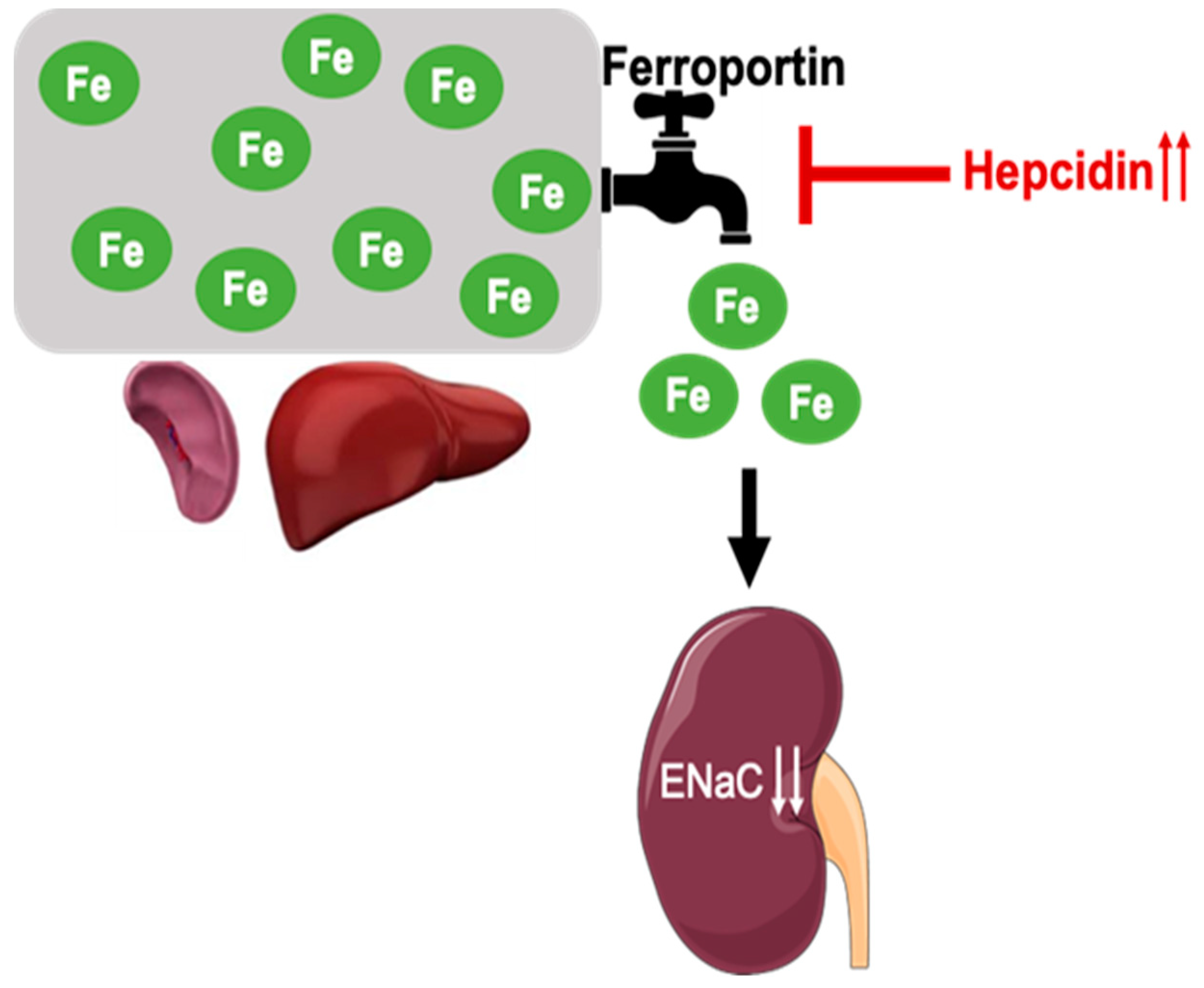
Iron Inhibits the Translation and Activity of the Renal Epithelial Sodium Channel
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
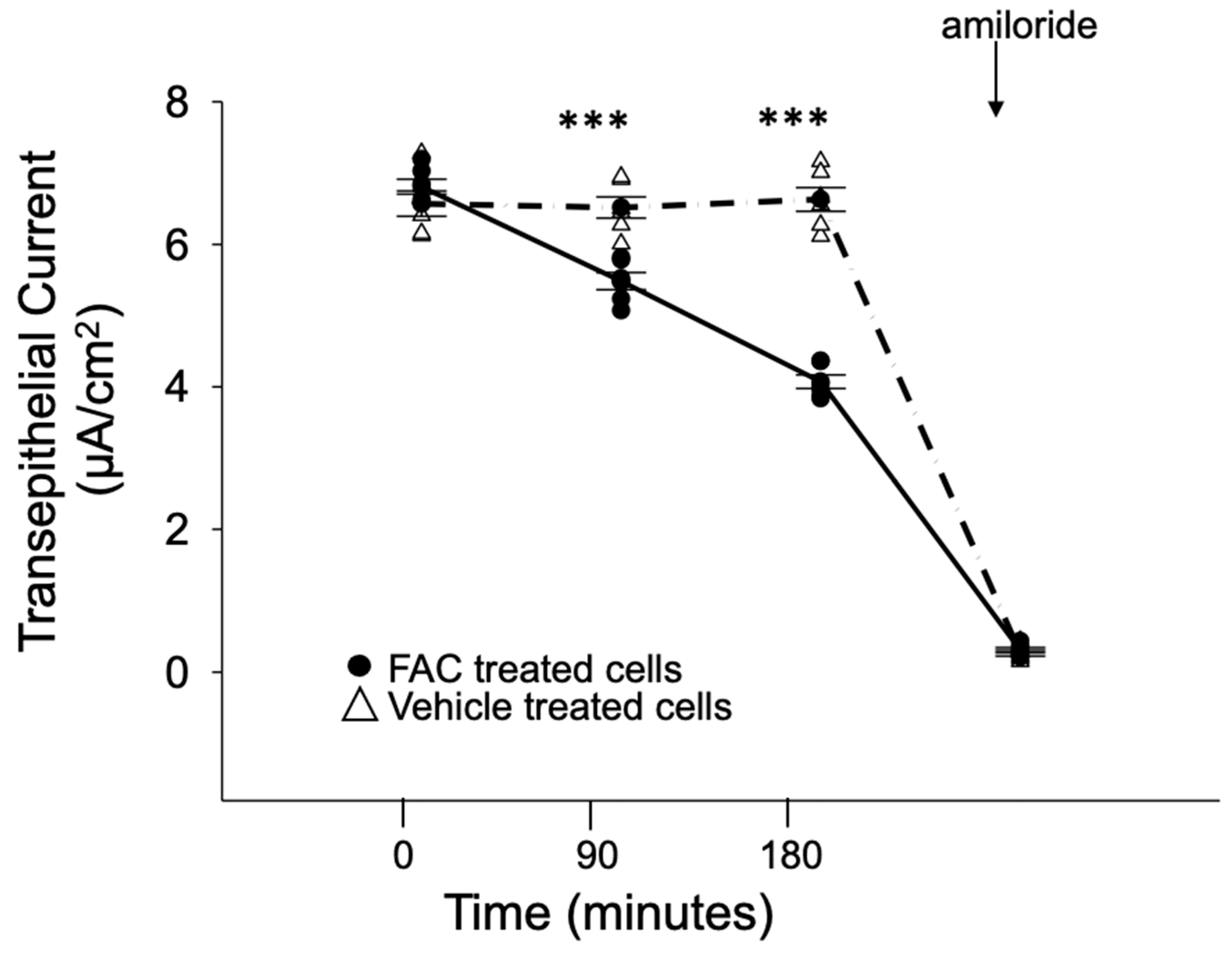
2.3. Amiloride-Sensitive Transepithelial Current Measurements
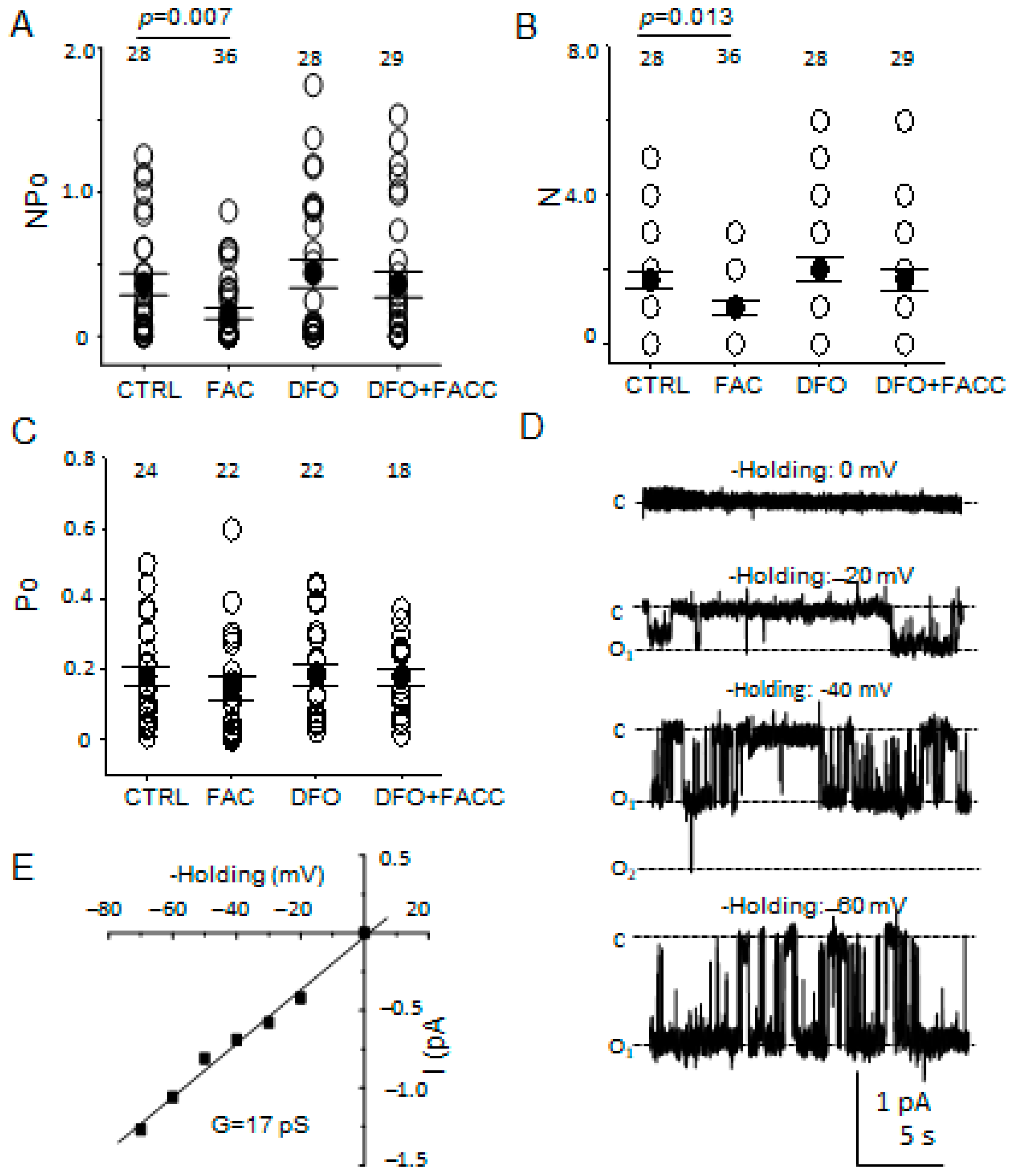
2.4. Electrophysiology
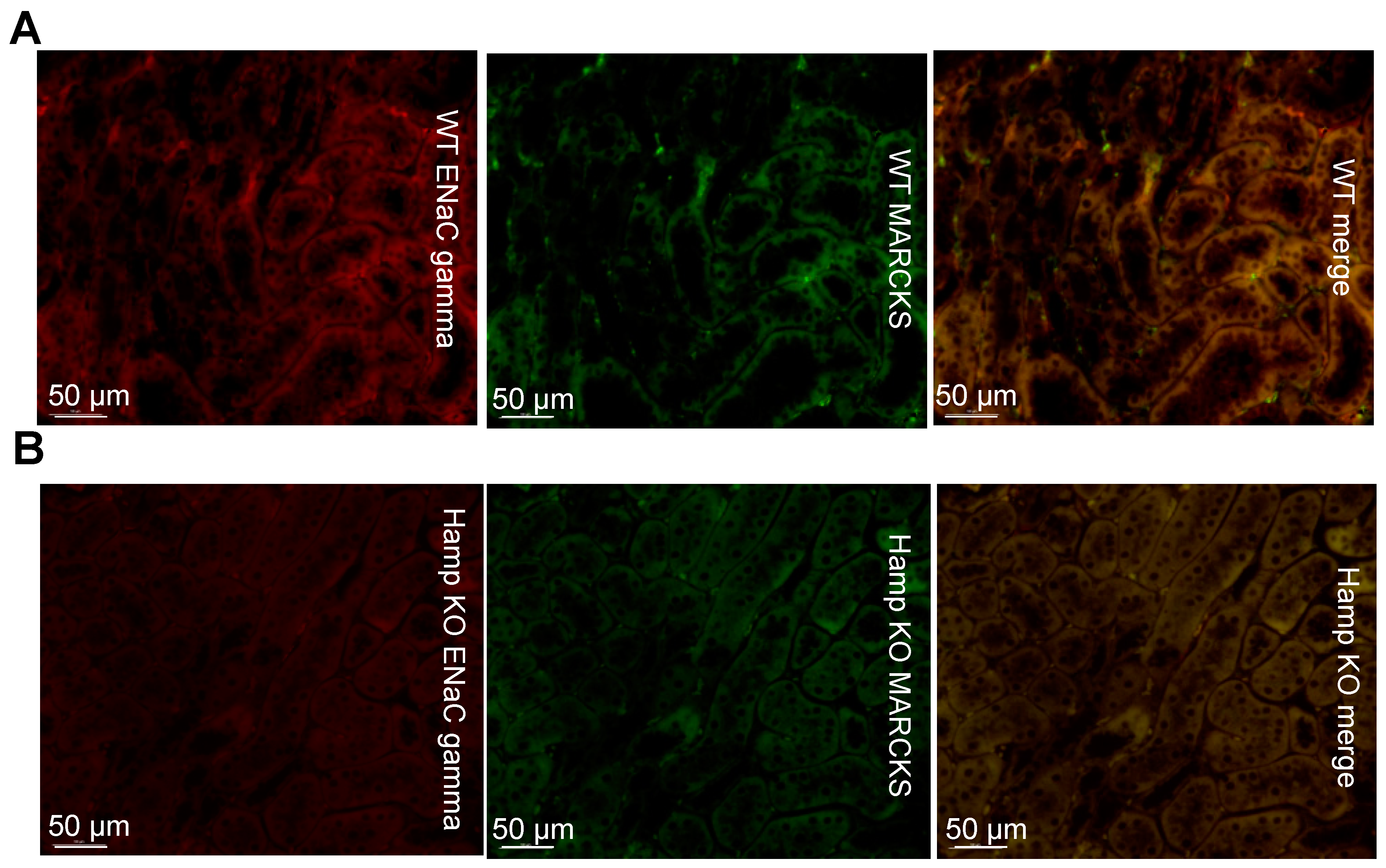
2.5. Immunohistochemistry and Immunofluorescence
2.6. Real Time PCR
2.7. Perls Detectable Iron Staining
2.8. Urinary and Serum Iron Measurements
2.9. Measurement of Urinary Electrolytes
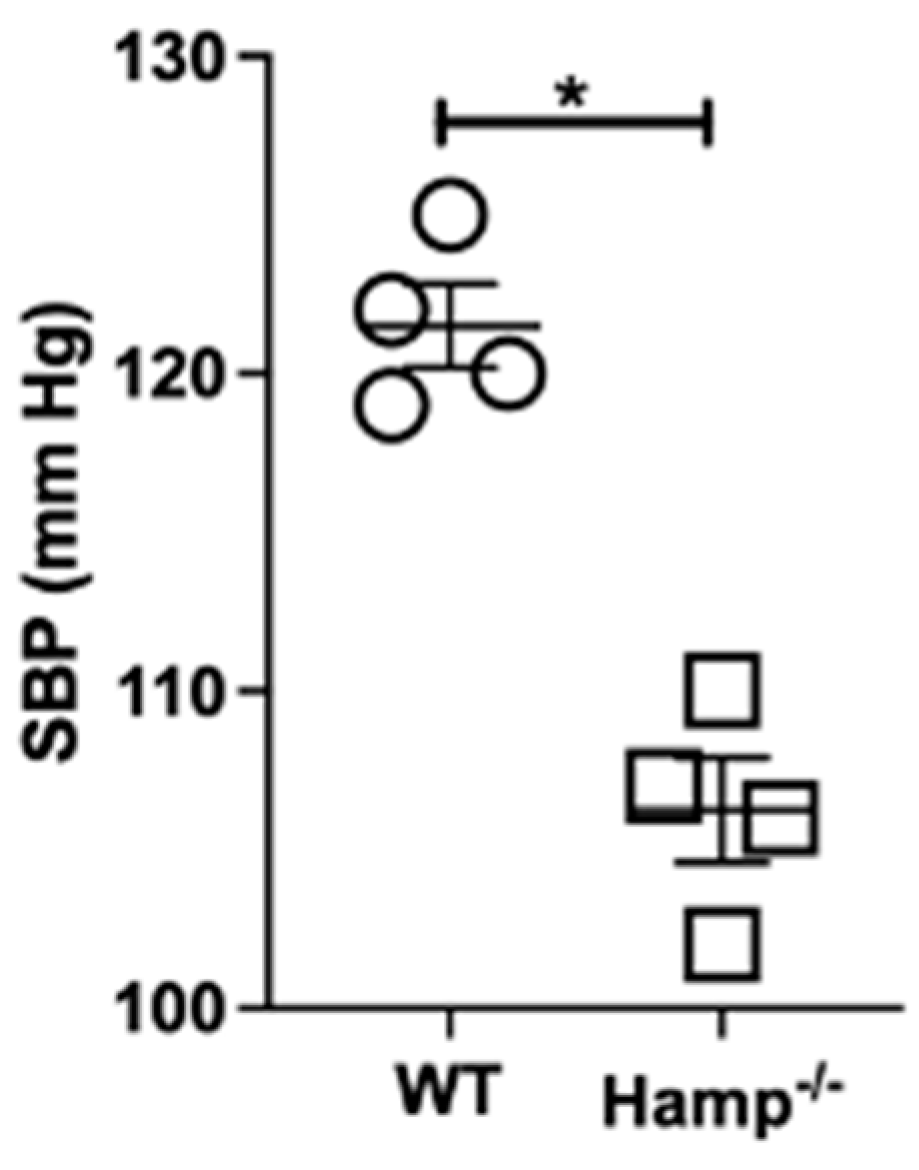
2.10. Blood Pressure Measurements
2.11. Statistical Analysis
3. Results
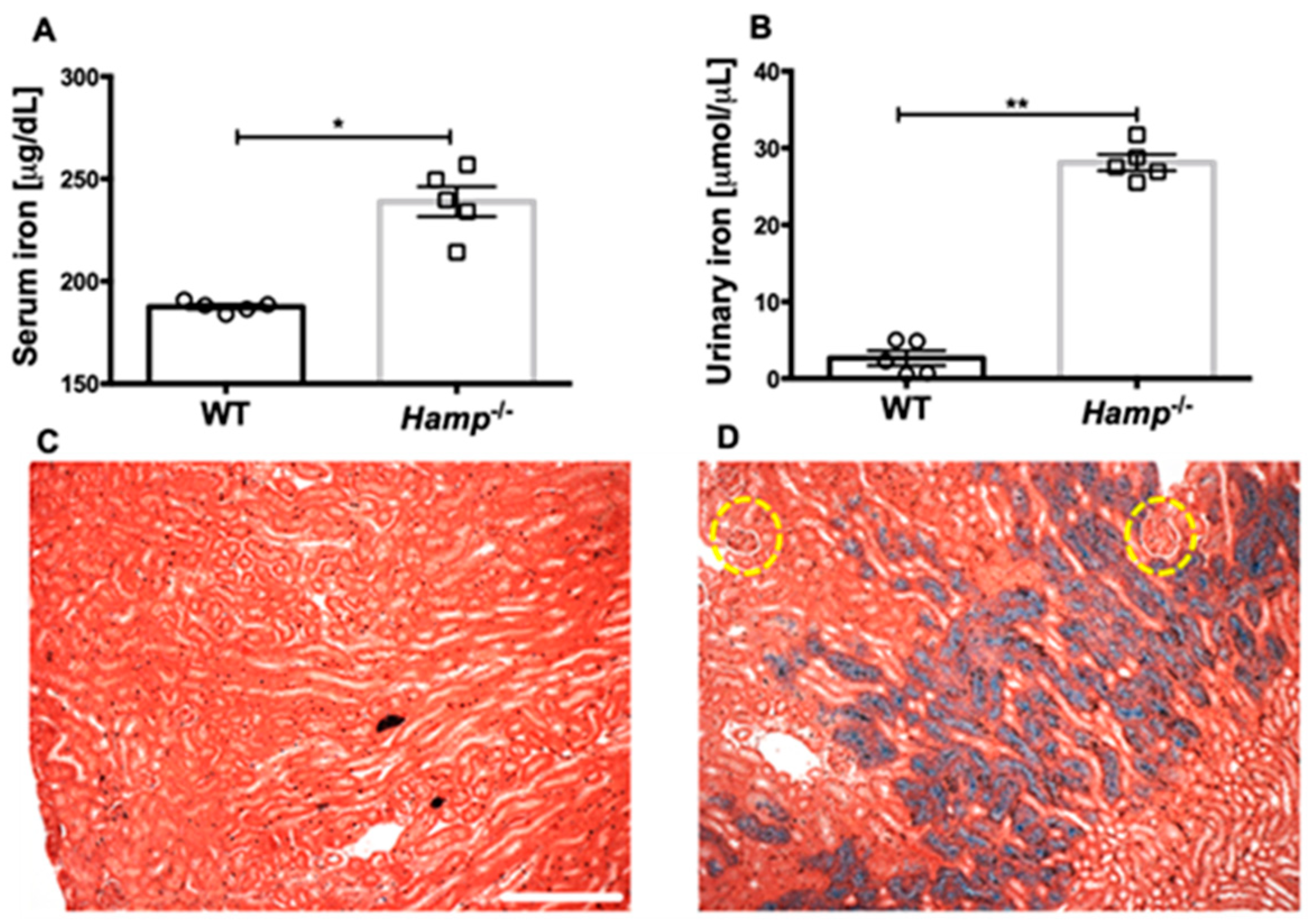
3.1. Hamp-/- Mice Are Iron Overloaded and Accumulate Iron in the Renal Tubules but Not the Glomeruli Compared to WT Controls
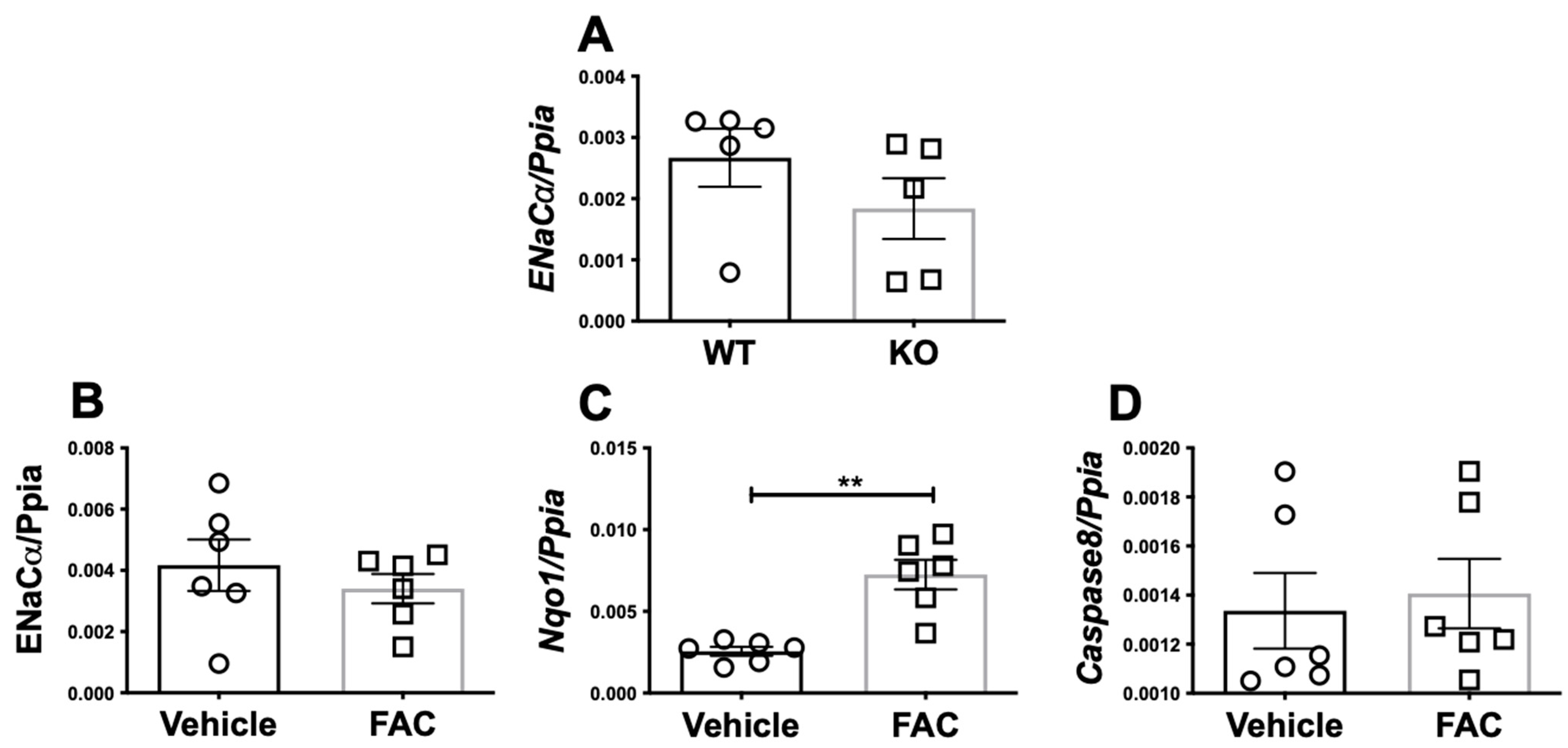
3.2. Iron Does Not Transcriptionally Regulate ENaC Alpha Expression in Mice or A Cortical Collecting Duct Cell Line
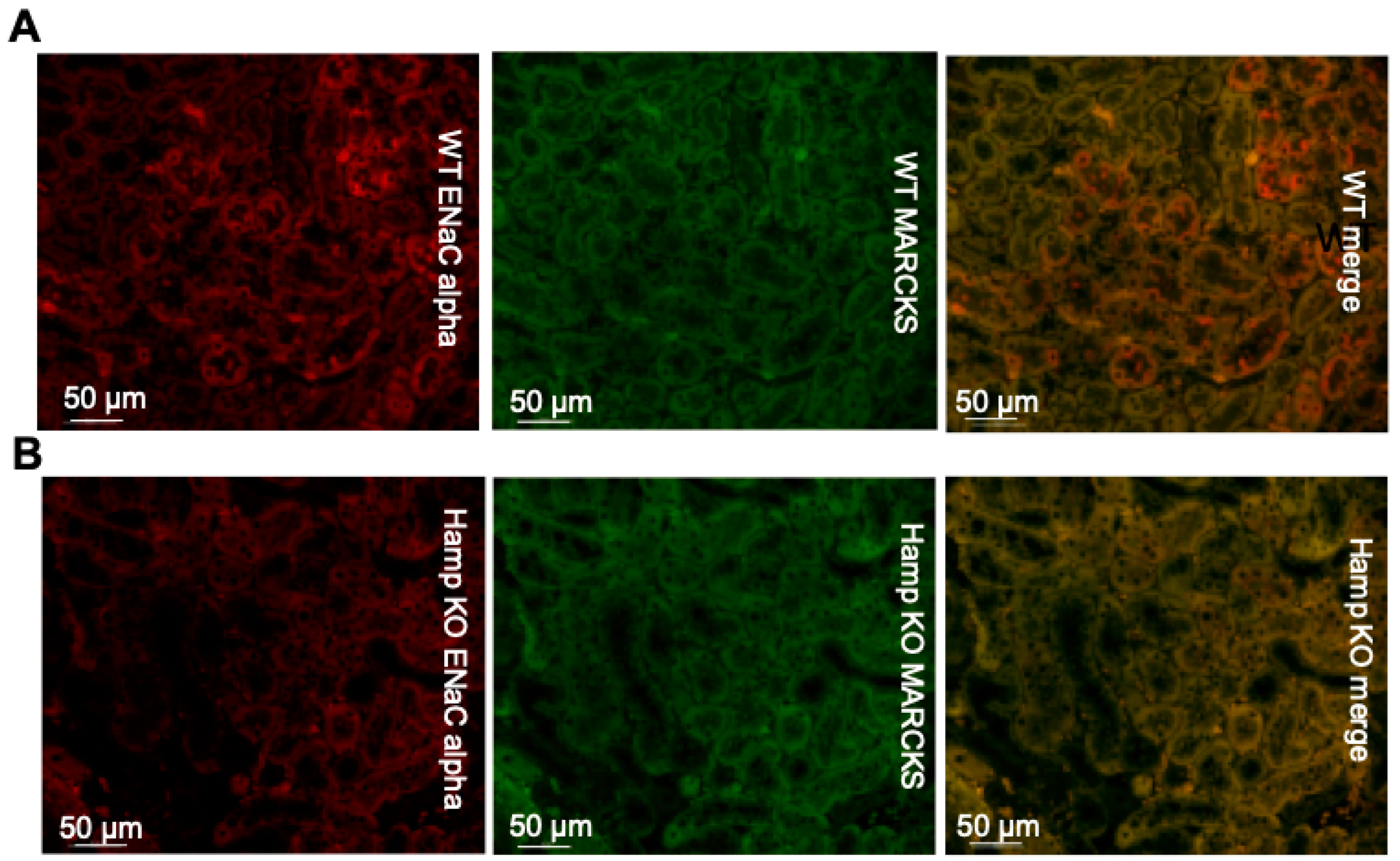
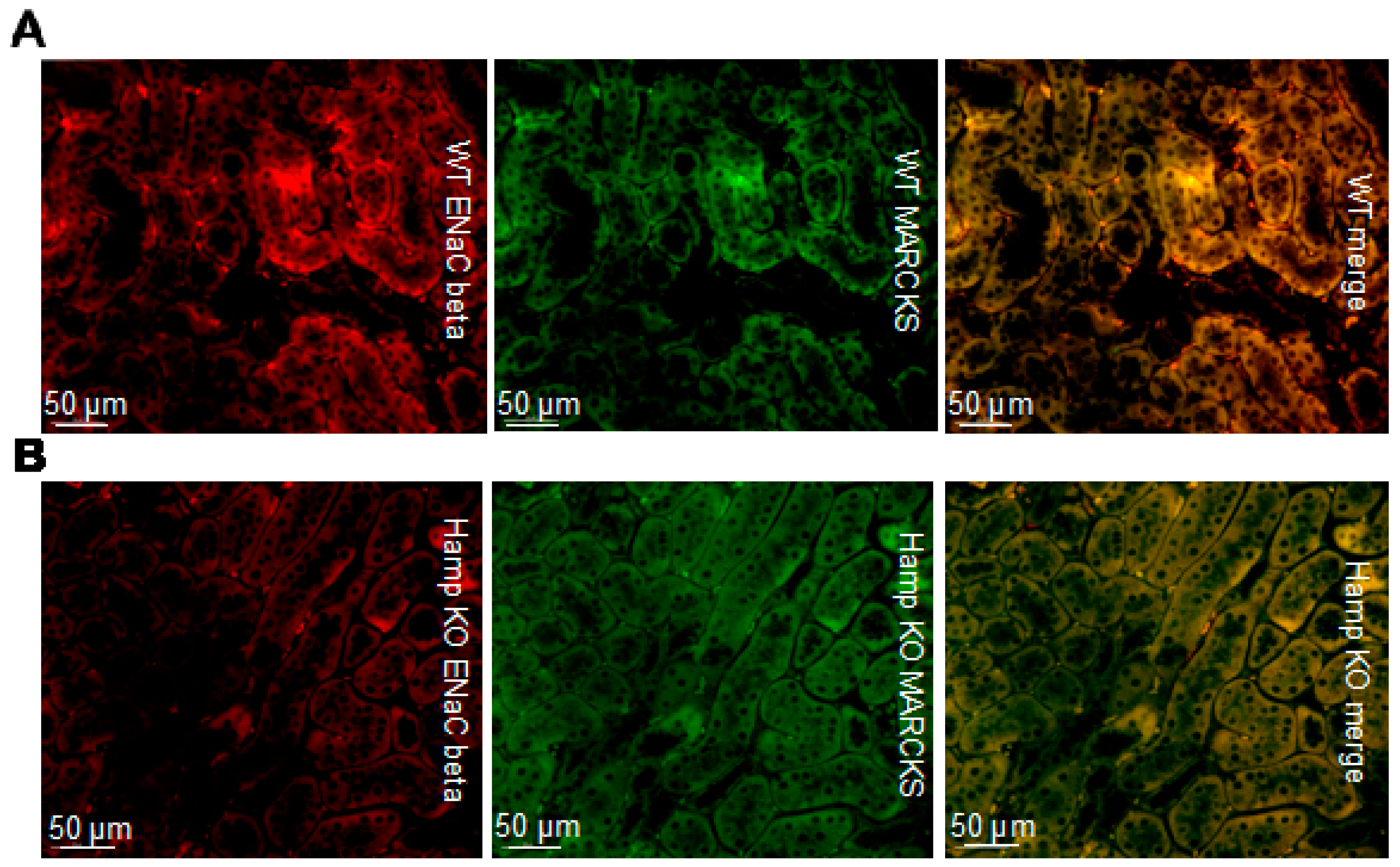
3.3. ENaC Protein Expression Is Reduced in the Kidneys of Iron Overloaded Hamp-/- Mice
3.4. ENaC Activity in Cultured Xenopus 2F3 Cells Is Inhibited by Iron
3.5. ENaC Activity in Cultured Mouse Cortical Collecting Ducts Cells Is Inhibited by Iron Loading
