The Prognostic Significance of Neutrophil to Lymphocyte Ratio (NLR), Monocyte to Lymphocyte Ratio (MLR) and Platelet to Lymphocyte Ratio (PLR) on Long-Term Survival in Off-Pump Coronary Artery Bypass Grafting (OPCAB) Procedures
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Outcomes
2.2. Statistical Analysis
3. Results
3.1. Univariable Analysis
3.2. Multivariable Analysis
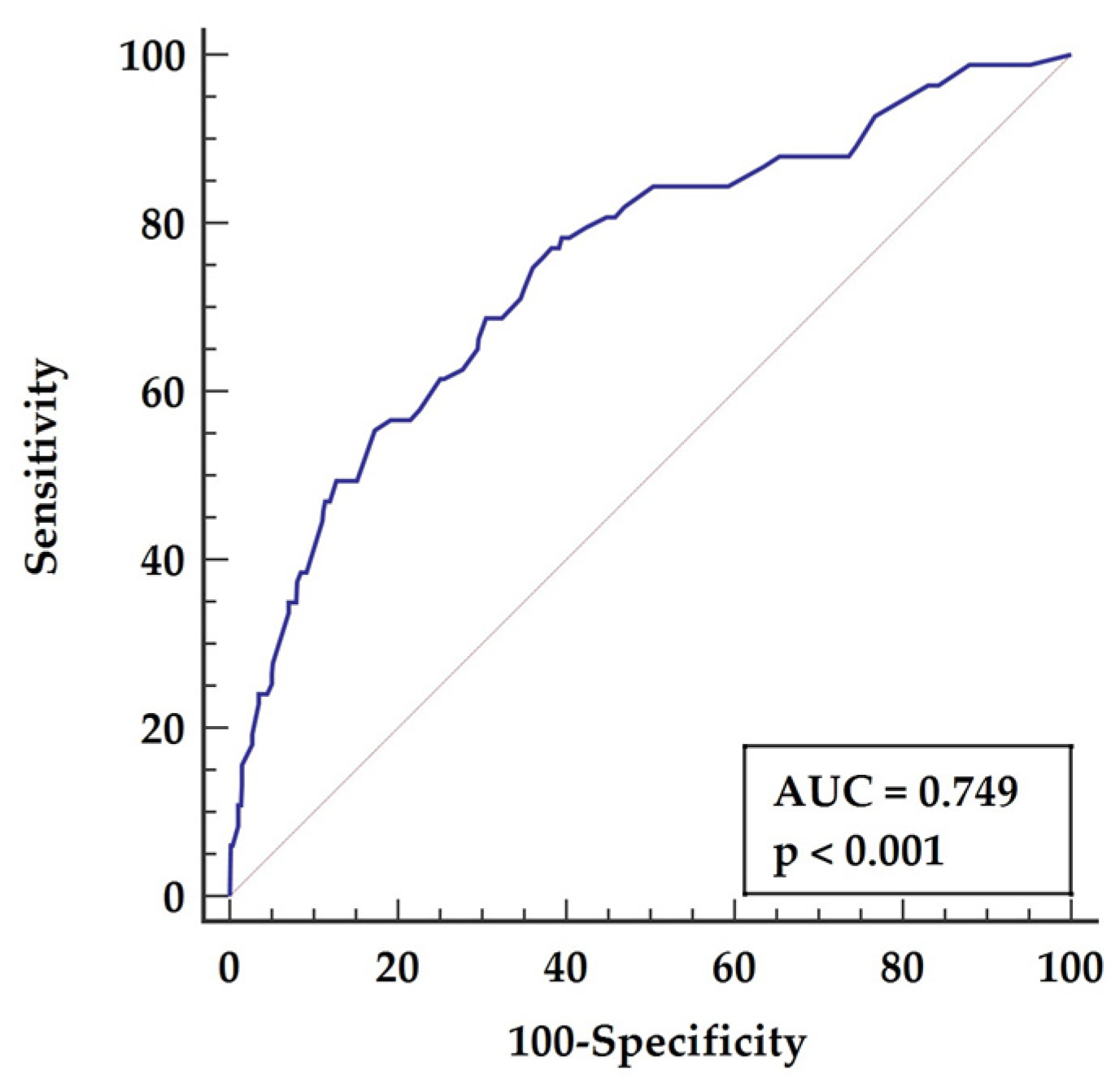
3.3. Receiver Operator Characteristics (ROC) Analysis
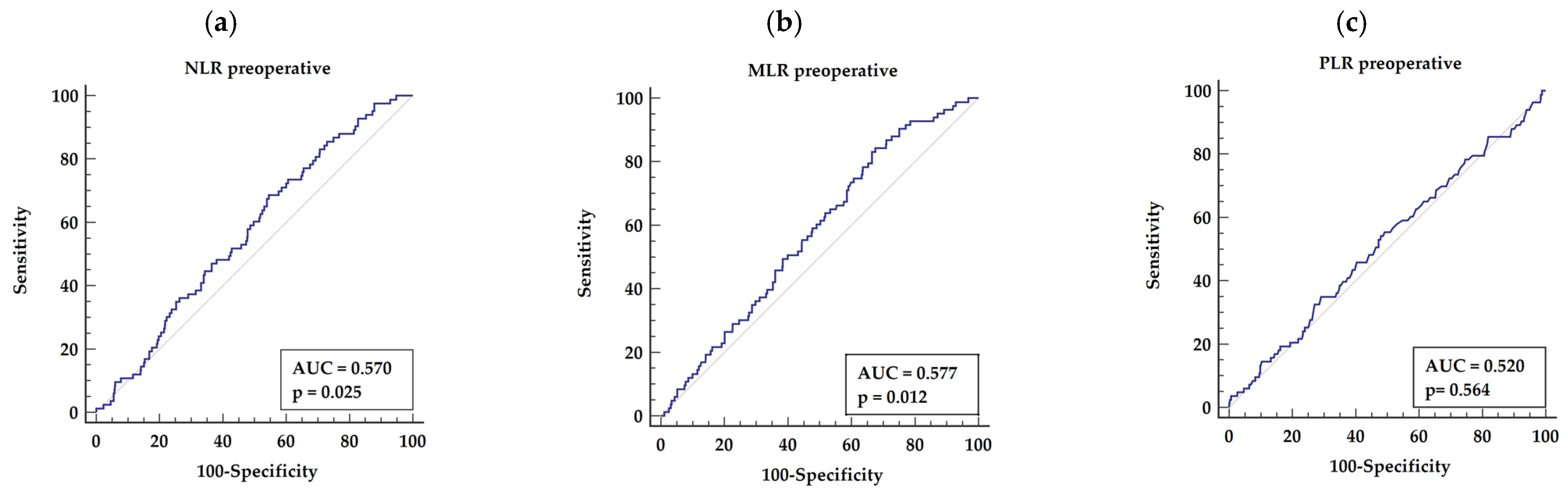
3.3.1. Preoperative NLR, MLR, and PLR
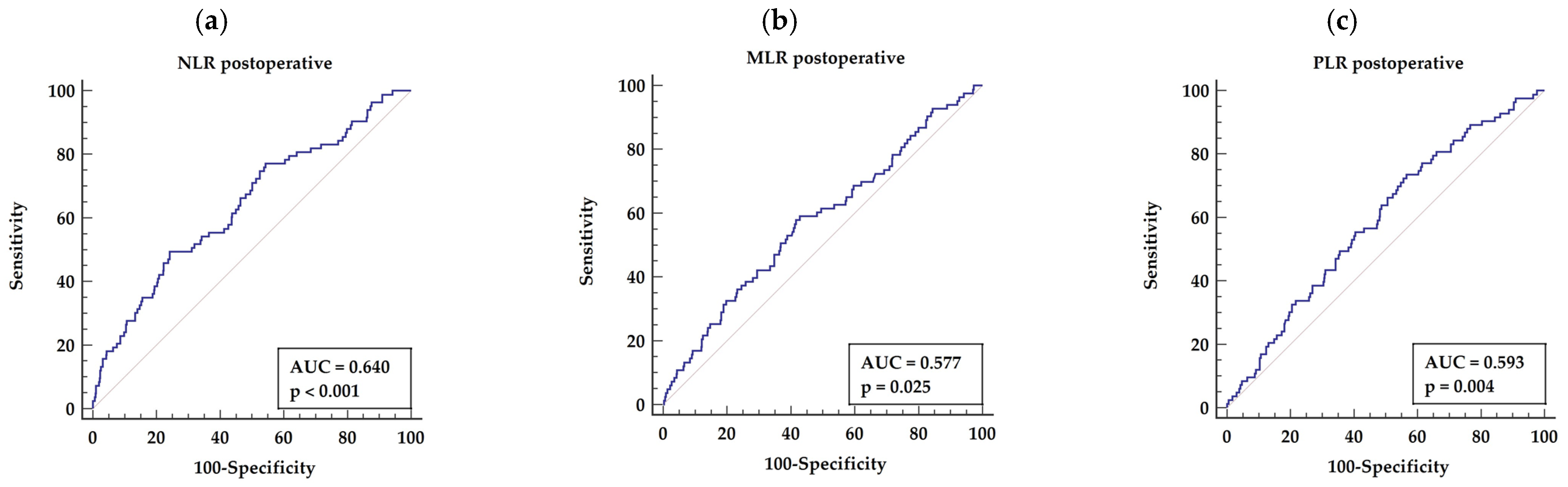
3.3.2. Postoperative NLR, MLR, and PLR
4. Discussion
4.1. Neutrophil to Lymphocyte Ratio (NLR)
4.2. Monocytes to Lymphocyte Ratio (MLR)
4.3. Platelets to Lymphocyte Ratio (PLR)
4.4. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef]
- Francula-Zaninovic, S.; Nola, I.A. Management of Measurable Variable Cardiovascular Disease’ Risk Factors. Curr. Cardiol. Rev. 2018, 14, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Moriya, J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019, 73, 22–27. [Google Scholar] [CrossRef]
- Tabas, I.; Bornfeldt, K.E. Intracellular and Intercellular Aspects of Macrophage Immunometabolism in Atherosclerosis. Circ. Res. 2020, 126, 1209–1227. [Google Scholar] [CrossRef]
- Mathis, D.; Shoelson, S.E. Immunometabolism: An emerging frontier. Nat. Rev. Immunol. 2011, 11, 81–86. [Google Scholar] [CrossRef]
- Adamstein, N.H.; MacFadyen, J.G.; Rose, L.M.; Glynn, R.J.; Dey, A.K.; Libby, P.; Tabas, I.A.; Mehta, N.N.; Ridker, P.M. The neutrophil-lymphocyte ratio and incident atherosclerotic events: Analyses from five contemporary randomized trials. Eur. Heart J. 2021, 42, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Fan, W.; Han, C.; Liu, J.; Sun, L. Atherogenic Index of Plasma, Triglyceride-Glucose Index and Monocyte-to-Lymphocyte Ratio for Predicting Subclinical Coronary Artery Disease. Am. J. Med. Sci. 2021, 362, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, M.; Yıldız, A.; Oylumlu, M.; Akyüz, A.; Aydın, M.; Kaya, H.; Acet, H.; Polat, N.; Bilik, M.Z.; Alan, S. The association between platelet/lymphocyte ratio and coronary artery disease severity. Anatol. J. Cardiol. 2015, 15, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Kose, N.; Akin, F.; Yildirim, T.; Ergun, G.; Altun, I. The association between the lymphocyte-to-monocyte ratio and coronary artery disease severity in patients with stable coronary artery disease. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2570–2575. [Google Scholar]
- Qiao, S.; Gao, W.; Guo, S. Neutrophil-Lymphocyte Ratio (NLR) for Predicting Clinical Outcomes in Patients with Coronary Artery Disease and Type 2 Diabetes Mellitus: A Propensity Score Matching Analysis. Ther. Clin. Risk Manag. 2020, 16, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Jiang, Y.; Jiang, X.; Yang, R.; Wu, Y.; Xu, Y.; Cheng, X. Relationship Between Platelet to Lymphocyte Ratio and Stable Coronary Artery Disease: Meta-Analysis of Observational Studies. Angiology 2020, 71, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Margraf, A.; Zarbock, A. Perioperative Inflammation [Perioperative inflammation]. Anaesthesist 2019, 68, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, J.H.; Forman, J.L.; Holm, L.M.; Kessel, L. Effect of anti-inflammatory regimen on early postoperative inflammation after cataract surgery. J. Cataract. Refract. Surg. 2021, 47, 323–330. [Google Scholar] [CrossRef]
- Finnerty, C.C.; Mabvuure, N.T.; Ali, A.; Kozar, R.A.; Herndon, D.N. The surgically induced stress response. JPEN J. Parenter. Enteral Nutr. 2013, 37, 21S–29S. [Google Scholar] [CrossRef]
- Forget, P.; Moreau, N.; Engel, H.; Cornu, O.; Boland, B.; De Kock, M.; Yombi, J.C. The neutrophil-to-lymphocyte ratio (NLR) after surgery for hip fracture (HF). Arch. Gerontol. Geriatr. 2015, 60, 366–371. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Fu, Z. Impact of enhanced recovery after surgery on postoperative neutrophil-lymphocyte ratio in patients with colorectal cancer. J. Int. Med. Res. 2020, 48, 300060520925941. [Google Scholar] [CrossRef]
