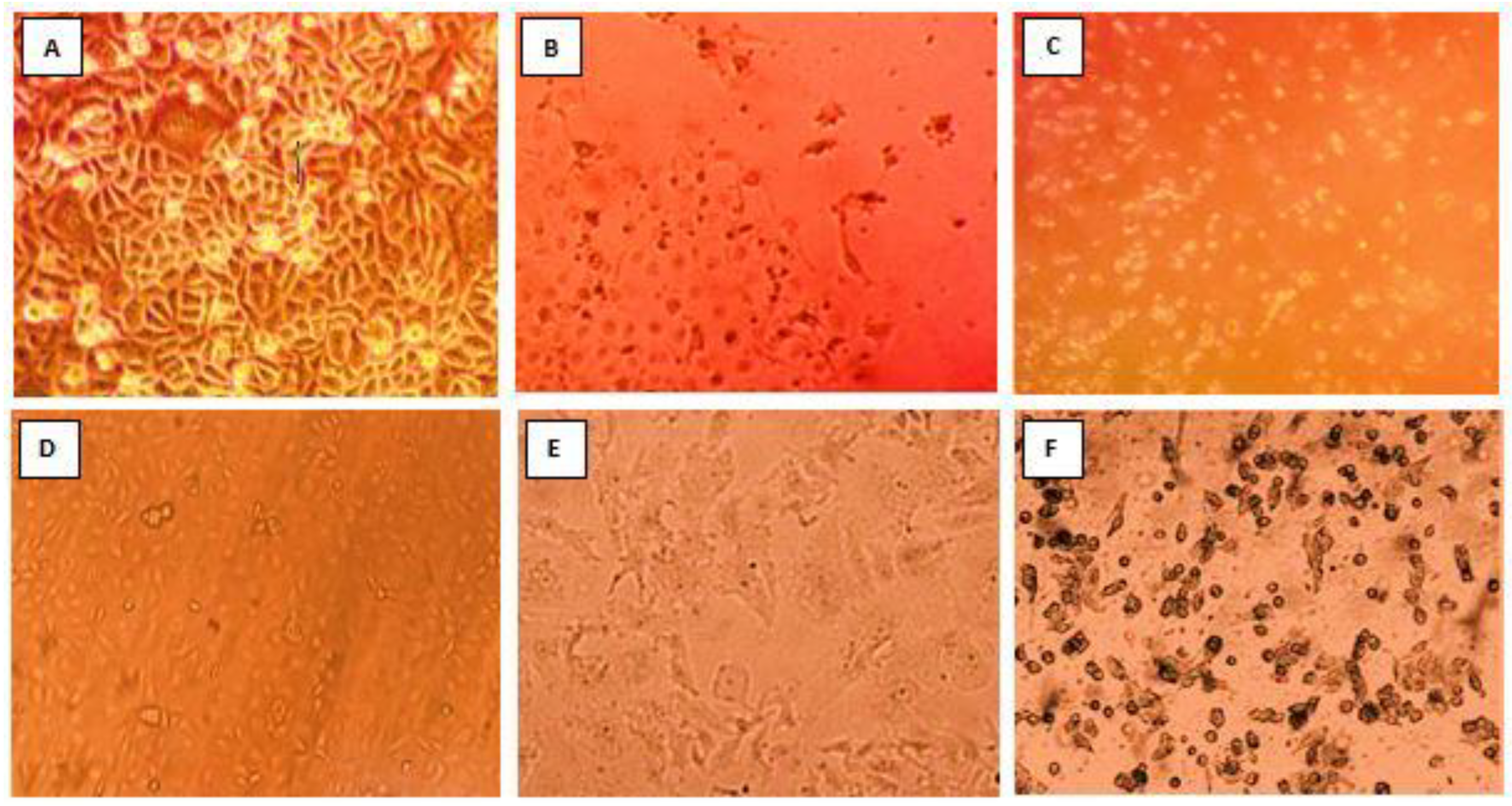
3.2. Isolation and Propagation of Detected Rotavirus Using Different Cell Lines
3.3. G/P Typing of the Isolated Rotavirus Strains
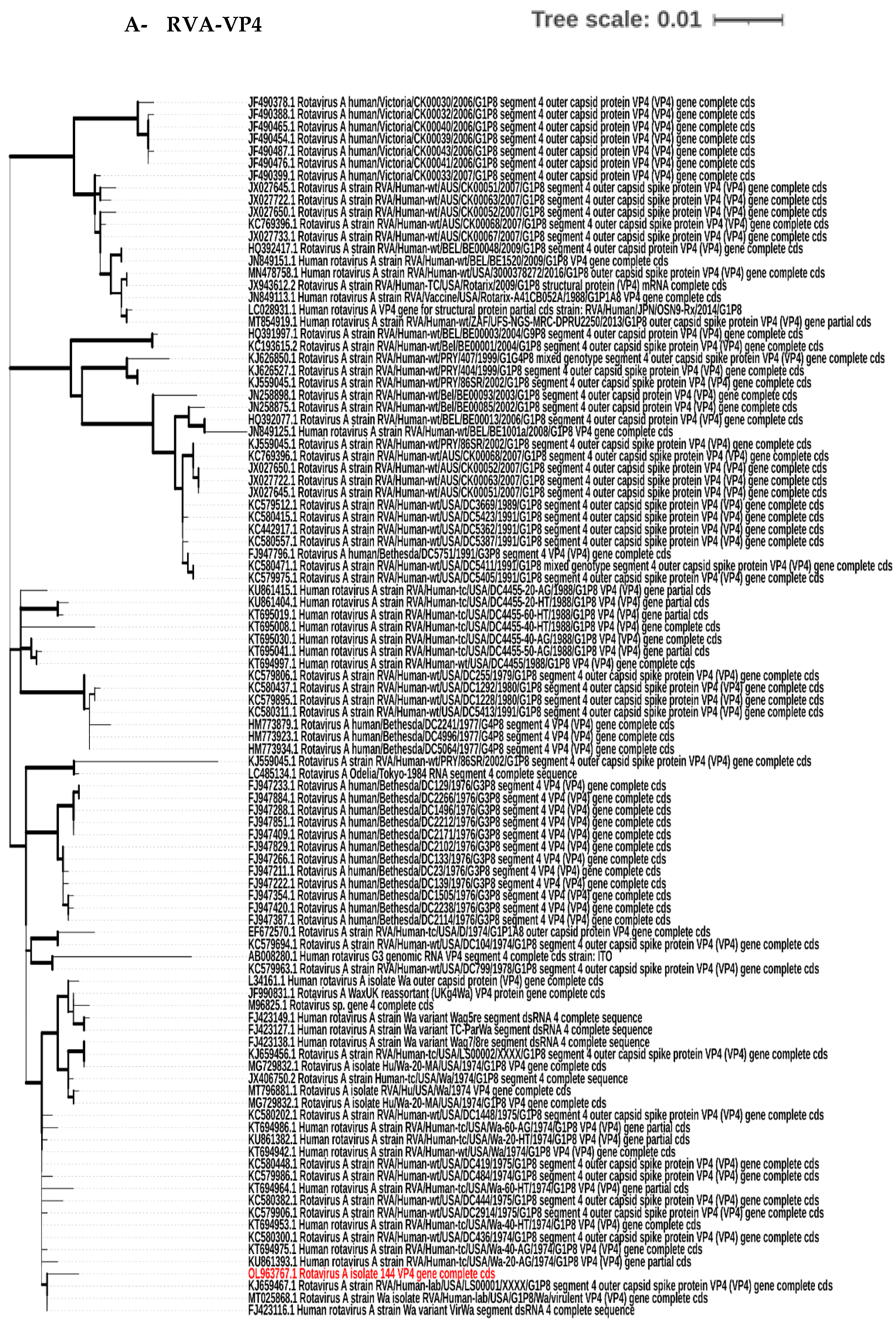
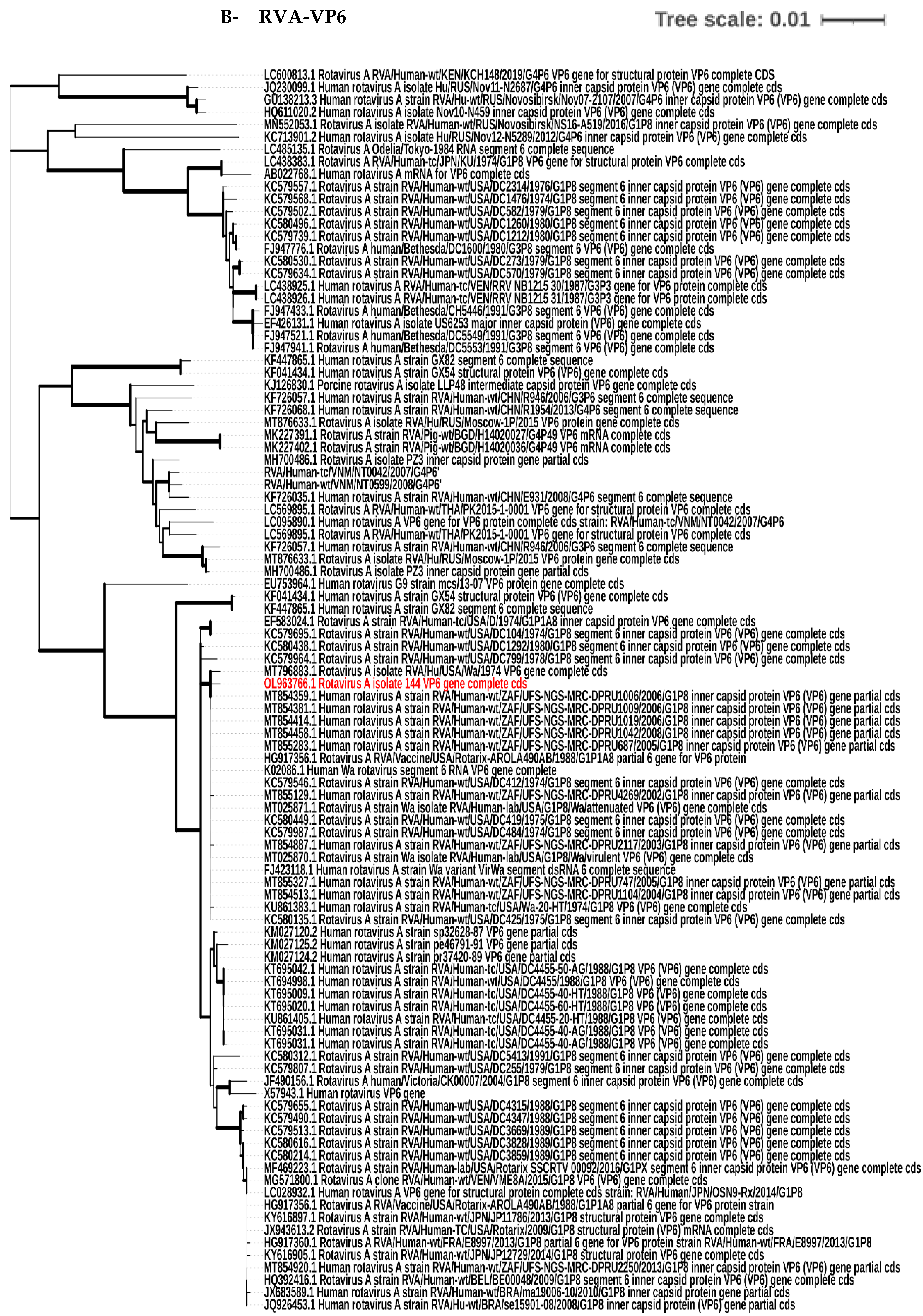
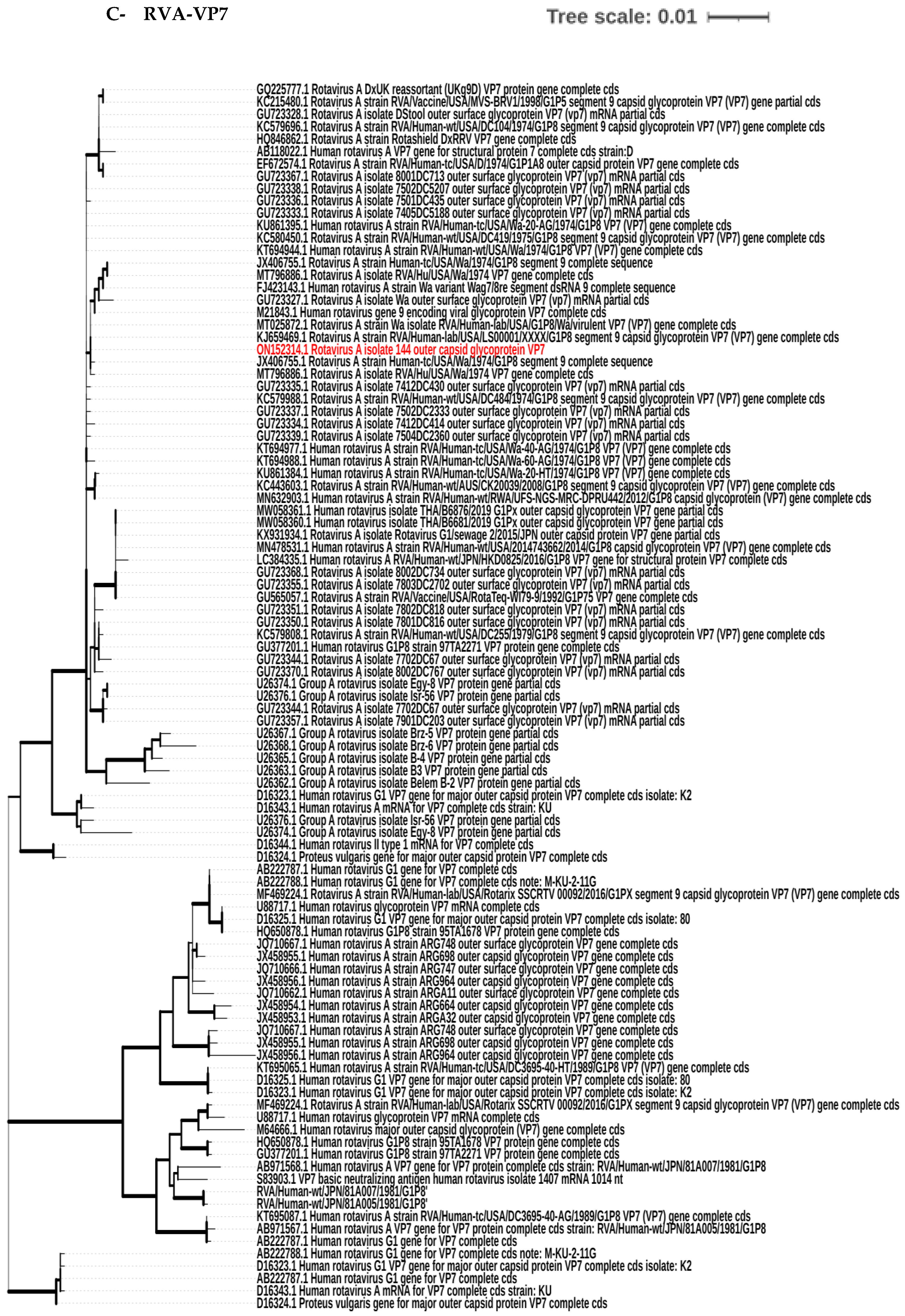
3.4. Phylogenetic Tree Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Prim. 2017, 3, 17083. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q. Medical Molecular Virology; Science Press: Beijing, China, 2001. [Google Scholar]
- Huang, W.L. Molecular Virology (for Postgraduate Use); People’s Medical Publishing House: Beijing, China, 2002. [Google Scholar]
- Chen, S.-M.; Ni, Y.-H.; Chen, H.-L.; Chang, M.-H. Microbial etiology of acute gastroenteritis in hospitalized children in Taiwan. J. Formos. Med. Assoc. 2006, 105, 964–970. [Google Scholar] [CrossRef]
- Parashar, U.D.; Hummelman, E.G.; Bresee, J.S.; Miller, M.A.; Glass, R.I. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 2003, 9, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.D.; Gibson, C.J.; Bresee, J.S.; Glass, R.I. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 2006, 12, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Bishop, R.F.; Davidson, G.P.; Holmes, I.H.; Ruck, B.J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet 1973, 2, 1281–1283. [Google Scholar] [CrossRef]
- Kalica, A.R.; Greenberg, H.B.; Wyatt, R.G.; Flores, J.; Sereno, M.M.; Kapikian, A.Z.; Chanock, R.M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology 1981, 112, 385–390. [Google Scholar] [CrossRef]
- Rotavirus Classification Working Group: RCWG. Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg (accessed on 10 September 2022).