3.6. The Number of Patches with ENaC Activity but Not Its Open Probability Is Attenuated after Iron Loading
3.7. Base-Line Systolic Blood Pressure Is Lower in Hamp-/- Mice Compared to Wild-Type Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pratt, J.H. Central role for ENaC in development of hypertension. J. Am. Soc. Nephrol. JASN 2005, 16, 3154–3159. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, T.S.; Staruschenko, A. Involvement of ENaC in the development of salt-sensitive hypertension. Am. J. Physiol.-Ren. Physiol. 2017, 313, F135–F140. [Google Scholar] [CrossRef] [PubMed]
- Shimkets, R.A.; Lifton, R.; Canessa, C.M. In vivo phosphorylation of the epithelial sodium channel. Proc. Natl. Acad. Sci. USA 1998, 95, 3301–3305. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, R.; Marunaka, Y. Interactive Actions of Aldosterone and Insulin on Epithelial Na(+) Channel Trafficking. Int. J. Mol. Sci. 2020, 21, 3407. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hu, X.; Riazi, S.; Tiwari, S.; Wade, J.B.; Ecelbarger, C.A. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am. J. Physiol.-Ren. Physiol. 2006, 290, F1055–F1064. [Google Scholar] [CrossRef]
- Blazer-Yost, B.L.; Esterman, M.A.; Vlahos, C.J. Insulin-stimulated trafficking of ENaC in renal cells requires PI 3-kinase activity. Am. J. Physiol. Cell Physiol. 2003, 284, C1645–C1653. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.J.; Alli, A.A.; Eaton, D.C.; Bao, H.F. ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling. Am. J. Physiol.-Ren. Physiol. 2013, 304, F930–F937. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sorokin, A.; Staruschenko, A. Inhibition of ENaC by endothelin-1. Vitam. Horm. 2015, 98, 155–187. [Google Scholar] [CrossRef] [PubMed]
- Bugaj, V.; Pochynyuk, O.; Mironova, E.; Vandewalle, A.; Medina, J.L.; Stockand, J.D. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am. J. Physiol.-Ren. Physiol. 2008, 295, F1063–F1070. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, T.S.; Chahdi, A.; Ilatovskaya, D.V.; Levchenko, V.; Vandewalle, A.; Pochynyuk, O.; Sorokin, A.; Staruschenko, A. Endothelin-1 inhibits the epithelial Na+ channel through betaPix/14-3-3/Nedd4-2. J. Am. Soc. Nephrol. JASN 2010, 21, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Mironova, E.; Chen, Y.; Pao, A.C.; Roos, K.P.; Kohan, D.E.; Bugaj, V.; Stockand, J.D. Activation of ENaC by AVP contributes to the urinary concentrating mechanism and dilution of plasma. Am. J. Physiol.-Ren. Physiol. 2015, 308, F237–F243. [Google Scholar] [CrossRef]
- Montgomery, D.S.; Yu, L.; Ghazi, Z.M.; Thai, T.L.; Al-Khalili, O.; Ma, H.P.; Eaton, D.C.; Alli, A.A. ENaC activity is regulated by calpain-2 proteolysis of MARCKS proteins. Am. J. Physiol. Cell Physiol. 2017, 313, C42–C53. [Google Scholar] [CrossRef]