- Bekki, H.; Arizono, T.; Hama, D.; Inokuchi, A.; Hamada, T.; Imamura, R. Association of Postoperative Neutrophil Lymphocyte Ratio (NLR) and Monocyte Lymphocyte Ratio (MLR) with the Presence of Osteoporosis in Japanese Patients after Hip Fracture Surgery: A Retrospective Cohort Study. J. Osteoporos. 2021, 2021, 5524069. [Google Scholar] [CrossRef]
- Cananzi, F.C.M.; Minerva, E.M.; Samà, L.; Ruspi, L.; Sicoli, F.; Conti, L.; Fumagalli Romario, U.; Quagliuolo, V.L. Preoperative monocyte-to-lymphocyte ratio predicts recurrence in gastrointestinal stromal tumors. J. Surg. Oncol. 2019, 119, 12–20. [Google Scholar] [CrossRef]
- Kang, Y.; Zhu, X.; Lin, Z.; Zeng, M.; Shi, P.; Cao, Y.; Chen, F. Compare the Diagnostic and Prognostic Value of MLR, NLR and PLR in CRC Patients. Clin. Lab. 2021, 67, 78–89. [Google Scholar] [CrossRef]
- Chen, J.H.; Zhai, E.T.; Yuan, Y.J.; Wu, K.M.; Xu, J.B.; Peng, J.J.; Chen, C.Q.; He, Y.L.; Cai, S.R. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol. 2017, 23, 6261–6272. [Google Scholar] [CrossRef]
- Schwartz, P.B.; Poultsides, G.; Roggin, K.; Howard, J.H.; Fields, R.C.; Clarke, C.N.; Votanopoulos, K.; Cardona, K.; Winslow, E.R. PLR and NLR Are Poor Predictors of Survival Outcomes in Sarcomas: A New Perspective From the USSC. J. Surg. Res. 2020, 251, 228–238. [Google Scholar] [CrossRef]
- Kirmani, B.H.; Guo, H.; Ahmadyur, O.; Bittar, M.N. Long-term survival following on-pump and off-pump coronary artery bypass graft surgery: A propensity score-matched analysis. Eur. J. Cardio-Thorac. Surg. 2018, 56, 1147–1153. [Google Scholar] [CrossRef]
- Napolitano, M.A.; Lee, K.B.; Rosenfeld, E.S.; Chen, S.W.; Sparks, A.D.; Nagy, C.D.; Greenberg, M.D.; Trachiotis, G.D. Long-Term Outcomes of Coronary Artery Bypass Grafting in Veterans with Ischemic Cardiomyopathy. Heart Surg. Forum 2020, 23, E323–E328. [Google Scholar] [CrossRef] [PubMed]
- Kirmani, B.H.; Holmes, M.V.; Muir, A.D. Long-Term Survival and Freedom from Reintervention After Off-Pump Coronary Artery Bypass Grafting: A Propensity-Matched Study. Circulation 2016, 134, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Haverich, A.; Serruys, P.; Bonow, R.O.; Kappetein, P.; Falk, V.; Velazquez, E.; Diegeler, A.; Sigusch, H. PCI and CABG for Treating Stable Coronary Artery Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 964–976. [Google Scholar] [CrossRef]
- Mei, Y.Q.; Ji, Q.; Liu, H.; Wang, X.; Feng, J.; Long, C.; Cheng, B.; Xing, Y.; Li, J.; Hu, D. Study on the relationship of APACHE III and levels of cytokines in patients with systemic inflammatory response syndrome after coronary artery bypass grafting. Biol. Pharm. Bull. 2007, 30, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Uyar, I.S.; Onal, S.; Uysal, A.; Ozdemir, U.; Burma, O.; Bulut, V. Evaluation of systemic inflammatory response in cardiovascular surgery via interleukin-6, interleukin-8, and neopterin. Heart Surg. Forum 2014, 17, E13–E17. [Google Scholar] [CrossRef]
- Rossaint, J.; Berger, C.; Van Aken, H.; Scheld, H.H.; Zahn, P.K.; Rukosujew, A.; Zarbock, A. Cardiopulmonary bypass during cardiac surgery modulates systemic inflammation by affecting different steps of the leukocyte recruitment cascade. PLoS ONE 2012, 7, e45738. [Google Scholar] [CrossRef]
- Møller, C.H.; Steinbrüchel, D.A. Off-pump versus on-pump coronary artery bypass grafting. Curr. Cardiol. Rep. 2014, 16, 455–462. [Google Scholar] [CrossRef]
- Matsuura, K.; Imamaki, M.; Ishida, A.; Shimura, H.; Fujita, H.; Niitsuma, Y.; Miyazaki, M. Low systemic vascular resistance state following off-pump coronary artery bypass grafting. Ann. Thorac. Cardiovasc. Surg. 2008, 14, 15–21. [Google Scholar]
