Aging as a Risk Factor on the Immunoexpression of Pro-Inflammatory IL-1β, IL-6 and TNF-α Cytokines in Chronic Apical Periodontitis Lesions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Study Population
2.3. Sample Collection and Processing
2.4. Histological Process
2.5. Biomarker Analysis
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Biomarker Levels at Study Time Point
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tibúrcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The Global Prevalence of Apical Periodontitis: A Systematic Review and Meta-Analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef]
- Ricucci, D.; Lopes, W.S.P.; Loghin, S.; Rôças, I.N.; Siqueira, J.F. Large Bacterial Floc Causing an Independent Extraradicular Infection and Posttreatment Apical Periodontitis: A Case Report. J. Endod. 2018, 44, 1308–1316. [Google Scholar] [CrossRef]
- Laukkanen, E.; Vehkalahti, M.M.; Kotiranta, A.K. Impact of Systemic Diseases and Tooth-Based Factors on Outcome of Root Canal Treatment. Int. Endod. J. 2019, 52, 1417–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminoshariae, A.; Kulild, J.C.; Mickel, A.; Fouad, A.F. Association between Systemic Diseases and Endodontic Outcome: A Systematic Review. J. Endod. 2017, 43, 514–519. [Google Scholar] [CrossRef]
- Naqvi, A.R.; Shango, J.; Seal, A.; Shukla, D.; Nares, S. Herpesviruses and MicroRNAs: New Pathogenesis Factors in Oral Infection and Disease? Front. Immunol. 2018, 9, 2099. [Google Scholar] [CrossRef]
- Hernández Vigueras, S.; Donoso Zúñiga, M.; Jané-Salas, E.; Salazar Navarrete, L.; Segura-Egea, J.J.; Velasco-Ortega, E.; López-López, J. Viruses in Pulp and Periapical Inflammation: A Review. Odontology 2016, 104, 184–191. [Google Scholar] [CrossRef]