- Alli, A.A.; Bao, H.F.; Liu, B.C.; Yu, L.; Aldrugh, S.; Montgomery, D.S.; Ma, H.P.; Eaton, D.C. Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am. J. Physiol.-Ren. Physiol. 2015, 309, F456–F463. [Google Scholar] [CrossRef]
- Alli, A.A.; Bao, H.F.; Alli, A.A.; Aldrugh, Y.; Song, J.Z.; Ma, H.P.; Yu, L.; Al-Khalili, O.; Eaton, D.C. Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein. Am. J. Physiol.-Ren. Physiol. 2012, 303, F800–F811. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, G.; Lankhorst, S.; Boonstra, A.; Postmus, P.E.; Zweegman, S.; Westerhof, N.; van der Laarse, W.J.; Vonk-Noordegraaf, A. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2011, 37, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J.; Howard, L.S.; Busbridge, M.; Ashby, D.; Kondili, E.; Gibbs, J.S.; Wharton, J.; Wilkins, M.R. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: Clinical prevalence, outcomes, and mechanistic insights. J. Am. Coll. Cardiol. 2011, 58, 300–309. [Google Scholar] [CrossRef]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C.; Vaja, V.; Babitt, J.L.; Lin, H.Y. Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am. J. Hematol. 2012, 87, 392–400. [Google Scholar] [CrossRef]
