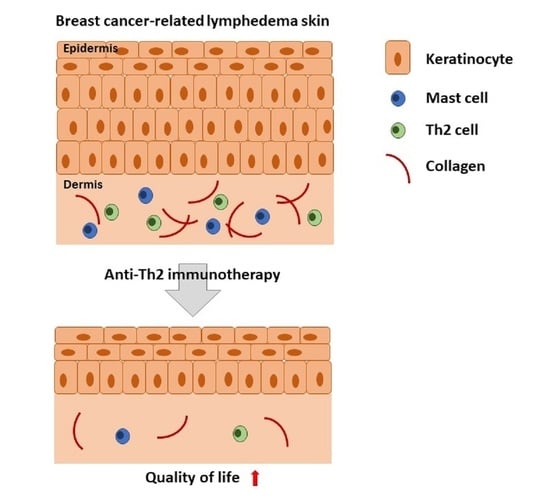
Pilot Study of Anti-Th2 Immunotherapy for the Treatment of Breast Cancer-Related Upper Extremity Lymphedema
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Approval
2.2. Pretreatment Evaluation and Measurements
2.3. Treatment Plan
2.4. Outcomes
2.4.1. Calculation of Arm Volume Changes
2.4.2. Analysis of Secondary Objectives
2.4.3. Histologic Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Adverse Events
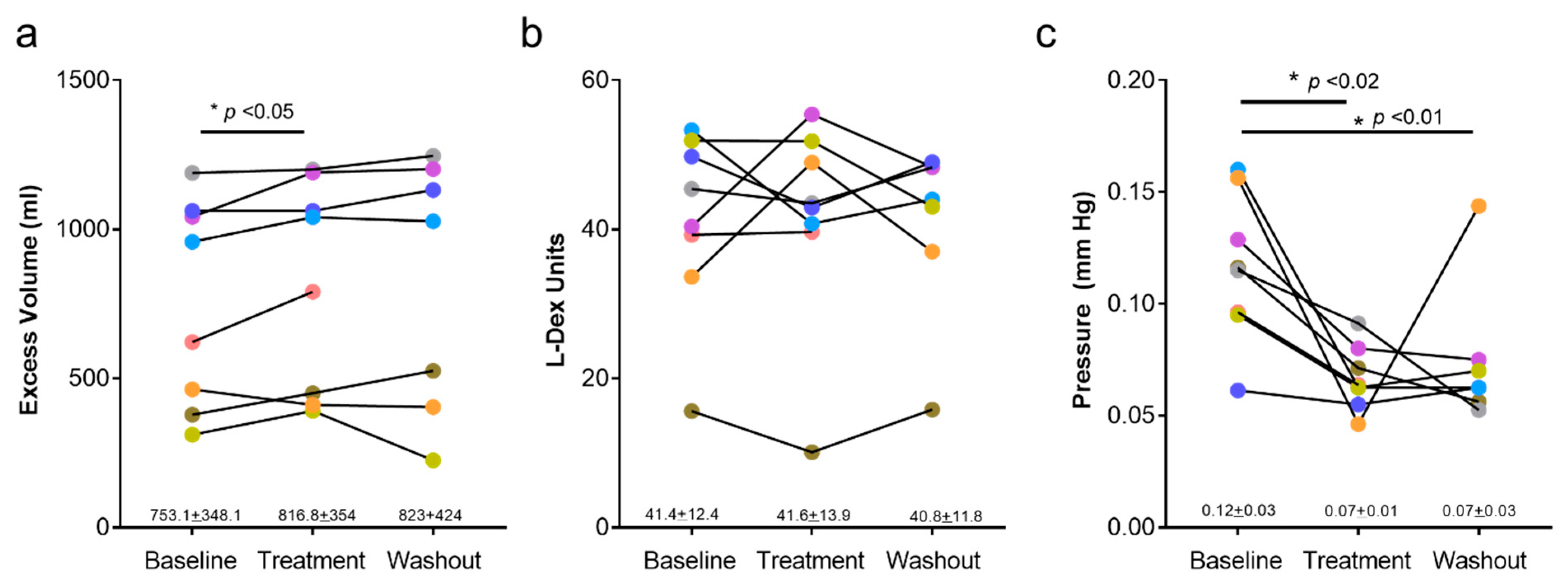
3.3. Arm Volume and Bioimpedance Measurements
3.4. Quality of Life Outcomes
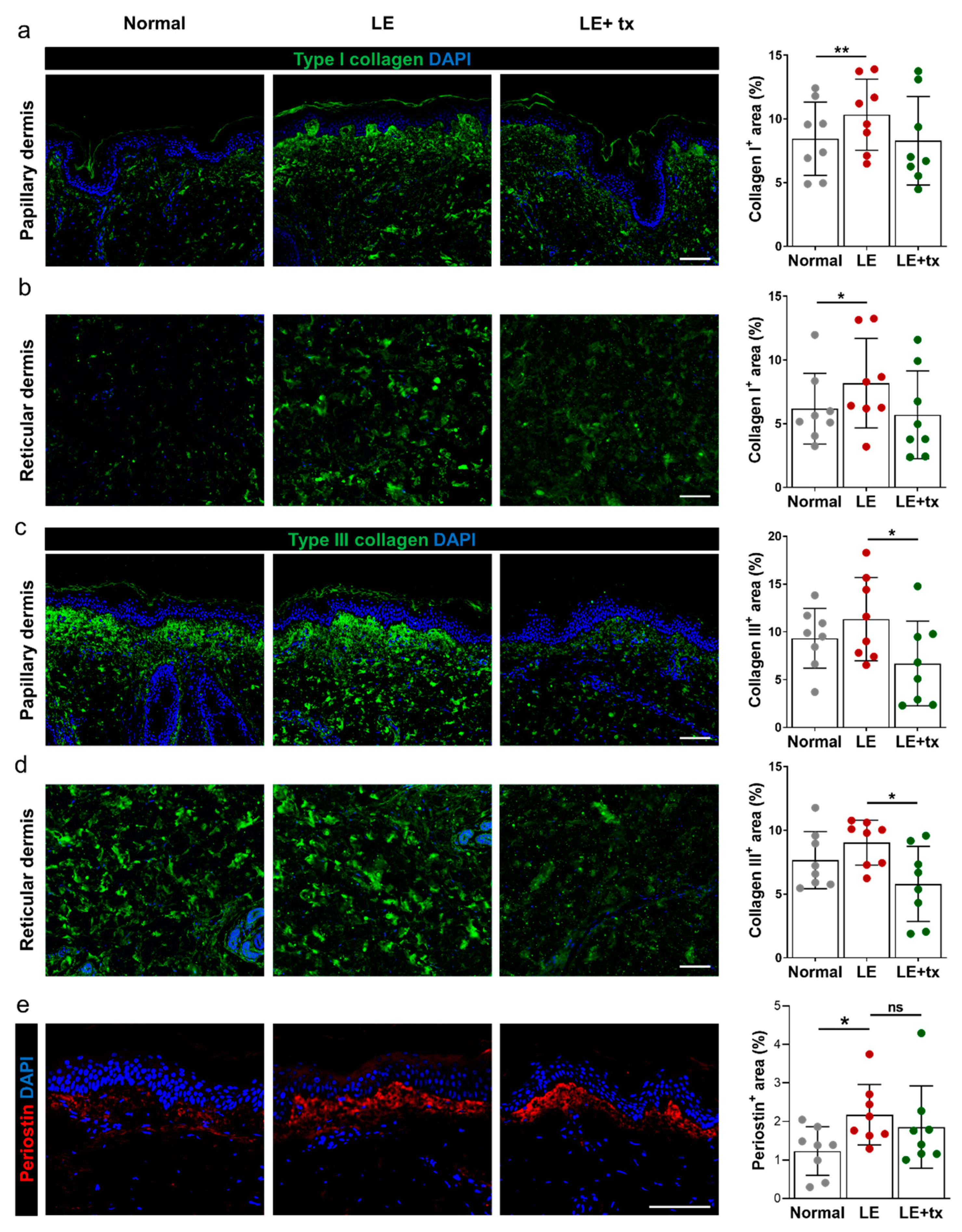
3.5. Histologic Analysis
QBX258 Treatment Decreases Hyperkeratosis and Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrek, J.A.; Senie, R.T.; Peters, M.; Rosen, P.P. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001, 92, 1368–1377. [Google Scholar] [CrossRef]
- Rockson, S.G.; Rivera, K.K. Estimating the population burden of lymphedema. Ann. N. Y. Acad. Sci. 2008, 1131, 147–154. [Google Scholar] [CrossRef]
- McLaughlin, S.A.; Wright, M.J.; Morris, K.T.; Giron, G.L.; Sampson, M.R.; Brockway, J.P.; Hurley, K.E.; Riedel, E.R.; Van Zee, K.J. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J. Clin. Oncol. 2008, 26, 5213–5219. [Google Scholar] [CrossRef] [PubMed]
- Cormier, J.N.; Askew, R.L.; Mungovan, K.S.; Xing, Y.; Ross, M.I.; Armer, J.M. Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010, 116, 5138–5149. [Google Scholar] [CrossRef]
- Goffman, T.E.; Laronga, C.; Wilson, L.; Elkins, D. Lymphedema of the arm and breast in irradiated breast cancer patients: Risks in an era of dramatically changing axillary surgery. Breast J. 2004, 10, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Janda, M.; Cornish, B.; Battistutta, D.; Newman, B. Lymphedema after breast cancer: Incidence, risk factors, and effect on upper body function. J. Clin. Oncol. 2008, 26, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Soran, A.; D’Angelo, G.; Begovic, M.; Ardic, F.; Harlak, A.; Samuel Wieand, H.; Vogel, V.G.; Johnson, R.R. Breast cancer-related lymphedema—What are the significant predictors and how they affect the severity of lymphedema? Breast J. 2006, 12, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.L.; Prizment, A.; Lazovich, D.; Schmitz, K.H.; Folsom, A.R. Lymphedema and quality of life in breast cancer survivors: The Iowa Women’s Health Study. J. Clin. Oncol. 2008, 26, 5689–5696. [Google Scholar] [CrossRef]
- Shih, Y.C.; Xu, Y.; Cormier, J.N.; Giordano, S.; Ridner, S.H.; Buchholz, T.A.; Perkins, G.H.; Elting, L.S. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: A 2-year follow-up study. J. Clin. Oncol. 2009, 27, 2007–2014. [Google Scholar] [CrossRef]
- Szuba, A.; Rockson, S.G. Lymphedema: Anatomy, physiology and pathogenesis. Vasc. Med. 1997, 2, 321–326. [Google Scholar] [CrossRef]