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; Junglen, S.; et al. Changes to virus taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2019). Arch. Virol. 2019, 164, 2417–2429. [Google Scholar] [CrossRef]
- Santos, N.; Hoshino, Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol. 2005, 15, 29–56. [Google Scholar] [CrossRef]
- El-Senousy, W.M.; Abu Senna, A.S.M.; Mohsen, N.A.; Hasan, S.F.; Sidkey, N.M. Clinical and environmental surveillance of rotavirus common genotypes showed high prevalence of common P genotypes in Egypt. Food Environ. Virol. 2020, 12, 99–117. [Google Scholar] [CrossRef]
- Parashar, U.D.; Nelson, E.A.S.; Kang, G. Diagnosis, management, and prevention of rotavirus gastroenteritis in children. BMJ 2013, 347, f7204. [Google Scholar] [CrossRef]
- Cunliffe, N.A.; Witte, D.; Ngwira, B.M.; Todd, S.; Bostock, N.J.; Turner, A.M.; Chimpeni, P.; Victor, J.C.; Steele, A.D.; Bouckenooghe, A.; et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: A randomised, double-blind, placebo-controlled trial. Vaccine 2012, 30, A36–A43. [Google Scholar] [CrossRef] [PubMed]
- Varghese, T.; Kang, G.; Steele, A.D. Understanding rotavirus vaccine efficacy and effectiveness in countries with high child mortality. Vaccines 2022, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- WHO Prequalified Vaccines. WHO. Available online: https://extranet.who.int/gavi/PQ_Web/Default.aspx?nav=1 (accessed on 11 September 2022).
- International Vaccine Access Center (IVAC). Johns Hopkins Bloomberg School of Public Health. Available online: www.view-hub.org (accessed on 25 July 2022).
- Greenberg, H.B.; Estes, M.K. Rotaviruses: From pathogenesis to vaccination. Gastroenterology 2009, 136, 1939–1951. [Google Scholar] [CrossRef]
- Steele, A.D.; Neuzil, K.M.; Cunliffe, N.A.; Madhi, S.A.; Bos, P.; Ngwira, B.; Witte, D.; Todd, S.; Louw, C.; Kirsten, M.; et al. Human rotavirus vaccine RotarixTM provides protection against diverse circulating rotavirus strains in African infants: A randomized controlled trial. BMC Infect. Dis. 2012, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Widdowson, M.-A.; Steele, D.; Vojdani, J.; Wecker, J.; Parashar, U. Global rotavirus surveillance: Determining the need and measuring the impact of rotavirus vaccines. J. Infect. Dis. 2009, 200 (Suppl. 1), S1–S8. [Google Scholar] [CrossRef]
- Allayeh, A.; Elbaz, R.; Saeed, M.; Osman, M.E. Detection and genotyping of viral gastroenteritis in hospitalized children below five years old in Cairo, Egypt. Arch. Pediatr. Infect. Dis. 2018, 6, e60288. [Google Scholar]
- World Health Organization (WHO). Manual of Rotavirus Detection and Characterization Methods; WHO: Geneva, Switzerland, 2009.