- Weinstein, D.A.; Roy, C.N.; Fleming, M.D.; Loda, M.F.; Wolfsdorf, J.I.; Andrews, N.C. Inappropriate expression of hepcidin is associated with iron refractory anemia: Implications for the anemia of chronic disease. Blood 2002, 100, 3776–3781. [Google Scholar] [CrossRef]
- Andrews, N.C. Anemia of inflammation: The cytokine-hepcidin link. J. Clin. Investig. 2004, 113, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- van Swelm, R.P.L.; Wetzels, J.F.M.; Swinkels, D.W. The multifaceted role of iron in renal health and disease. Nat. Rev. Nephrol. 2020, 16, 77–98. [Google Scholar] [CrossRef]
- van Raaij, S.; van Swelm, R.; Bouman, K.; Cliteur, M.; van den Heuvel, M.C.; Pertijs, J.; Patel, D.; Bass, P.; van Goor, H.; Unwin, R.; et al. Tubular iron deposition and iron handling proteins in human healthy kidney and chronic kidney disease. Sci. Rep. 2018, 8, 9353. [Google Scholar] [CrossRef] [PubMed]
- Wlazlo, E.; Mehrad, B.; Morel, L.; Scindia, Y. Iron Metabolism: An Under Investigated Driver of Renal Pathology in Lupus Nephritis. Front. Med. 2021, 8, 643686. [Google Scholar] [CrossRef] [PubMed]
- van Raaij, S.E.G.; Srai, S.K.S.; Swinkels, D.W.; van Swelm, R.P.L. Iron uptake by ZIP8 and ZIP14 in human proximal tubular epithelial cells. Biometals 2019, 32, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Zarjou, A.; Hull, T.D.; Traylor, A.M.; Perianayagam, A.; Joseph, R.; Kamal, A.I.; Arosio, P.; Soares, M.P.; Jeney, V.; et