- Szuba, A.; Rockson, S.G. Lymphedema: Classification, diagnosis and therapy. Vasc. Med. 1998, 3, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Norman, S.A.; Localio, A.R.; Potashnik, S.L.; Simoes Torpey, H.A.; Kallan, M.J.; Weber, A.L.; Miller, L.T.; Demichele, A.; Solin, L.J. Lymphedema in breast cancer survivors: Incidence, degree, time course, treatment, and symptoms. J. Clin. Oncol. 2009, 27, 390–397. [Google Scholar] [CrossRef]
- Nakamura, K.; Radhakrishnan, K.; Wong, Y.M.; Rockson, S.G. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS ONE 2009, 4, e8380. [Google Scholar] [CrossRef] [PubMed]
- Avraham, T.; Zampell, J.C.; Yan, A.; Elhadad, S.; Weitman, E.S.; Rockson, S.G.; Bromberg, J.; Mehrara, B.J. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013, 27, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Zampell, J.C.; Yan, A.; Elhadad, S.; Avraham, T.; Weitman, E.; Mehrara, B.J. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS ONE 2012, 7, e49940. [Google Scholar] [CrossRef]
- Garcia Nores, G.D.; Ly, C.L.; Cuzzone, D.A.; Kataru, R.P.; Hespe, G.E.; Torrisi, J.S.; Huang, J.J.; Gardenier, J.C.; Savetsky, I.L.; Nitti, M.D.; et al. CD4(+) T cells are activated in regional lymph nodes and migrate to skin to initiate lymphedema. Nat. Commun. 2018, 9, 1970. [Google Scholar] [CrossRef]
- Gardenier, J.C.; Kataru, R.P.; Hespe, G.E.; Savetsky, I.L.; Torrisi, J.S.; Nores, G.D.; Jowhar, D.K.; Nitti, M.D.; Schofield, R.C.; Carlow, D.C.; et al. Topical tacrolimus for the treatment of secondary lymphedema. Nat. Commun. 2017, 8, 14345. [Google Scholar] [CrossRef]
- Ghanta, S.; Cuzzone, D.A.; Torrisi, J.S.; Albano, N.J.; Joseph, W.J.; Savetsky, I.L.; Gardenier, J.C.; Chang, D.; Zampell, J.C.; Mehrara, B.J. Regulation of inflammation and fibrosis by macrophages in lymphedema. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1065–H1077. [Google Scholar] [CrossRef]
- Ogata, F.; Fujiu, K.; Matsumoto, S.; Nakayama, Y.; Shibata, M.; Oike, Y.; Koshima, I.; Watabe, T.; Nagai, R.; Manabe, I. Excess lymphangiogenesis cooperatively induced by macrophages and CD4(+) T cells drives the pathogenesis of lymphedema. J. Investig. Dermatol. 2016, 136, 706–714. [Google Scholar] [CrossRef]
- Furlong-Silva, J.; Cross, S.D.; Marriott, A.E.; Pionnier, N.; Archer, J.; Steven, A.; Schulte-Merker, S.; Mack, M.; Hong, Y.K.; Taylor, M.J.; et al. Tetracyclines improve experimental lymphatic filariasis pathology by disrupting interleukin-4 receptor-mediated lymphangiogenesis. J. Clin. Investig. 2021, 131, e140853. [Google Scholar] [CrossRef]
- Ly, C.L.; Nores, G.D.G.; Kataru, R.P.; Mehrara, B.J. T helper 2 differentiation is necessary for development of lymphedema. Transl. Res. 2019, 206, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Savetsky, I.L.; Ghanta, S.; Gardenier, J.C.; Torrisi, J.S.; Garcia Nores, G.D.; Hespe, G.E.; Nitti, M.D.; Kataru, R.P.; Mehrara, B.J. Th2 cytokines inhibit lymphangiogenesis. PLoS ONE 2015, 10, e0126908. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef]