- Karamifar, K.; Tondari, A.; Saghiri, M.A. Endodontic Periapical Lesion: An Overview on the Etiology, Diagnosis and Current Treatment Modalities. Eur. Endod. J. 2020, 5, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Segura-Egea, J.J.; Jiménez-Pinzón, A.; Ríos-Santos, J.V.; Velasco-Ortega, E.; Cisneros-Cabello, R.; Poyato-Ferrera, M.M. High Prevalence of Apical Periodontitis amongst Smokers in a Sample of Spanish Adults. Int. Endod. J. 2008, 41, 310–316. [Google Scholar] [CrossRef]
- Segura-Egea, J.-J.; Castellanos-Cosano, L.; Machuca, G.; López-López, J.; Martín-González, J.; Velasco-Ortega, E.; Sánchez-Domínguez, B.; López-Frías, F.-J. Diabetes Mellitus, Periapical Inflammation and Endodontic Treatment Outcome. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e356–e361. [Google Scholar] [CrossRef] [Green Version]
- Bjørndal, L.; Kirkevang, L.-L.; Whitworth, J.M. Textbook of Endodontology; Wiley-Blackwell: Oxfort, UK, 2018; ISBN 978-1-119-05732-1. [Google Scholar]
- Hamedy, R.; Shakiba, B.; Pak, J.G.; Barbizam, J.V.; Ogawa, R.S.; White, S.N. Prevalence of Root Canal Treatment and Periapical Radiolucency in Elders: A Systematic Review. Gerodontology 2016, 33, 116–127. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-Aging: An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Fuentes, E.; Fuentes, M.; Alarcón, M.; Palomo, I. Immune System Dysfunction in the Elderly. An. Acad. Bras. Cienc. 2017, 89, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Fouad, A.F.; Khan, A.A.; Silva, R.M.; Kang, M.K. Genetic and Epigenetic Characterization of Pulpal and Periapical Inflammation. Front. Physiol. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, A.; Knezevic, A.; Karalic, D.; Soldatovic, I.; Popovic, B.; Milasin, J.; Andric, M. Pro-Inflammatory Cytokine Levels in Human Apical Periodontitis: Correlation with Clinical and Histological Findings. Aust. Endod. J. 2015, 41, 72–77. [Google Scholar] [CrossRef]