al. Macrophage and epithelial cell H-ferritin expression regulates renal inflammation. Kidney Int. 2015, 88, 95–108. [Google Scholar] [CrossRef]
- Scindia, Y.; Dey, P.; Thirunagari, A.; Liping, H.; Rosin, D.L.; Floris, M.; Okusa, M.D.; Swaminathan, S. Hepcidin Mitigates Renal Ischemia-Reperfusion Injury by Modulating Systemic Iron Homeostasis. J. Am. Soc. Nephrol. JASN 2015, 26, 2800–2814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Meyron-Holtz, E.; Rouault, T.A. Renal iron metabolism: Transferrin iron delivery and the role of iron regulatory proteins. J. Am. Soc. Nephrol. JASN 2007, 18, 401–406. [Google Scholar] [CrossRef]
- Groebler, L.K.; Liu, J.; Shanu, A.; Codd, R.; Witting, P.K. Comparing the potential renal protective activity of desferrioxamine B and the novel chelator desferrioxamine B-N-(3-hydroxyadamant-1-yl)carboxamide in a cell model of myoglobinuria. Biochem. J. 2011, 435, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, R.; Saha, A.; Chamani, A.; Schneider, N.; Shah, R.; Nanjundan, M. Iron Pathways and Iron Chelation Approaches in Viral, Microbial, and Fungal Infections. Pharmaceuticals 2020, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Moulouel, B.; Houamel, D.; Delaby, C.; Tchernitchko, D.; Vaulont, S.; Letteron, P.; Thibaudeau, O.; Puy, H.; Gouya, L.; Beaumont, C.; et