- Sastre, J.; Davila, I. Dupilumab: A new paradigm for the treatment of allergic diseases. J. Investig. Allergol. Clin. Immunol. 2018, 28, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Mannent, L.; Naclerio, R.M.; Mullol, J.; Ferguson, B.J.; Gevaert, P.; Hellings, P.; Jiao, L.; Wang, L.; Evans, R.R.; et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: A randomized clinical trial. JAMA 2016, 315, 469–479. [Google Scholar] [CrossRef]
- Kaye, A.; Gordon, S.C.; Deverapalli, S.C.; Her, M.J.; Rosmarin, D. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018, 154, 1225–1226. [Google Scholar] [CrossRef]
- Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Deltombe, T.; Jamart, J.; Recloux, S.; Legrand, C.; Vandenbroeck, N.; Theys, S.; Hanson, P. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology 2007, 40, 26–34. [Google Scholar]
- Stanton, A.W.; Northfield, J.W.; Holroyd, B.; Mortimer, P.S.; Levick, J.R. Validation of an optoelectronic limb volumeter (Perometer). Lymphology 1997, 30, 77–97. [Google Scholar] [PubMed]
- Cornish, B. Bioimpedance analysis: Scientific background. Lymphat. Res. Biol. 2006, 4, 47–50. [Google Scholar] [CrossRef]
- Wiser, I.; Mehrara, B.J.; Coriddi, M.; Kenworthy, E.; Cavalli, M.; Encarnacion, E.; Dayan, J.H. Preoperative Assessment of Upper Extremity Secondary Lymphedema. Cancers 2020, 12, 135. [Google Scholar] [CrossRef]
- Cornish, B.H.; Chapman, M.; Hirst, C.; Mirolo, B.; Bunce, I.H.; Ward, L.C.; Thomas, B.J. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology 2001, 34, 2–11. [Google Scholar]
- Gerber, L.H. A review of measures of lymphedema. Cancer 1998, 83, 2803–2804. [Google Scholar] [CrossRef]
- Carati, C.J.; Anderson, S.N.; Gannon, B.J.; Piller, N.B. Treatment of postmastectomy lymphedema with low-level laser therapy: A double blind, placebo-controlled trial. Cancer 2003, 98, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Moseley, A.; Piller, N. Reliability of bioimpedance spectroscopy and tonometry after breast conserving cancer treatment. Lymphat. Res. Biol. 2008, 6, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Pusic, A.L.; Cemal, Y.; Albornoz, C.; Klassen, A.; Cano, S.; Sulimanoff, I.; Hernandez, M.; Massey, M.; Cordeiro, P.; Morrow, M.; et al. Quality of life among breast cancer patients with lymphedema: A systematic review of patient-reported outcome instruments and outcomes. J. Cancer Surviv. 2013, 7, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Viehoff, P.B.; van Genderen, F.R.; Wittink, H. Upper limb lymphedema 27 (ULL27): Dutch translation and validation of an illness-specific health-related quality of life questionnaire for patients with upper limb lymphedema. Lymphology 2008, 41, 131–138. [Google Scholar]
- Launois, R.; Mègnigbêto, A.; Pocquet, K.; Alliot, F. A specific quality of life scale in upper limb lymphedema: The ULL-27 questionnaire. Lymphology 2002, 35, 181–187. [Google Scholar]
- Andersen, L.; Hojris, I.; Erlandsen, M.; Andersen, J. Treatment of breast-cancer-related lymphedema with or without manual lymphatic drainage—A randomized study. Acta Oncol. 2000, 39, 399–405. [Google Scholar] [CrossRef]