- Akila D’souza, B.; Vishwanath, P.; D’souza, V. Oxidative injury and antioxidants in coronary artery bypass graft surgery: Off-pump CABG significantly reduces oxidative stress. Clin. Chim. Acta 2007, 375, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Hadem, J.; Rossnick, R.; Hesse, B.; Herr, M.; Hansen, M.; Bergmann, A.; Kensah, G.; Maess, C.; Baraki, H.; Kümpers, P.; et al. Endothelial dysfunction following coronary artery bypass grafting: Influence of patient and procedural factors. Herz 2020, 45, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Kerbaul, F.; Giorgi, R.; Oddoze, C.; Collart, F.; Guidon, C.; Lejeune, P.J.; Villacorta, J.; Gouin, F. High concentrations of N-BNP are related to non-infectious severe SIRS associated with cardiovascular dysfunction occurring after off-pump coronary artery surgery. Br. J. Anaesth. 2004, 93, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.T.; Milojevic, M.; Thuijs, D.; van Mieghem, N.M.; Daemen, J.; van Domburg, R.T.; Kappetein, A.P.; Head, S.J. Heart Team decision making and long-term outcomes for 1000 consecutive cases of coronary artery disease. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 206–213. [Google Scholar] [CrossRef]
- ISCHEMIA Trial Research Group; Maron, D.J.; Hochman, J.S.; O’Brien, S.M.; Reynolds, H.R.; Boden, W.E.; Stone, G.W.; Bangalore, S.; Spertus, J.A.; Mark, D.B.; et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am. Heart J. 2018, 201, 124–135. [Google Scholar]
- Nording, H.; Baron, L.; Langer, H.F. Platelets as therapeutic targets to prevent atherosclerosis. Atherosclerosis 2020, 307, 97–108. [Google Scholar] [CrossRef]
- Gómez-Moreno, D.; Adrover, J.M.; Hidalgo, A. Neutrophils as effectors of vascular inflammation. Eur. J. Clin. Investig. 2018, 48, e12940. [Google Scholar] [CrossRef]
- Döring, Y.; Soehnlein, O.; Weber, C. Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ. Res. 2017, 120, 736–743. [Google Scholar] [CrossRef]
- Darbousset, R.; Thomas, G.M.; Mezouar, S.; Frère, C.; Bonier, R.; Mackman, N.; Renné, T.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood 2012, 120, 2133–2143. [Google Scholar] [CrossRef]
- Lievens, D.; von Hundelshausen, P. Platelets in atherosclerosis. Thromb Haemost. 2011, 106, 827–838. [Google Scholar]
- Drechsler, M.; Megens, R.T.; van Zandvoort, M.; Weber, C.; Soehnlein, O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 2010, 122, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Pamukcu, B.; Lip, G.Y.; Devitt, A.; Griffiths, H.; Shantsila, E. The role of monocytes in atherosclerotic coronary artery disease. Ann. Med. 2010, 42, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Woollard, K.J.; Geissmann, F. Monocytes in atherosclerosis: Subsets and functions. Nat. Rev. Cardiol. 2010, 7, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Moroni, F.; Magnoni, M.; Camici, P.G. The role of T and B cells in human atherosclerosis and atherothrombosis. Clin. Exp. Immunol. 2015, 179, 173–187. [Google Scholar] [CrossRef]
- de Boer, O.J.; Becker, A.E.; van der Wal, A.C. T lymphocytes in atherogenesis-functional aspects and antigenic repertoire. Cardiovasc. Res. 2003, 60, 78–86. [Google Scholar] [CrossRef]
- Wigren, M.; Nilsson, J.; Kolbus, D. Lymphocytes in atherosclerosis. Clin. Chim. Acta 2012, 413, 1562–1568. [Google Scholar] [CrossRef]
- Núñez, J.; Miñana, G.; Bodí, V.; Núñez, E.; Sanchis, J.; Husser, O.; Llàcer, A. Low lymphocyte count and cardiovascular diseases. Curr. Med. Chem. 2011, 18, 3226–3233. [Google Scholar] [CrossRef]
- Weedle, R.C.; Da Costa, M.; Veerasingam, D.; Soo, A.W.S. The use of neutrophil lymphocyte ratio to predict complications post cardiac surgery. Ann. Transl. Med. 2019, 7, 778–788. [Google Scholar] [CrossRef]
- Ramlawi, B.; Otu, H.; Mieno, S.; Boodhwani, M.; Sodha, N.R.; Clements, R.T.; Bianchi, C.; Sellke, F.W. Oxidative stress and atrial fibrillation after cardiac surgery: A case-control study. Ann. Thorac. Surg. 2007, 84, 1166–1172. [Google Scholar] [CrossRef]
- Giakoumidakis, K.; Fotos, N.V.; Patelarou, A.; Theologou, S.; Argiriou, M.; Chatziefstratiou, A.A.; Katzilieri, C.; Brokalaki, H. Perioperative neutrophil to lymphocyte ratio as a predictor of poor cardiac surgery patient outcomes. Pragmat. Obs. Res. 2017, 8, 9–14. [Google Scholar] [CrossRef]
- Green, J.; Bin Mahmood, S.U.; Mori, M.; Yousef, S.; Mangi, A.A.; Geirsson, A. Stability across time of the neutrophil-lymphocyte and lymphocyte-neutrophil ratios and associations with outcomes in cardiac surgery patients. J. Cardiothorac. Surg. 2019, 14, 164–171. [Google Scholar] [CrossRef]