- Teixeira-Salum, T.B.; Rodrigues, D.B.R.; Gervásio, A.M.; Souza, C.J.A.; Rodrigues, V.; Loyola, A.M. Distinct Th1, Th2 and Treg Cytokines Balance in Chronic Periapical Granulomas and Radicular Cysts. J. Oral Pathol. Med. 2010, 39, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Dessaune Neto, N.; Porpino, M.T.M.; Antunes, H.D.S.; Rodrigues, R.C.V.; Perez, A.R.; Pires, F.R.; Siqueira, J.F.; Armada, L. Pro-Inflammatory and Anti-Inflammatory Cytokine Expression in Post-Treatment Apical Periodontitis. J. Appl. Oral Sci. 2018, 26, e20170455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuma, M.M.; Samuel, R.O.; Gomes-Filho, J.E.; Dezan-Junior, E.; Cintra, L.T.A. The Role of IL-6 on Apical Periodontitis: A Systematic Review. Int. Endod. J. 2014, 47, 615–621. [Google Scholar] [CrossRef]
- Kitaura, H.; Kimura, K.; Ishida, M.; Kohara, H.; Yoshimatsu, M.; Takano-Yamamoto, T. Immunological Reaction in TNF-α-Mediated Osteoclast Formation and Bone Resorption in Vitro and in Vivo. Clin. Dev. Immunol 2013, 2013, 181849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-Dependent Dysregulation of Innate Immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Nikolich-Žugich, J. The Twilight of Immunity: Emerging Concepts in Aging of the Immune System. Nat. Immunol. 2018, 19, 10–19. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Linton, P.J.; Dorshkind, K. Age-Related Changes in Lymphocyte Development and Function. Nat. Immunol. 2004, 5, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Filioreanu, A.M.; Stelea, C.; Maftei, G.A.; Popescu, E. Prevalence of Oral Lesions Modulated by Patient’s Age: The Young versus the Elderly. Rom. J. Oral Rehabil. 2018, 10, 50–56. [Google Scholar]
- Appay, V.; Sauce, D. Assessing Immune Aging in HIV-Infected Patients. Virulence 2017, 8, 529–538. [Google Scholar] [CrossRef]