al. Hepcidin regulates intrarenal iron handling at the distal nephron. Kidney Int. 2013, 84, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Lesbordes-Brion, J.C.; Viatte, L.; Bennoun, M.; Lou, D.Q.; Ramey, G.; Houbron, C.; Hamard, G.; Kahn, A.; Vaulont, S. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood 2006, 108, 1402–1405. [Google Scholar] [CrossRef] [PubMed]
- Turi, J.L.; Piantadosi, C.A.; Stonehuerner, J.D.; Ghio, A.J. Iron accumulation in bronchial epithelial cells is dependent on concurrent sodium transport. Biometals 2008, 21, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Goch, J.; Birgegard, G.; Danielson, B.G.; Wikstrom, B. Iron absorption in patients with chronic uremia on maintenance hemodialysis and in healthy volunteers measured with a simple oral iron load test. Nephron 1996, 73, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Locatelli, F.; Gallieni, M.; Bonofiglio, R.; Fuiano, G.; Oldrizzi, L.; Conte, G.; De Nicola, L.; Mangione, F.; Esposito, P.; et al. Anaemia management in non-dialysis chronic kidney disease (CKD) patients: A multicentre prospective study in renal clinics. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transplant Assoc. Eur. Ren. Assoc. 2013, 28, 3035–3045. [Google Scholar] [CrossRef]