- Bath, J.; Smith, J.B.; Kruse, R.L.; Vogel, T.R. Association of neutrophil-to-lymphocyte ratio with outcomes after elective abdominal aortic aneurysm repair. J. Vasc. Nurs. 2019, 37, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Gawdat, K.; Legere, S.; Wong, C.; Myers, T.; Marshall, J.S.; Hassan, A.; Brunt, K.R.; Kienesberger, P.C.; Pulinilkunnil, T.; Legare, J.F. Changes in Circulating Monocyte Subsets (CD16 Expression) and Neutrophil-to-Lymphocyte Ratio Observed in Patients Undergoing Cardiac Surgery. Front. Cardiovasc. Med. 2017, 4, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Haran, C.; Gimpel, D.; Clark, H.; McCormack, D.J. Preoperative Neutrophil and Lymphocyte Ratio as a Predictor of Mortality and Morbidity After Cardiac Surgery. Heart Lung Circ. 2021, 30, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Silberman, S.; Abu-Yunis, U.; Tauber, R.; Shavit, L.; Grenader, T.; Fink, D.; Bitran, D.; Merin, O. Neutrophil-Lymphocyte Ratio: Prognostic Impact in Heart Surgery. Early Outcomes and Late Survival. Ann Thorac Surg. 2018, 105, 581–586. [Google Scholar] [CrossRef]
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1682–1690. [Google Scholar] [CrossRef]
- Paparella, D.; Parolari, A.; Rotunno, C.; Vincent, J.; Myasoedova, V.; Guida, P.; De Palo, M.; Margari, V.; Devereaux, P.J.; Lamy, A.; et al. The Effects of Steroids on Coagulation Dysfunction Induced by Cardiopulmonary Bypass: A Steroids in Cardiac Surgery (SIRS) Trial Substudy. Semin. Thorac. Cardiovasc. Surg. 2017, 29, 35–44. [Google Scholar] [CrossRef]
- Aldemir, M.; Baki, E.D.; Adali, F.; Çarşanba, G.; Tecer, E.; Taş, H.U. Comparison of neutrophil:lymphocyte ratios following coronary artery bypass surgery with or without cardiopulmonary bypass. Cardiovasc. J. Afr. 2015, 26, 159–164. [Google Scholar] [CrossRef][Green Version]
- Urbanowicz, T.; Michalak, M.; Gąsecka, A.; Perek, B.; Rodzki, M.; Bociański, M.; Straburzyńska-Migaj, E.; Jemielity, M. Postoperative Neutrophil to Lymphocyte Ratio as an Overall Mortality Midterm Prognostic Factor following OPCAB Procedures. Clin. Pract. 2021, 11, 587–597. [Google Scholar] [CrossRef]
- Urbanowicz, T.K.; Michalak, M.; Gąsecka, A.; Olasińska-Wiśniewska, A.; Perek, B.; Rodzki, M.; Bociański, M.; Jemielity, M. A Risk Score for Predicting Long-Term Mortality Following Off-Pump Coronary Artery Bypass Grafting. J. Clin. Med. 2021, 10, 3032. [Google Scholar] [CrossRef]
- Parlar, H.; Şaşkın, H. Are Pre and Postoperative Platelet to Lymphocyte Ratio and Neutrophil to Lymphocyte Ratio Associated with Early Postoperative AKI Following CABG? Braz. J. Cardiovasc. Surg. 2018, 33, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Taşoğlu, I.; Turak, O.; Nazli, Y.; Ozcan, F.; Colak, N.; Sahin, S.; Kavasoglu, K.; Genc, B.; Sert, D.; Karahan, M.; et al. Preoperative neutrophil-lymphocyte ratio and saphenous vein graft patency after coronary artery bypass grafting. Clin. Appl. Thromb Hemost. 2014, 20, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.O.; Caputo, M.; Angelini, G.D.; George, S.J.; Zakkar, M. Activation and inflammation of the venous endothelium in vein graft disease. Atherosclerosis 2017, 265, 266–274. [Google Scholar] [CrossRef]
- Baganha, F.; de Jong, A.; Jukema, J.W.; Quax, P.H.A.; de Vries, M.R. The Role of Immunomodulation in Vein Graft Remodeling and Failure. J. Cardiovasc. Transl. Res. 2021, 14, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Guida, G.; Ward, A.O.; Bruno, V.D.; George, S.J.; Caputo, M.; Angelini, G.D.; Zakkar, M. Saphenous vein graft disease, pathophysiology, prevention, and treatment. A review of the literature. J. Card Surg. 2020, 35, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Spartalis, M.; Spartalis, E.; Tzatzaki, E.; Tsilimigras, D.I.; Moris, D.; Kontogiannis, C.; Iliopoulos, D.C.; Voudris, V.; Siasos, G. Cardiac allograft vasculopathy after heart transplantation: Current prevention and treatment strategies. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 303–311. [Google Scholar]