- Masuoka, M.; Shiraishi, H.; Ohta, S.; Suzuki, S.; Arima, K.; Aoki, S.; Toda, S.; Inagaki, N.; Kurihara, Y.; Hayashida, S.; et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J. Clin. Investig. 2012, 122, 2590–2600. [Google Scholar] [CrossRef]
- Takayama, G.; Arima, K.; Kanaji, T.; Toda, S.; Tanaka, H.; Shoji, S.; McKenzie, A.N.; Nagai, H.; Hotokebuchi, T.; Izuhara, K. Periostin: A novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J. Allergy Clin. Immunol. 2006, 118, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The staining of mast cells: A historical overview. Int. Arch. Allergy Immunol. 2018, 176, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A.; Spergel, J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev. Clin. Immunol. 2014, 10, 1463–1474. [Google Scholar] [CrossRef]
- Miyata, M.; Hatsushika, K.; Ando, T.; Shimokawa, N.; Ohnuma, Y.; Katoh, R.; Suto, H.; Ogawa, H.; Masuyama, K.; Nakao, A. Mast cell regulation of epithelial TSLP expression plays an important role in the development of allergic rhinitis. Eur. J. Immunol. 2008, 38, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.; et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef]
- Ramasamy, A.; Kuokkanen, M.; Vedantam, S.; Gajdos, Z.K.; Couto Alves, A.; Lyon, H.N.; Ferreira, M.A.; Strachan, D.P.; Zhao, J.H.; Abramson, M.J.; et al. Genome-wide association studies of asthma in population-based cohorts confirm known and suggested loci and identify an additional association near HLA. PLoS ONE 2012, 7, e44008. [Google Scholar] [CrossRef]
- Pedroza-Gonzalez, A.; Xu, K.; Wu, T.C.; Aspord, C.; Tindle, S.; Marches, F.; Gallegos, M.; Burton, E.C.; Savino, D.; Hori, T.; et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J. Exp. Med. 2011, 208, 479–490. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Pires, I.; Prada, J.; Queiroga, F.L. A role for T-lymphocytes in human breast cancer and in canine mammary tumors. BioMed Res. Int. 2014, 2014, 130894. [Google Scholar] [CrossRef]
- Aspord, C.; Pedroza-Gonzalez, A.; Gallegos, M.; Tindle, S.; Burton, E.C.; Su, D.; Marches, F.; Banchereau, J.; Palucka, A.K. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J. Exp. Med. 2007, 204, 1037–1047. [Google Scholar] [CrossRef]
- Blais, Y.; Gingras, S.; Haagensen, D.E.; Labrie, F.; Simard, J. Interleukin-4 and interleukin-13 inhibit estrogen-induced breast cancer cell proliferation and stimulate GCDFP-15 expression in human breast cancer cells. Mol. Cell. Endocrinol. 1996, 121, 11–18. [Google Scholar] [CrossRef]
- Minn, A.J.; Gupta, G.P.; Siegel, P.M.; Bos, P.D.; Shu, W.; Giri, D.D.; Viale, A.; Olshen, A.B.; Gerald, W.L.; Massague, J. Genes that mediate breast cancer metastasis to lung. Nature 2005, 436, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Terabe, M.; Matsui, S.; Noben-Trauth, N.; Chen, H.; Watson, C.; Donaldson, D.D.; Carbone, D.P.; Paul, W.E.; Berzofsky, J.A. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 2000, 1, 515–520. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kobayashi, H.; Pollard, R.B.; Suzuki, F. A pathogenic role of Th2 cells and their cytokine products on the pulmonary metastasis of murine B16 melanoma. J. Immunol. 1998, 160, 5869–5873. [Google Scholar] [PubMed]