- Matthijnssens, J.; Rahman, M.; Martella, V.; Xuelei, Y.; de Vos, S.; De Leener, K.; Ciarlet, M.; Buonavoglia, C.; Van Ranst, M. Full genomic analysis of human rotavirus strain B4106 and lapine rotavirus strain 30/96 provides evidence for interspecies transmission. J. Virol. 2006, 80, 3801–3810. [Google Scholar] [CrossRef]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Urasawa, T.; Urasawa, S.; Taniguchi, K. Sequential passages of human rotavirus in MA-104 cells. Microbiol. Immunol. 1981, 25, 1025–1035. [Google Scholar] [CrossRef]
- Feng, N.; Hu, L.; Ding, S.; Sanyal, M.; Zhao, B.; Sankaran, B.; Ramani, S.; McNeal, M.; Yasukawa, L.L.; Song, Y.; et al. Human VP8* mAbs neutralize rotavirus selectively in human intestinal epithelial cells. J. Clin. Investig. 2019, 129, 3839–3851. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, W. Isolation and characteristics of the human rotavirus isolate CY2017. Virus Dis. 2020, 31, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Babiuk, L.; Mohammed, K.; Spence, L.; Fauvel, M.; Petro, R. Rotavirus isolation and cultivation in the presence of trypsin. J. Clin. Microbiol. 1978, 6, 610–617. [Google Scholar] [CrossRef]
- Ennima, I.; Sebbar, G.; Harif, B.; Amzazi, S.; Loutfi, C.; Touil, N. Isolation and identification of group A rotaviruses among neonatal diarrheic calves, Morocco. BMC Res. Notes 2016, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.A. Methods for long-term virus preservation. Mol. Biotechnol. 1999, 13, 57–66. [Google Scholar] [CrossRef]
- Gouvea, V.; Glass, R.I.; Woods, P.; Taniguchi, K.; Clark, H.F.; Forrester, B.; Fang, Z.Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 1990, 28, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Gentsch, J.R.; Glass, R.I.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.K.; Bhan, M.K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Alkali, B.R.; Daneji, A.I.; Magaji, A.A.; Bilbis, L.S.; Bande, F. Molecular Characterization of Human Rotavirus from Children with Diarrhoeal Disease in Sokoto State, Nigeria. Mol. Biol. Int. 2016, 2016, 1876065. [Google Scholar] [CrossRef]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Oxford Academic: Oxford, UK, 1999. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Ahmed, S.F.; Mansour, A.M.; Klena, J.D.; Husain, T.S.; Hassan, K.A.; Mohamed, F.; Steele, D. Rotavirus genotypes associated with acute diarrhea in Egyptian infants. Pediatr. Infect. Dis. J. 2014, 33 (Suppl. 1), S62–S68. [Google Scholar] [CrossRef] [PubMed]
- Shoeib, A.R.S.; Hull, J.J.; Jiang, B. Rotavirus G and P types in children with acute diarrhea in Cairo, Egypt, 2011–2012. J. Egypt Public Health Assoc. 2015, 90, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.; B Barakat, A.; FM El-Sayed, A.; M El-Senousy, W.; EL-Farouk Rabia Elsayed, O. Molecular characterization of rotavirus Group A VP6 gene in Egyptian surface water, wastewater and diarrheal specimens. Egypt. J. Aquat. Biol. Fish. 2020, 24, 403–423. [Google Scholar] [CrossRef]
- Girish Kumar, C.P.; Giri, S.; Chawla-Sarkar, M.; Gopalkrishna, V.; Chitambar, S.D.; Ray, P.; Venkatasubramanian, S.; Borkakoty, B.; Roy, S.; Bhat, J.; et al. Epidemiology of rotavirus diarrhea among children less than 5 years hospitalized with acute gastroenteritis prior to rotavirus vaccine introduction in India. Vaccine 2020, 38, 8154–8160. [Google Scholar] [CrossRef]
- Chissaque, A.; Cassocera, M.; Gasparinho, C.; Langa, J.S.; Bauhofer, A.F.L.; Chilaúle, J.J.; João, E.D.; Munlela, B.A.; Sambo, J.A.M.; Boene, S.S.; et al. Rotavirus A infection in children under five years old with a double health problem: Undernutrition and diarrhoea, a cross-sectional study in four provinces of Mozambique. BMC Infect. Dis. 2021, 21, 18. [Google Scholar] [CrossRef]
- Abdulridha, A. Effects of age and gender on rotavirus infection among children in Iraq. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 701–709. [Google Scholar]