- Jackson, S.M.; Perry, L.A.; Borg, C.; Ramson, D.M.; Campbell, R.; Liu, Z.; Nguyen, J.; Douglas, N.; Kok, J.; Penny-Dimri, J. Prognostic Significance of Preoperative Neutrophil-Lymphocyte Ratio in Vascular Surgery: Systematic Review and Meta-Analysis. Vasc. Endovasc. Surg. 2020, 54, 697–706. [Google Scholar] [CrossRef]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “Western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 2014, 14, 404–417. [Google Scholar] [CrossRef]
- Dominguez-Andres, J.; Netea, M.G. Long-term reprogramming of the innate immune system. J. Leukoc. Biol. 2019, 105, 329–338. [Google Scholar] [CrossRef]
- Swirski, F.K.; Pittet, M.J.; Kircher, M.F.; Aikawa, E.; Jaffer, F.A.; Libby, P.; Weissleder, R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc. Natl. Acad. Sci. USA 2006, 103, 10340–10345. [Google Scholar] [CrossRef]
- Nagareddy, P.R.; Murphy, A.J.; Stirzaker, R.A.; Hu, Y.; Yu, S.; Miller, R.G.; Ramkhelawon, B.; Distel, E.; Westerterp, M.; Huang, L.S.; et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013, 17, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, Y.; Fan, Z.; Zuo, B.; Jian, X.; Li, L.; Liu, T. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: A syntax score assessment. BMC Cardiovasc. Disord. 2017, 17, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Kramer, M.C.; Woudstra, P.; Yahagi, K.; Ladich, E.; Finn, A.V.; de Winter, R.J.; Kolodgie, F.D.; Wight, T.N.; Davis, H.R.; et al. Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study. Atherosclerosis 2015, 41, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liang, M.; Wu, H.; Huang, S.; Weng, R.; Hou, J.; Wu, Z. Preoperative Lymphocyte-to-Monocyte Ratio as a Prognostic Predictor of Long-Term Mortality in Cardiac Surgery Patients: A Propensity Score Matching Analysis. Front. Cardiovasc. Med. 2021, 8, 639890–639900. [Google Scholar] [CrossRef]
- Oksuz, F.; Elcik, D.; Yarlioglues, M.; Duran, M.; Ozturk, S.; Celik, I.E.; Kurtul, A.; Kilic, A.; Murat, S.N. The relationship between lymphocyte-to-monocyte ratio and saphenous vein graft patency in patients with coronary artery bypass graft. Biomark Med. 2017, 11, 867–876. [Google Scholar] [CrossRef]
- Cai, M.; Liang, D.; Gao, F.; Hong, X.; Feng, X.; Yang, Y.; Wu, S.; Huang, W. Association of lymphocyte-to-monocyte ratio with the long-term outcome after hospital discharge in patients with ST-elevation myocardial infarction: A retrospective cohort study. Coron Artery Dis. 2020, 31, 248–254. [Google Scholar] [CrossRef]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Seruga, B.; Ocana, A.; Tannock, I.F.; Amir, E. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev 2014, 23, 1204–1212. [Google Scholar] [CrossRef]
- Nikolsky, E.; Grines, C.L.; Cox, D.A.; Garcia, E.; Tcheng, J.E.; Sadeghi, M.; Mehran, R.; Lansky, A.J.; Na, Y.; Stone, G.W. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial). Am. J. Cardiol. 2007, 99, 1055–1061. [Google Scholar] [CrossRef]
- Iijima, R.; Ndrepepa, G.; Mehilli, J.; Bruskina, O.; Schulz, S.; Schömig, A.; Kastrati, A. Relationship between platelet count and 30-day clinical outcomes after percutaneous coronary interventions. Pooled analysis of four ISAR trials. Thromb Haemost. 2007, 98, 852–857. [Google Scholar]
- Efe, E.; Kocayiğit, I.; Türker, P.M.; Murat, K.; Erkan, A.; Sedat, T.; Alper, Ç.; Necati, A.M.; Gökhan, V.M.; Bahri, A. Platelet-to-lymphocyte ratio but not neutrophil-to-lymphocyte ratio predicts high on-treatment platelet reactivity in clopidogrel-treated patients with acute coronary syndrome. Indian J. Pharmacol. 2016, 48, 355–359. [Google Scholar] [CrossRef]
- Zouridakis, E.G.; Garcia-Moll, X.; Kaski, J.C. Usefulness of the blood lymphocyte count in predicting recurrent instability and death in patients with unstable angina pectoris. Am. J. Cardiol. 2000, 86, 449–451. [Google Scholar] [CrossRef]