- Kawakami, K.; Kawakami, M.; Husain, S.R.; Puri, R.K. Potent antitumor activity of IL-13 cytotoxin in human pancreatic tumors engineered to express IL-13 receptor alpha2 chain in vivo. Gene Ther. 2003, 10, 1116–1128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wynn, T.A. IL-13 effector functions. Annu. Rev. Immunol. 2003, 21, 425–456. [Google Scholar] [CrossRef]
- Lee, T.S.; Morris, C.M.; Czerniec, S.A.; Mangion, A.J. Does lymphedema severity affect quality of life? Simple question. Challenging answers. Lymphat. Res. Biol. 2018, 16, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tabibiazar, R.; Cheung, L.; Han, J.; Swanson, J.; Beilhack, A.; An, A.; Dadras, S.S.; Rockson, N.; Joshi, S.; Wagner, R.; et al. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 2006, 3, e254. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G.; Tian, W.; Jiang, X.; Kuznetsova, T.; Haddad, F.; Zampell, J.; Mehrara, B.; Sampson, J.P.; Roche, L.; Kim, J.; et al. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight 2018, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Rockson, S.G.; Jiang, X.; Kim, J.; Begaye, A.; Shuffle, E.M.; Tu, A.B.; Cribb, M.; Nepiyushchikh, Z.; Feroze, A.H.; et al. Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci. Transl. Med. 2017, 9, eaal3920. [Google Scholar] [CrossRef] [PubMed]
- Mand, S.; Debrah, A.Y.; Klarmann, U.; Batsa, L.; Marfo-Debrekyei, Y.; Kwarteng, A.; Specht, S.; Belda-Domene, A.; Fimmers, R.; Taylor, M.; et al. Doxycycline improves filarial lymphedema independent of active filarial infection: A randomized controlled trial. Clin. Infect. Dis. 2012, 55, 621–630. [Google Scholar] [CrossRef]
- Taghian, N.R.; Miller, C.L.; Jammallo, L.S.; O’Toole, J.; Skolny, M.N. Lymphedema following breast cancer treatment and impact on quality of life: A review. Crit. Rev. Oncol. Hematol. 2014, 92, 227–234. [Google Scholar] [CrossRef]
- Toyserkani, N.M.; Jensen, C.H.; Andersen, D.C.; Sheikh, S.P.; Sorensen, J.A. Treatment of breast cancer-related lymphedema with adipose-derived regenerative cells and fat grafts: A feasibility and safety study. Stem Cells Transl. Med. 2017, 6, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, J.S.; Joseph, W.J.; Ghanta, S.; Cuzzone, D.A.; Albano, N.J.; Savetsky, I.L.; Gardenier, J.C.; Skoracki, R.; Chang, D.; Mehrara, B.J. Lymphaticovenous bypass decreases pathologic skin changes in upper extremity breast cancer-related lymphedema. Lymphat. Res. Biol. 2015, 13, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimaki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A.; et al. IL-33 and ST2 in atopic dermatitis: Expression profiles and modulation by triggering factors. J. Investig. Dermatol. 2012, 132, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Kottyan, L.C.; Davis, B.P.; Sherrill, J.D.; Liu, K.; Rochman, M.; Kaufman, K.; Weirauch, M.T.; Vaughn, S.; Lazaro, S.; Rupert, A.M.; et al. Genome-wide association analysis of eosinophilic esophagitis provides insight into the tissue specificity of this allergic disease. Nat. Genet. 