- Cunha, L.L.; Perazzio, S.F.; Azzi, J.; Cravedi, P.; Riella, L.V. Remodeling of the Immune Response with Aging: Immunosenescence and Its Potential Impact on COVID-19 Immune Response. Front. Immunol. 2020, 11, 1748. [Google Scholar] [CrossRef]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The Integration of Inflammaging in Age-Related Diseases. Semin. Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- Ajuz, N.C.; Antunes, H.; Mendonça, T.A.; Pires, F.R.; Siqueira, J.F.; Armada, L. Immunoexpression of Interleukin 17 in Apical Periodontitis Lesions. J. Endod. 2014, 40, 1400–1403. [Google Scholar] [CrossRef]
- Sasaki, H.; Hirai, K.; Martins, C.M.; Furusho, H.; Battaglino, R.; Hashimoto, K. Interrelationship Between Periapical Lesion and Systemic Metabolic Disorders. Curr. Pharm. Des. 2016, 22, 2204–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, J.F.; Rôças, I.N. Clinical Implications and Microbiology of Bacterial Persistence after Treatment Procedures. J. Endod. 2008, 34, 1291–1301.e3. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.J.; Palmer, J.L.; Fortin, C.F.; Fülöp, T.; Goldstein, D.R.; Linton, P.-J. Aging and Innate Immunity in the Mouse: Impact of Intrinsic and Extrinsic Factors. Trends Immunol. 2009, 30, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Chelvarajan, R.L.; Liu, Y.; Popa, D.; Getchell, M.L.; Getchell, T.V.; Stromberg, A.J.; Bondada, S. Molecular Basis of Age-Associated Cytokine Dysregulation in LPS-Stimulated Macrophages. J. Leukoc. Biol. 2006, 79, 1314–1327. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Sharma, N.; Upadhyay, C.; Kumar, S.; Rathi, B. Small Molecules Effective against Liver and Blood Stage Malarial Infection. Curr. Top. Med. Chem. 2019, 18, 2008–2021. [Google Scholar] [CrossRef]
- Duque, G.; Troen, B.R. Understanding the Mechanisms of Senile Osteoporosis: New Facts for a Major Geriatric Syndrome. J. Am. Geriatr. Soc. 2008, 56, 935–941. [Google Scholar] [CrossRef]
- Lin, J.T.; Lane, J.M. Rehabilitation of the Older Adult with an Osteoporosis-Related Fracture. Clin. Geriatr. Med. 2006, 22, 435–447. [Google Scholar] [CrossRef]