- Akboga, M.K.; Canpolat, U.; Yayla, C.; Ozcan, F.; Ozeke, O.; Topaloglu, S.; Aras, D. Association of Platelet to Lymphocyte Ratio With Inflammation and Severity of Coronary Atherosclerosis in Patients With Stable Coronary Artery Disease. Angiology 2016, 67, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sari, I.; Sunbul, M.; Mammadov, C.; Durmus, E.; Bozbay, M.; Kivrak, T.; Gerin, F. Relation of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio with coronary artery disease severity in patients undergoing coronary angiography. Kardiol. Pol. 2015, 73, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, G.; Fan, Y.; Wan, Z.; Liu, X. Platelet to lymphocyte ratio is associated with the severity of coronary artery disease and clinical outcomes of percutaneous coronary intervention in the Chinese Han population. Exp. Ther. Med. 2017, 13, 731–738. [Google Scholar] [CrossRef]
- Açar, G.; Kalkan, M.E.; Avci, A.; Alizade, E.; Tabakci, M.M.; Toprak, C.; Özkan, B.; Alici, G.; Esen, A.M. The relation of platelet-lymphocyte ratio and coronary collateral circulation in patients with stable angina pectoris and chronic total occlusion. Clin. Appl. Thromb Hemost. 2015, 21, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Azab, B.; Shah, N.; Akerman, M.; McGinn, J.T., Jr. Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J. Thromb Thrombolysis. 2012, 34, 326–334. [Google Scholar] [CrossRef]
- Yildiz, A.; Yuksel, M.; Oylumlu, M.; Polat, N.; Akyuz, A.; Acet, H.; Aydin, M.; Ülgen, M.S. The Utility of the Platelet-Lymphocyte Ratio for Predicting No Reflow in Patients With ST-Segment Elevation Myocardial Infarction. Clin. Appl. Thromb Hemost. 2015, 21, 223–228. [Google Scholar] [CrossRef]
- Çiçek, G.; Açıkgoz, S.K.; Bozbay, M.; Altay, S.; Uğur, M.; Uluganyan, M.; Uyarel, H. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio combination can predict prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology 2015, 66, 441–447. [Google Scholar] [CrossRef]
- Osadnik, T.; Wasilewski, J.; Lekston, A.; Strzelczyk, J.; Kurek, A.; Gonera, M.; Gawlita, M.; Reguła, R.; Bujak, K.; Szyguła-Jurkiewicz, B.; et al. The platelet-to-lymphocyte ratio as a predictor of all-cause mortality in patients with coronary artery disease undergoing elective percutaneous coronary intervention and stent implantation. J. Saudi Heart Assoc. 2015, 27, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.G.; Baradi, A.; Peverelle, M.; Sultani, R.; Adams, H.; Garlick, J.; Wilson, A.M. Usefulness of Platelet-to-Lymphocyte Ratio to Predict Long-Term All-Cause Mortality in Patients at High Risk of Coronary Artery Disease Who Underwent Coronary Angiography. Am. J. Cardiol. 2018, 121, 1021–1026. [Google Scholar] [CrossRef]
- Cho, K.I.; Ann, S.H.; Singh, G.B.; Her, A.Y.; Shin, E.S. Combined Usefulness of the Platelet-to-Lymphocyte Ratio and the Neutrophil-to-Lymphocyte Ratio in Predicting the Long-Term Adverse Events in Patients Who Have Undergone Percutaneous Coronary Intervention with a Drug-Eluting Stent. PLoS ONE 2015, 10, e0133934. [Google Scholar]
- Wang, Y.; Peng, Z. Prognostic value of platelet/lymphocyte ratio and CAMI-STEMI score for major adverse cardiac events in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: A prospective observational study. Medicine 2021, 100, e26942. [Google Scholar] [CrossRef] [PubMed]
- Şaşkın, H.; Düzyol, Ç.; Özcan, K.S.; Aksoy, R.; Idiz, M. Preoperative Platelet to Lymphocyte Ratio Is Associated with Early Morbidity and Mortality after Coronary Artery Bypass Grafting. Heart Surg. Forum 2015, 18, E255–E262. [Google Scholar] [CrossRef]
- Engin, M. Are Pre and Postoperative Platelet to Lymphocyte Ratio and Neutrophil to Lymphocyte Ratio Associated with Early Postoperative AKI Following CABG? Braz. J. Cardiovasc. Surg. 2020, 35, 239. [Google Scholar] [CrossRef] [PubMed]
- Gungor, H.; Babu, A.S.; Zencir, C.; Akpek, M.; Selvi, M.; Erkan, M.H.; Durmaz, S. Association of Preoperative Platelet-to-Lymphocyte Ratio with Atrial Fibrillation after Coronary Artery Bypass Graft Surgery. Med. Princ. Pract. 2017, 26, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Parlar, H.; Arıkan, A.A.; Önmez, A. Dynamic Changes in Perioperative Cellular Inflammation and Acute Kidney Injury after Coronary Artery Bypass Grafting. Braz. J. Cardiovasc. Surg. 2021, 36, 354–364. [Google Scholar] [CrossRef]