2014, 46, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdi, Z.; Comeau, M.R.; Jessup, H.K.; Yoon, B.R.; Brewer, A.; Chartier, S.; Paquette, N.; Ziegler, S.F.; Sarfati, M.; Delespesse, G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007, 204, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.C.; Zaph, C.; Troy, A.E.; Du, Y.; Guild, K.J.; Comeau, M.R.; Artis, D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 2009, 206, 655–667. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef]
- Yoo, J.; Omori, M.; Gyarmati, D.; Zhou, B.; Aye, T.; Brewer, A.; Comeau, M.R.; Campbell, D.J.; Ziegler, S.F. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 2005, 202, 541–549. [Google Scholar] [CrossRef]
- Jessup, H.K.; Brewer, A.W.; Omori, M.; Rickel, E.A.; Budelsky, A.L.; Yoon, B.R.; Ziegler, S.F.; Comeau, M.R. Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. J. Immunol. 2008, 181, 4311–4319. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lenig, D.; Mackay, C.R.; Lanzavecchia, A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 1998, 187, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in adults with uncontrolled asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Parnes, J.R.; She, D.; Crouch, S.; Rees, W.; Mo, M.; van der Merwe, R. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J. Am. Acad. Dermatol. 2019, 80, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Pushparaj, P.N.; Tay, H.K.; H’Ng, S.C.; Pitman, N.; Xu, D.; McKenzie, A.; Liew, F.Y.; Melendez, A.J. The cytokine interleukin-33 mediates anaphylactic shock. Proc. Natl. Acad. Sci. USA 2009, 106, 9773–9778. [Google Scholar] [CrossRef]
- Martin, L.J.; He, H.; Collins, M.H.; Abonia, J.P.; Biagini Myers, J.M.; Eby, M.; Johansson, H.; Kottyan, L.C.; Khurana Hershey, G.K.; Rothenberg, M.E. Eosinophilic esophagitis (EoE) genetic susceptibility is mediated by synergistic interactions between EoE-specific and general atopic disease loci. J. Allergy Clin. Immunol. 2018, 141, 1690–1698. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Tamagawa-Mineoka, R.; Okuzawa, Y.; Masuda, K.; Katoh, N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdi, Z.; Smith, D.E.; Comeau, M.R.; Delespesse, G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J. Immunol. 2007, 179, 2051–2054. [Google Scholar] [CrossRef]
- Kakkar, R.; Hei, H.; Dobner, S.; Lee, R.T. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J. Biol. Chem. 2012, 287, 6941–6948. [Google Scholar] [CrossRef]
- Lefrancais, E.; Roga, S.; Gautier, V.; Gonzalez-de-Peredo, A.; Monsarrat, B.; Girard, J.P.; Cayrol, C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl. Acad. Sci. USA 2012, 109, 1673–1678. [Google Scholar] [CrossRef]
- Fort, M.M.; Cheung, J.; Yen, D.; Li, J.; Zurawski, S.M.; Lo, S.; Menon, S.; Clifford, T.; Hunte, B.; Lesley, R.; et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 2001, 15, 985–995. [Google Scholar] [CrossRef]