- Pietschmann, P.; Skalicky, M.; Kneissel, M.; Rauner, M.; Hofbauer, G.; Stupphann, D.; Viidik, A. Bone Structure and Metabolism in a Rodent Model of Male Senile Osteoporosis. Exp. Gerontol. 2007, 42, 1099–1108. [Google Scholar] [CrossRef]
- Raisz, L.G. Pathogenesis of Osteoporosis: Concepts, Conflicts, and Prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef] [Green Version]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1α and the Inflammatory Process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Langeland, K.; Block, R.M.; Grossman, L.I. A Histopathologic and Histobacteriologic Study of 35 Periapical Endodontic Surgical Specimens. J. Endod. 1977, 3, 8–23. [Google Scholar] [CrossRef]
- Martinho, F.C.; Leite, F.R.M.; Nascimento, G.G.; Cirelli, J.A.; Gomes, B.P.F.A. Clinical Investigation of Bacterial Species and Endotoxin in Endodontic Infection and Evaluation of Root Canal Content Activity against Macrophages by Cytokine Production. Clin. Oral Investig. 2014, 18, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, N.F.; Brasil, S.D.; Ferreira, D.D.; Armada, L. Aging Effects in the Expression of Macrophages in Post-Treatment Apical Periodontitis Lesions. Spec. Care Dent. 2017, 37, 230–235. [Google Scholar] [CrossRef]
- Meghji, S.; Qureshi, W.; Henderson, B.; Harris, M. The Role of Endotoxin and Cytokines in the Pathogenesis of Odontogenic Cysts. Arch. Oral Biol. 1996, 41, 523–531. [Google Scholar] [CrossRef]
- Takahashi, K. Microbiological, Pathological, Inflammatory, Immunological and Molecular Biological Aspects of Periradicular Disease. Int. Endod. J. 1998, 31, 311–325. [Google Scholar] [CrossRef]
- de Andrade, A.L.; Nonaka, C.F.; Gordón-Núñez, M.A.; de Almeida Freitas, R.; Galvão, H.C. Immunoexpression of Interleukin 17, Transforming Growth Factor Β1, and Forkhead Box P3 in Periapical Granulomas, Radicular Cysts, and Residual Radicular Cysts. J. Endod. 2013, 39, 990–994. [Google Scholar] [CrossRef]