- El Sherif, M.; Esona, M.D.; Wang, Y.; Gentsch, J.R.; Jiang, B.; Glass, R.I.; Abou Baker, S.; Klena, J.D. Detection of the first G6P[14] human rotavirus strain from a child with diarrhea in Egypt. Infect. Genet. Evol. 2011, 11, 1436–1442. [Google Scholar] [CrossRef]
- Alaoui Amine, S.; Melloul, M.; El Alaoui, M.A.; Boulahyaoui, H.; Loutfi, C.; Touil, N.; El Fahime, E. Evidence for zoonotic transmission of species A rotavirus from goat and cattle in nomadic herds in Morocco, 2012–2014. Virus Genes 2020, 56, 582–593. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; Heiman, E.; Arijs, I.; Delbeke, T.; McDonald, S.M.; Palombo, E.A.; Iturriza-Gómara, M.; Maes, P.; Patton, J.T.; et al. Full genome-based classification of rotaviruses reveals a common origin between human Wa-like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008, 82, 3204–3219. [Google Scholar] [CrossRef]
- Isa, P.; Pérez-Delgado, A.; Quevedo, I.; López, S.; Arias, C. Rotaviruses associate with distinct types of extracellular vesicles. Viruses 2020, 12, 763. [Google Scholar] [CrossRef]
- Guerrero, C.A.; Guerrero, R.A.; Silva, E.; Acosta, O.; Barreto, E. Experimental adaptation of rotaviruses to tumor cell lines. PLoS ONE 2016, 11, e0147666. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.A.; Guerrero, C.A.; Guzmán, F.; Acosta, O. Assessing the oncolytic potential of rotavirus on mouse myeloma cell line Sp2/0-Ag14. Biomédica 2020, 40, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, F. Close relationship of Group A rotaviruses between bovine and human based on VP7 gene sequence in Egypt. Pak. Vet. J. 2014, 34, 391–393. [Google Scholar]
| Variable | RV Positive n = 34 N (%) | RV Negative n = 196 N (%) | p-Value | |
|---|---|---|---|---|
| Gender | Male | 23 (18.0) | 105 (82.0) | 0.130 |
| Female | 11 (10.8) | 91 (89.2) | ||
| Age | ≤1 year | 25 (28.0) | 64 (72.0) | <0.001 ** |
| >1 year–2 years | 8 (6.0) | 125 (94.0) | ||
| >2 years | 1 (12.5) | 7 (87.5) | ||
| Season | Autumn | 3 (7.3) | 38 (92.7) | 0.100 |
| Spring | 8 (15.1) | 45 (84.9) | ||
| Summer | 0 (0) | 9 (100) | ||
| Winter | 23 (18.1) | 104 (81.9) | ||
| Residence | Cairo | 15 (15.6) | 81 (84.4) | 0.300 |
| Giza | 17 (16.2) | 88 (83.8) | ||
| Others | 2 (6.9) | 27(93.1) | ||
| Cell Line | Passage Number | No. of RVA Strain(s) Isolated | RVA Strain (s) |
|---|---|---|---|
| MA104 | First passage | 0 | None |
| Second passage | 3 | RVA144, 113, 206 | |
| Third passage | 2 | RVA 111, 163 | |
| Caco-2 | First passage | 5 | RVA144, 113, 163, 111, 206 |
| Second passage | 5 | RVA144, 113, 163, 111, 206 | |
| Third passage | 5 | RVA144, 113, 163, 111, 206 |
| RVA Strain | MA104 | Caco-2 |
|---|---|---|
| 144 * | Complete | Complete |
| 113 | Partial | Complete |
| 206 | Complete | Partial |
| 111 | Partial | Partial |
| 163 | Partial | Partial |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gayar, M.H.; Saleh, S.E.; Mohamed, A.F.; Aboulwafa, M.M.; Hassouna, N.A.; Allayeh, A.K. Isolation, Propagation and Genotyping of Human Rotaviruses Circulating among Children with Gastroenteritis in Two Egyptian University Hospitals. Biology 2022, 11, 1413. https://doi.org/10.3390/biology11101413
El-Gayar MH, Saleh SE, Mohamed AF, Aboulwafa MM, Hassouna NA, Allayeh AK. Isolation, Propagation and Genotyping of Human Rotaviruses Circulating among Children with Gastroenteritis in Two Egyptian University Hospitals. Biology. 2022; 11(10):1413. https://doi.org/10.3390/biology11101413
Chicago/Turabian StyleEl-Gayar, Mona H., Sarra E. Saleh, Aly F. Mohamed, Mohammad M. Aboulwafa, Nadia A. Hassouna, and Abdou Kamal Allayeh. 2022. "Isolation, Propagation and Genotyping of Human Rotaviruses Circulating among Children with Gastroenteritis in Two Egyptian University Hospitals" Biology 11, no. 10: 1413. https://doi.org/10.3390/biology11101413
APA StyleEl-Gayar, M. H., Saleh, S. E., Mohamed, A. F., Aboulwafa, M. M., Hassouna, N. A., & Allayeh, A. K. (2022). Isolation, Propagation and Genotyping of Human Rotaviruses Circulating among Children with Gastroenteritis in Two Egyptian University Hospitals. Biology, 11(10), 1413. https://doi.org/10.3390/biology11101413