- Hong, H.; Liao, S.; Chen, F.; Yang, Q.; Wang, D.Y. Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy 2020, 75, 2794–2804. [Google Scholar] [CrossRef] [PubMed]
- Cemal, Y.; Jewell, S.; Albornoz, C.R.; Pusic, A.; Mehrara, B.J. Systematic review of quality of life and patient reported outcomes in patients with oncologic related lower extremity lymphedema. Lymphat. Res. Biol. 2013, 11, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Green, J.M.; Paladugu, S.; Shuyu, X.; Stewart, B.R.; Shyu, C.R.; Armer, J.M. Using temporal mining to examine the development of lymphedema in breast cancer survivors. Nurs. Res. 2013, 62, 122–129. [Google Scholar] [CrossRef] [PubMed]




| Inclusion | Exclusion |
|---|---|
| Women 18–70 with unilateral stage I or II BCRL | Bilateral lymphedema or history of bilateral axillary lymph node dissection |
| Volume difference of at least 300 mL between the normal and lymphedema limb | Recent (within last 3 months) history of cellulitis |
| BMI 18–30 | Current (within last month) use of chemotherapy or radiation |
| No current evidence of breast cancer | Recent (within last month) or current intensive manual lymphatic massage and/or short stretch bandage use |
| At least 6 months postop from axillary lymph node dissection | Unstable lymphedema (i.e., worsening symptoms/measurements in the past 3 months) |
| Pregnant or nursing (lactating) women | |
| Stage III lymphedema | |
| Chronic use of acetaminophen (>1 gm/day for ≥3/7 days, or >2 gm/day for ≥1 day) | |
| Use of other investigational drugs ≤30 days or 5 half-lives of enrollment (whichever is longer) | |
| History of hypersensitivity to study drugs or to drugs of similar chemical classes (e.g., monoclonal antibodies, polyclonal gamma globulin, polysorbates). |
| Assessment | Enrollment | Infusion 1 | Infusion 2 | Infusion 3 | Infusion 4 | Outcome | Washout |
|---|---|---|---|---|---|---|---|
| Week −2–0 | Week 0 | Week 4 | Week 8 | Week 12 | Week 12–15 | Week 2835 | |
| Demographics | x | ||||||
| Physical exam | x | x | x | x | x | x | x |
| Pregnancy test | x | x | x | x | x | ||
| ECG | x | x | |||||
| Blood tests, Urinalysis | x | x | |||||
| Arm volumes | x | x | x | ||||
| Bioimpedance | x | x | x | ||||
| Tonometry | x | x | x | ||||
| QOL questionnaire | x | x | x | ||||
| Skin biopsy | x | x | |||||
| Adverse event check | x | x | x | x | x | x | x |
| Physical Functioning | Psychological Dimension | Social Dimension |
|---|---|---|
| Difficulties grasping high objects | Feeling sad | Difficulty taking advantage of good weather, such as outside the house |
| Difficulties maintaining certain positions | Feeling Discouraged | Difficulty with personal projects, holidays, hobbies |
| Arm feels heavy | Feeling a lack of self-confidence | Difficulties in emotional life with spouse or partner |
| Arm feels swollen | Feeling well in one’s self | Difficulty in social life |
| Difficulties dressing | Feeling a wish to be angry | Fearful of looking in the mirror |