- Marçal, J.R.B.; Samuel, R.O.; Fernandes, D.; de Araujo, M.S.; Napimoga, M.H.; Pereira, S.A.L.; Clemente-Napimoga, J.T.; Alves, P.M.; Mattar, R.; Rodrigues, V.; et al. T-Helper Cell Type 17/Regulatory T-Cell Immunoregulatory Balance in Human Radicular Cysts and Periapical Granulomas. J. Endod. 2010, 36, 995–999. [Google Scholar] [CrossRef]
- Popovska, L.; Dimova, C.; Evrosimoska, B.; Stojanovska, V.; Muratovska, I.; Ćetenović, B.; Marković, D. Relationship between IL-1β Production and Endodontic Status of Human Periapical Lesions. Vojnosanit. Pregl. 2017, 74, 1134–1139. [Google Scholar] [CrossRef]
- de Oliveira Rodini, C.; Batista, A.C.; Lara, V.S. Comparative Immunohistochemical Study of the Presence of Mast Cells in Apical Granulomas and Periapical Cysts: Possible Role of Mast Cells in the Course of Human Periapical Lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 59–63. [Google Scholar] [CrossRef]
- Patidar, K.A.; Parwani, R.N.; Wanjari, S.P.; Patidar, A.P. Mast Cells in Human Odontogenic Cysts. Biotechnic Histochem. 2012, 87, 397–402. [Google Scholar] [CrossRef]
- Caruso, C.; Accardi, G.; Virruso, C.; Candore, G. Sex, Gender and Immunosenescence: A Key to Understand the Different Lifespan between Men and Women? Immun. Ageing 2013, 10, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
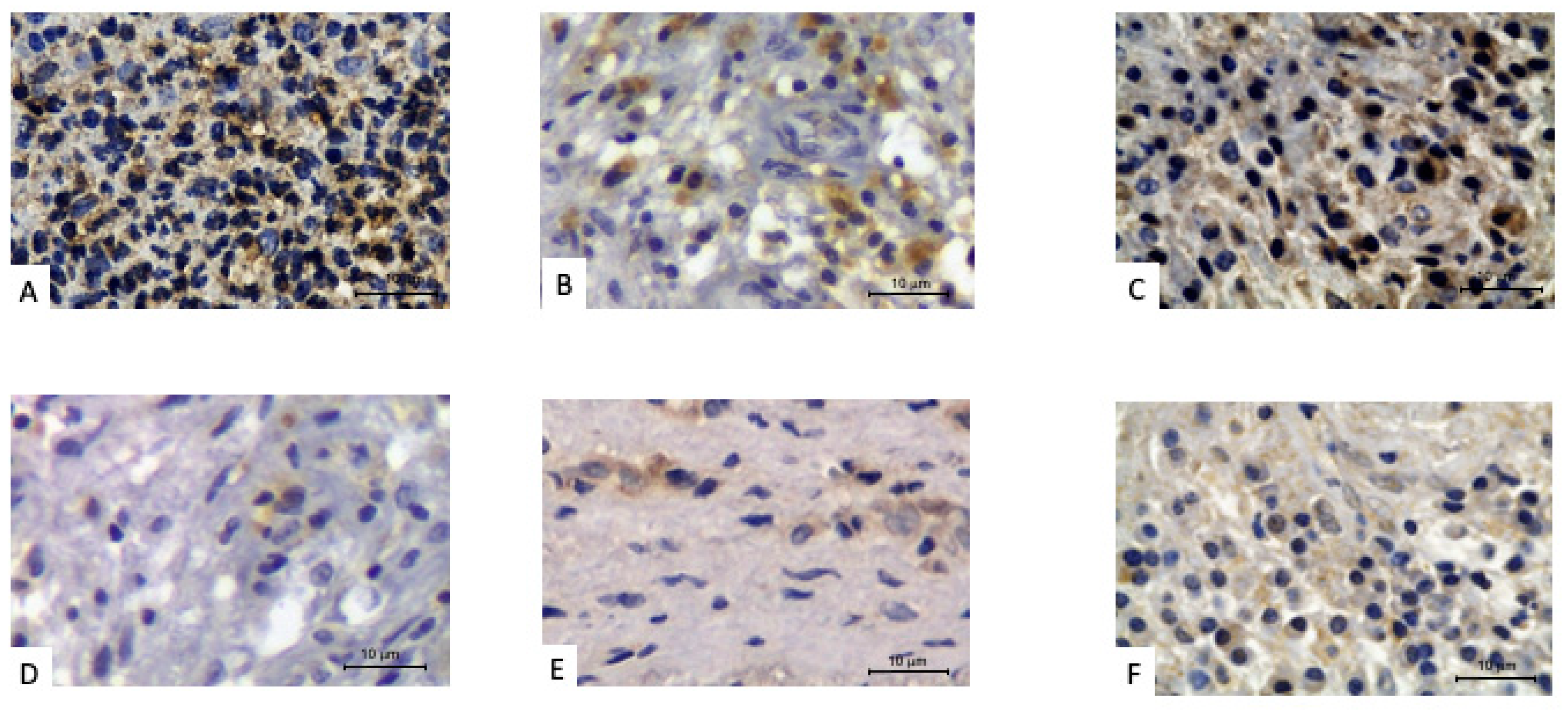
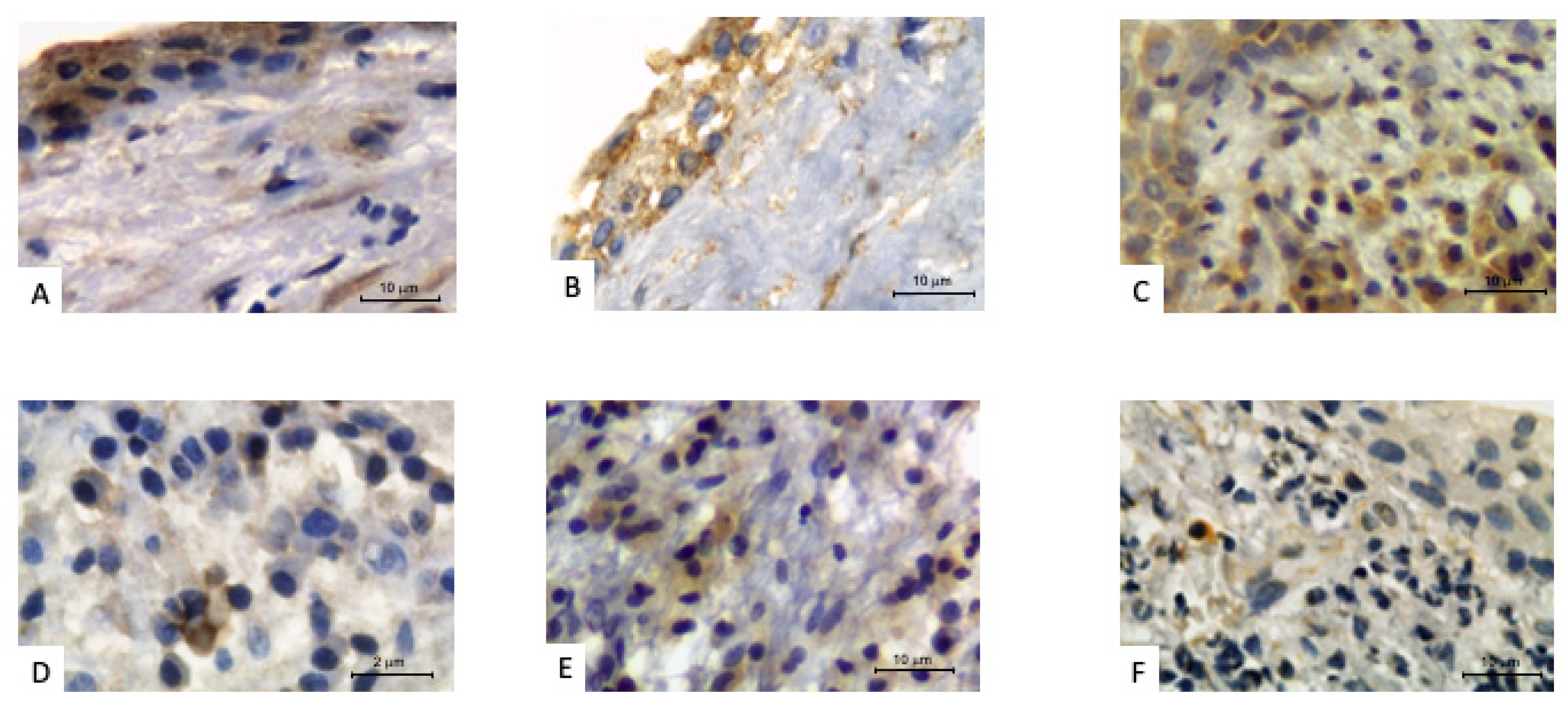
| Parameter | Elderly | Young/Middle-Aged | p-Value |
|---|---|---|---|
| Age 1 | 70.4 ± 5.34 | 39.9 ± 9.3 | <0.00001 |
| Gender 2 | 19 females 11 males | 18 females 12 males | 1.0 |
| Localization 2 | 18 anterior 12 posterior | 19 anterior 11 posterior | 1.0 |
| Anatomical location 2 | 17 maxilla 13 mandible | 20 maxilla 10 mandible | 0.59 |
| Cytokine | Groups | |||||
|---|---|---|---|---|---|---|
| Elderly | Young/Middle-Aged | |||||