- Zaritsky, J.; Young, B.; Wang, H.J.; Westerman, M.; Olbina, G.; Nemeth, E.; Ganz, T.; Rivera, S.; Nissenson, A.R.; Salusky, I.B. Hepcidin—A potential novel biomarker for iron status in chronic kidney disease. Clin. J. Am. Soc. Nephrol. CJASN 2009, 4, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alli, A.A.; Yu, L.; Wlazlo, E.; Kasem, S.; Gholam, M.F.; Desai, D.; Lugo, C.I.; Vaulont, S.; Scindia, Y.M. Iron Inhibits the Translation and Activity of the Renal Epithelial Sodium Channel. Biology 2022, 11, 123. https://doi.org/10.3390/biology11010123
Alli AA, Yu L, Wlazlo E, Kasem S, Gholam MF, Desai D, Lugo CI, Vaulont S, Scindia YM. Iron Inhibits the Translation and Activity of the Renal Epithelial Sodium Channel. Biology. 2022; 11(1):123. https://doi.org/10.3390/biology11010123
Chicago/Turabian StyleAlli, Abdel A., Ling Yu, Ewa Wlazlo, Sadat Kasem, Mohammed F. Gholam, Dhruv Desai, Carlos I. Lugo, Sophie Vaulont, and Yogesh M. Scindia. 2022. "Iron Inhibits the Translation and Activity of the Renal Epithelial Sodium Channel" Biology 11, no. 1: 123. https://doi.org/10.3390/biology11010123
APA StyleAlli, A. A., Yu, L., Wlazlo, E., Kasem, S., Gholam, M. F., Desai, D., Lugo, C. I., Vaulont, S., & Scindia, Y. M. (2022). Iron Inhibits the Translation and Activity of the Renal Epithelial Sodium Channel. Biology, 11(1), 123. https://doi.org/10.3390/biology11010123