| Difficulties going to sleep | Having confidence in the future | |
| Difficulties holding objects | ||
| Difficulties walking/heavy arm | ||
| Difficulties washing | ||
| Difficulties taking public transport | ||
| Tingling, burning feelings | ||
| Feeling of swollen, hard, tense skin | ||
| Difficulties in working relationship and tasks |
| Patient | Age | Stage | Years with Disease | Time to Develop Lymphedema | History of Cellulitis? | BMI |
|---|---|---|---|---|---|---|
| 1 | 62 | II | 8 | ~3 years | No | 22.1 |
| 2 | 59 | II | 3 | ~8 months | No | 27.5 |
| 3 | 59 | II | 5 | <1 year | Yes | 25.3 |
| 5 | 36 | II | 3 | <1 year | No | 29.7 |
| 6 | 49 | II | 9 | <1 year | No | 29.8 |
| 7 | 59 | II | 18 | <1 year | Yes | 24.7 |
| 8 | 46 | II | 11 | <1 year | Yes | 27.7 |
| 9 | 60 | II | 4 | <1 year | Yes | 29.1 |
| Patient | Baseline Lymphedema Treatment |
|---|---|
| 1 | Massage, exercise |
| 2 | Physical therapy 2 × a week, lymphedema pump (~3 × a week) |
| 3 | Sleeve at night, self-manual lymphatic massage (MLD) |
| 5 | MLD 1 × a week, ACE bandage at night, occasionally pump |
| 6 | Sleeve/glove 24 h/day, compression pump, intermittent self-MLD |
| 7 | Wrapping at nighttime, lymph node transfer |
| 8 | Compression sleeve |
| 9 | Sleeve during the day, short stretch bandage 4 nights/week, lymph node transplant |
| Adverse Event | Patients (n) |
|---|---|
| Restless legs | 2 |
| Tiredness | 1 |
| Dyspnea on exertion | 1 |
| Heartburn | 1 |
| Food poisoning | 1 |
| Rash | 1 |
| Trouble sleeping | 1 |
| Light-headedness | 1 |
| Sore throat | 1 |
| Upper lip numbness | 1 |
| Cellulitis | 1 |
| Pulmonary metastases | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehrara, B.J.; Park, H.J.; Kataru, R.P.; Bromberg, J.; Coriddi, M.; Baik, J.E.; Shin, J.; Li, C.; Cavalli, M.R.; Encarnacion, E.M.; et al. Pilot Study of Anti-Th2 Immunotherapy for the Treatment of Breast Cancer-Related Upper Extremity Lymphedema. Biology 2021, 10, 934. https://doi.org/10.3390/biology10090934
Mehrara BJ, Park HJ, Kataru RP, Bromberg J, Coriddi M, Baik JE, Shin J, Li C, Cavalli MR, Encarnacion EM, et al. Pilot Study of Anti-Th2 Immunotherapy for the Treatment of Breast Cancer-Related Upper Extremity Lymphedema. Biology. 2021; 10(9):934. https://doi.org/10.3390/biology10090934
Chicago/Turabian StyleMehrara, Babak J., Hyeung Ju Park, Raghu P. Kataru, Jacqueline Bromberg, Michelle Coriddi, Jung Eun Baik, Jinyeon Shin, Claire Li, Michele R. Cavalli, Elizabeth M. Encarnacion, and et al. 2021. "Pilot Study of Anti-Th2 Immunotherapy for the Treatment of Breast Cancer-Related Upper Extremity Lymphedema" Biology 10, no. 9: 934. https://doi.org/10.3390/biology10090934
APA StyleMehrara, B. J., Park, H. J., Kataru, R. P., Bromberg, J., Coriddi, M., Baik, J. E., Shin, J., Li, C., Cavalli, M. R., Encarnacion, E. M., Lee, M., Van Zee, K. J., Riedel, E., & Dayan, J. H. (2021). Pilot Study of Anti-Th2 Immunotherapy for the Treatment of Breast Cancer-Related Upper Extremity Lymphedema. Biology, 10(9), 934. https://doi.org/10.3390/biology10090934