| Cysts 1 | Granulomas 1 | p-Value 2 | Cysts 1 | Granulomas 1 | p-Value 2 | |
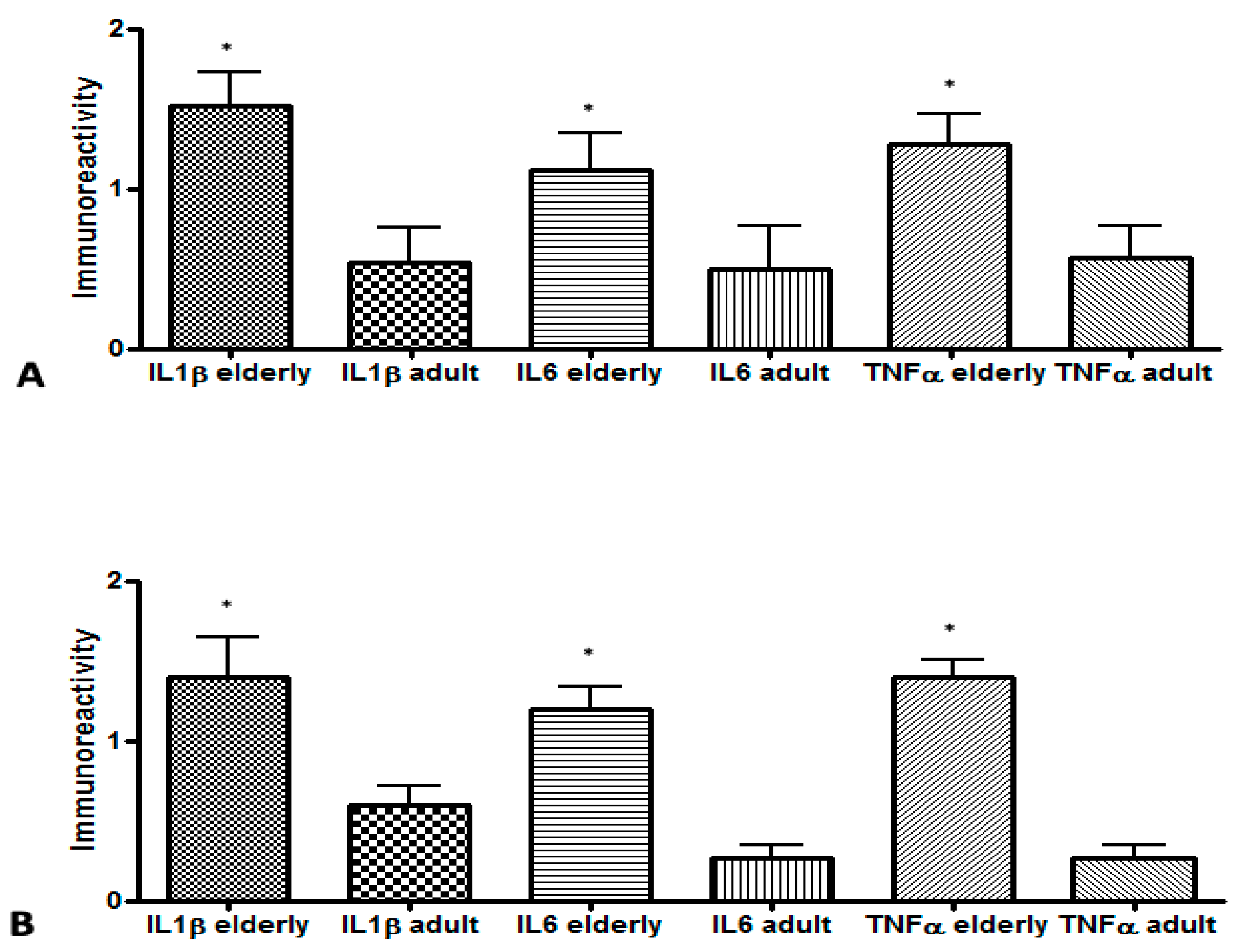
| IL-1β | 1.52 ± 0.46 | 1.40 ± 0.59 | 0.792 | 0.32 ± 0.17 | 0.60 ± 0.26 | 0.019 |
| IL-6 | 1.12 ± 0.50 | 1.20 ± 0.33 | 0.930 | 0.24 ± 0.16 | 0.26 ± 0.20 | 0.937 |
| TNF-α | 1.28 ± 0.41 | 1.40 ± 0.25 | 0.662 | 0.40 ± 0.26 | 0.26 ± 0.20 | 0.536 |
| p-value 3 | 0.500 | 0.420 | 0.690 | 0.060 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, Q.E.; Ferreira, D.d.C.; da Silva, A.M.P.; Gonçalves, L.S.; Pires, F.R.; Carrouel, F.; Bourgeois, D.; Sufiawati, I.; Armada, L. Aging as a Risk Factor on the Immunoexpression of Pro-Inflammatory IL-1β, IL-6 and TNF-α Cytokines in Chronic Apical Periodontitis Lesions. Biology 2022, 11, 14. https://doi.org/10.3390/biology11010014
Teixeira QE, Ferreira DdC, da Silva AMP, Gonçalves LS, Pires FR, Carrouel F, Bourgeois D, Sufiawati I, Armada L. Aging as a Risk Factor on the Immunoexpression of Pro-Inflammatory IL-1β, IL-6 and TNF-α Cytokines in Chronic Apical Periodontitis Lesions. Biology. 2022; 11(1):14. https://doi.org/10.3390/biology11010014
Chicago/Turabian StyleTeixeira, Quésia Euclides, Dennis de Carvalho Ferreira, Alexandre Marques Paes da Silva, Lucio Souza Gonçalves, Fabio Ramoa Pires, Florence Carrouel, Denis Bourgeois, Irna Sufiawati, and Luciana Armada. 2022. "Aging as a Risk Factor on the Immunoexpression of Pro-Inflammatory IL-1β, IL-6 and TNF-α Cytokines in Chronic Apical Periodontitis Lesions" Biology 11, no. 1: 14. https://doi.org/10.3390/biology11010014
APA StyleTeixeira, Q. E., Ferreira, D. d. C., da Silva, A. M. P., Gonçalves, L. S., Pires, F. R., Carrouel, F., Bourgeois, D., Sufiawati, I., & Armada, L. (2022). Aging as a Risk Factor on the Immunoexpression of Pro-Inflammatory IL-1β, IL-6 and TNF-α Cytokines in Chronic Apical Periodontitis Lesions. Biology, 11(1), 14. https://doi.org/10.3390/biology11010014