- Navani, R.V.; Baradi, A.; Colin Huang, K.L.; Jin, D.; Jiao, Y.; Nguyen, J.K.; Ellis, Z.C.; Newcomb, A.E.; Wilson, A.M. Preoperative Platelet-to-Lymphocyte Ratio Is Not Associated with Postoperative Atrial Fibrillation. Ann. Thorac. Surg. 2020, 110, 1265–1270. [Google Scholar] [CrossRef]

| Parameters | Survivors | Deaths | p-Value |
|---|---|---|---|
| No = 598 (%) | No = 84 (%) | ||
| Demographical data | |||
| 1. Gender (M/F) | 480 (80%)/118 (20%) | 71 (84%)/13 (16%) | 0.589 |
| 2. Age (years) | 64 (59–70) | 67 (62–73) | 0.021 * |
| Comorbidities | |||
| 1. Arterial hypertension (n (%)) | 459 ((78%) | 71 (85%) | 0.109 |
| 2. Diabetes mellitus (n (%)) | 209 (35%) | 31 (37%) | 0.676 |
| 3. Hypercholesterolemia (n (%)) | 353 (59%) | 43 (51%) | 0.173 |
| 4. COPD (n (%)) | 45 (8%) | 18 (21%) | <0.001 * |
| 5. PAD (n (%)) | 84 (14%) | 18 (21%) | <0.001 * |
| 6. kidney failure (n (%)) | 33 (6%) | 5 (6%) | 0.768 |
| Echocardiography: | |||
| 1. preoperative LV diameter (%) | 48 (44–52) | 50 (45–54) | 0.006 * |
| 2. preoperative LVEF (%) | 55 (50–60) | 50 (45–60) | <0.001 * |
| Preoperative laboratory tests: | |||
| 1. WBC × 109/L (median (Q1–Q3)) | 7.7 (6.5–9.1) | 7.9 (6.8–9.2) | 0.657 |
| 2. Lymphocytes × 109/L (median (Q1–Q3)) | 1.8 (1.4–2.3) | 1.8 (1.4–2.0) | 0.196 |
| 3. Neutrophils × 109/L (median (Q1–Q3)) | 4.9 (4–6.2) | 5.2 (4.2–6.4) | 0.165 |
| 3. NLR (median (Q1–Q3)) | 2.7 (2–3.7) | 2.9 (2.3–4.0) | 0.039 * |
| 4. Hb × 109/L (median (Q1–Q3)) | 8.8 (8.2–9.3) | 8.6 (7.9–9.2) | 0.266 |
| 5. Platelets × 103/uL (median (Q1–Q3)) | 221 (189–266) | 228 (192–268) | 0.559 |
| 6. Monocytes × 109/L (median (Q1–Q3)) | 0.5 (0.4–0.6) | 0.5 (0.4–0.6) | 0.022 * |
| 7. MLR (median (Q1–Q3)) | 0.3 (0.2–0.3) | 0.3 (0.2–0.3) | <0.001 * |
| 8. MCHC (mmol/dL) (median (Q1–Q3)) | 21.2 (20.7–21.6) | 20.9 (20.5–21.3) | 0.378 |
| 9. PLR (mean +/− SD) | 134 +/− 63 | 148 +/− 79 | 0.105 |
| Parameters | Survivors | Deaths | p-Value |
|---|---|---|---|
| No = 598 | No = 83 | ||
| Surgical characteristics: | |||
| 1.number of grafts (n) (mean/SD) | 2.3 +/− 0.2 | 2.3 +/− 0.2 | 0.843 |
| 2. skin-to-skin time (h) (mean/SD) | 2.4 +/− 0.4 | 2.4 +/− 0.3 | 0.782 |
| Laboratory test results: | |||
| 1. WBC × 109/L (median (Q1–Q3)) | 8.4 (6.9–10.2) | 8.8 (7.4–10.7) | 0.118 |
| 2. Lymphocytes × 109/L (median (Q1–Q3)) | 1.9 (1.5–2.5) | 1.8 (1.3–2.2) | 0018 * |
| 3. Neutrophils × 109/L (median (Q1–Q3)) | 5 (3.7–6.4) | 5 (3.7–6.4) | 0.003 * |
| 3. NLR (median (Q1–Q3)) | 2.6 (1.8–3.5) | 5.6 (4.5–7.2) | <0.001 * |
| 4. Hb (mmol/dL) (median (Q1–Q3)) | 6.9 (6.5–7.3) | 6.9 (6.5–7.3) | 0.609 |
| 5. Platelets × 103/uL (median (Q1–Q3)) | 278 (230–343) | 290 (221–382) | 0.457 |
| 6. Monocytes × 109/L (median (Q1–Q3)) | 0.9 (0.7–1.1) | 0.9 (0.7–1.2) | 0.696 |
| 7. MLR (median (Q1–Q3)) | 0.5 (0.3–0.6) | 0.5 (0.4–0.7) | 0.023 * |
| 8. MCHC (mmol/dL) (median (Q1–Q3)) | 21.3 (20.8–21.7) | 21.1(20.7–21.6) | 0.006 * |
| 9. PLR (mean +/− SD) | 134 +/− 63 | 186 +/− 96 | 0.869 |
| 10. Troponin-I (median (Q1–Q3)) | 1.6 (0.8–3.8) | 1.8 (0.6–5.4) | 0.578 |
| Echocardiography: | |||
| 1. LV diameter (mm) (median (Q1–Q3)) | 47 (44–51) | 50 (46–55) | <0.001 * |
| 2. postoperative LVEF (%) (median (Q1–Q3)) | 60 (50–60) | 45 (40–55) | <0.001 * |
| Overall duration of hospitalization (days) (median (Q1–Q3)) | 12 (7–13) | 12 8–13) | p = 0.783 |
| Parameter | Cut-off Point | AUC | Sensitivity (%) | Specificity (%) | p-Value |
|---|---|---|---|---|---|
| Demographical: | |||||
| 1. Age | >62 years | 0.578 | 73.81 | 41.14 | p = 0.019 |
| Echocardiography: | |||||
| 1. Preoperative LV | LV > 49 mm | 0.594 | 51.81 | 63.16 | <0.005 |
| 2. Preoperative LVEF | LVEF <50% | 0.639 | 61.45 | 60.27 | <0.001 |
| 3. Postoperative LV | LV > 48 mm | 0.633 | 67.47 | 58.74 | <0.001 |
| 4. Postoperative LVEF | LVEF < 45% | 0.718 | 53.01 | 83.02 | <0.001 |
| Haematological indices: | |||||
| 1. Preoperative NLR | NLR >2.5 | 0.570 | 68.67 | 45.52 | 0.025 |
| 2. Postoperative NLR | NLR >3.5 | 0.640 | 49.40 | 75.80 | <0.001 |
| 3. Preoperative MLR | MLR >0.2 | 0.577 | 84.34 | 32.49 | 0.012 |
| 4. Postoperative MLR | MLR> 0.49 | 0.577 | 84.34 | 57.19 | 0.025 |
| 5. Preoperative MCHC | MCHC < 21.1 | 0.613 | 59.04 | 56.80 | <0.001 |
| 6. Postoperative MCHC | MCHC < 21.2 | 0.581 | 65.48 | 60.10 | 0.016 |
| 7. Postoperative PLR | PLR >136 | 0.593 | 55.95 | 57.79 | 0.039 |
| Parameter | HR | 95% CI | p-Value |
|---|---|---|---|
| Demographical and clinical: | |||
| 1. Age | 1.04 | 1.01–1.07 | 0.006 |
| 2. Age >62 years | 1.97 | 1.19–3.24 | 0.007 |
| 3. COPD | 2.77 | 1.64–4.68 | <0.001 |
| 4.PAD | 2.36 | 1.47–3.79 | <0.001 |
| Preoperative parameters: | |||
| 1. WBC | 1 | 0.93–1.07 | 0.984 |
| 2. NLR > 2.5 | 1.75 | 1.09–2.79 | 0.019 |
| 3. MLR > 0.2 | 2.46 | 1.33–4.55 | 0.004 |
| 4. MCHC | 0.67 | 0.47–0.94 | 0.021 |
| 5. MCHC > 21.1 | 1.05–2.65 | 0.028 | |
| 6. PLR | 1.01 | 1.00–1.01 | 0.039 |
| Postoperative parameters: | |||
| 1. WBC | 1.05 | 1.02–1.08 | 0.004 |
| 2. Lymphocytes | 0.67 | 0.47–0.94 | 0.02 |
| 3. Lymphocytes < 2.6 | 2.34 | 1.08–5.08 | 0.032 |
| 4. Neutrophils | 1.12 | 1.07–1.16 | <0.001 |
| 5. Neutrophils > 5.3 | 1.92 | 1.23–3.00 | 0.004 |
| 6. NLR | 1.16 | 1.10–1.22 | <0.001 |
| 7. NLR > 3.5 | 2.66 | 1.72–4.12 | <0.001 |
| 8. MLR > 0.49 | 1.78 | 1.13–2.78 | 0.012 |
| 9. PLR | 1 | 1.00–1.01 | 0.005 |
| 10. PLR > 136 | 2.18 | 1.31–3.62 | 0.003 |
| Echocardiography: | |||
| 1. Preoperative LV | 1.04 | 1.01–1.07 | 0.21 |
| 2. Preoperative LV > 49 mm | 1.53 | 0.98–2.38 | 0.058 |
| 3. Preoperative LVEF | 0.96 | 0.94–0.98 | <0.001 |
| 4. LVEF < 50% | 2.31 | 1.47–3.61 | <0.001 |
| 5. Postoperative LV | 1.06 | 1.03–1.09 | <0.001 |
| 6. Postoperative LV > 48 mm | 2.41 | 1.50–3.87 | <0.001 |
| 7. Postoperative LVEF | 0.94 | 0.93–0.96 | <0.001 |
| 8. Postoperative LVEF < 45% | 3.49 | 2.21–5.52 | <0.001 |
| Parameter | HR | 95% CI | p-Value |
|---|---|---|---|
| Demographical and clinical: | |||
| 1. Age above 62 years | 1.75 | 1.05–2.91 | 0.03 |
| 2. COPD | 3.01 | 1.74–5.24 | 0 |
| Laboratory parameters: | |||
| 1. Postoperative WBC | 1.05 | 0.93–1.07 | 0.984 |
| 2. Postoperative NLR > 3.5 | 1.75 | 1.09–2.79 | 0.018 |
| 3. Preoperative MLR > 0.2 | 1.98 | 1.05–3.68 | 0.034 |
| Echocardiography: | |||
| 1. Preoperative LV > 49 mm | 0.46 | 0.24–0.85 | 0.014 |
| 2. Postoperative LV > 48 mm | 2.53 | 1.29–4.95 | 0.007 |
| 3. Postoperative LVEF | 0.95 | 0.93–0.97 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanowicz, T.; Olasińska-Wiśniewska, A.; Michalak, M.; Rodzki, M.; Witkowska, A.; Straburzyńska-Migaj, E.; Perek, B.; Jemielity, M. The Prognostic Significance of Neutrophil to Lymphocyte Ratio (NLR), Monocyte to Lymphocyte Ratio (MLR) and Platelet to Lymphocyte Ratio (PLR) on Long-Term Survival in Off-Pump Coronary Artery Bypass Grafting (OPCAB) Procedures. Biology 2022, 11, 34. https://doi.org/10.3390/biology11010034
Urbanowicz T, Olasińska-Wiśniewska A, Michalak M, Rodzki M, Witkowska A, Straburzyńska-Migaj E, Perek B, Jemielity M. The Prognostic Significance of Neutrophil to Lymphocyte Ratio (NLR), Monocyte to Lymphocyte Ratio (MLR) and Platelet to Lymphocyte Ratio (PLR) on Long-Term Survival in Off-Pump Coronary Artery Bypass Grafting (OPCAB) Procedures. Biology. 2022; 11(1):34. https://doi.org/10.3390/biology11010034
Chicago/Turabian StyleUrbanowicz, Tomasz, Anna Olasińska-Wiśniewska, Michał Michalak, Michał Rodzki, Anna Witkowska, Ewa Straburzyńska-Migaj, Bartłomiej Perek, and Marek Jemielity. 2022. "The Prognostic Significance of Neutrophil to Lymphocyte Ratio (NLR), Monocyte to Lymphocyte Ratio (MLR) and Platelet to Lymphocyte Ratio (PLR) on Long-Term Survival in Off-Pump Coronary Artery Bypass Grafting (OPCAB) Procedures" Biology 11, no. 1: 34. https://doi.org/10.3390/biology11010034
APA StyleUrbanowicz, T., Olasińska-Wiśniewska, A., Michalak, M., Rodzki, M., Witkowska, A., Straburzyńska-Migaj, E., Perek, B., & Jemielity, M. (2022). The Prognostic Significance of Neutrophil to Lymphocyte Ratio (NLR), Monocyte to Lymphocyte Ratio (MLR) and Platelet to Lymphocyte Ratio (PLR) on Long-Term Survival in Off-Pump Coronary Artery Bypass Grafting (OPCAB) Procedures. Biology, 11(1), 34. https://doi.org/10.3390/biology11010034






