Human Periodontal Ligament Stem Cells Response to Titanium Implant Surface: Extracellular Matrix Deposition
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Cell Culture
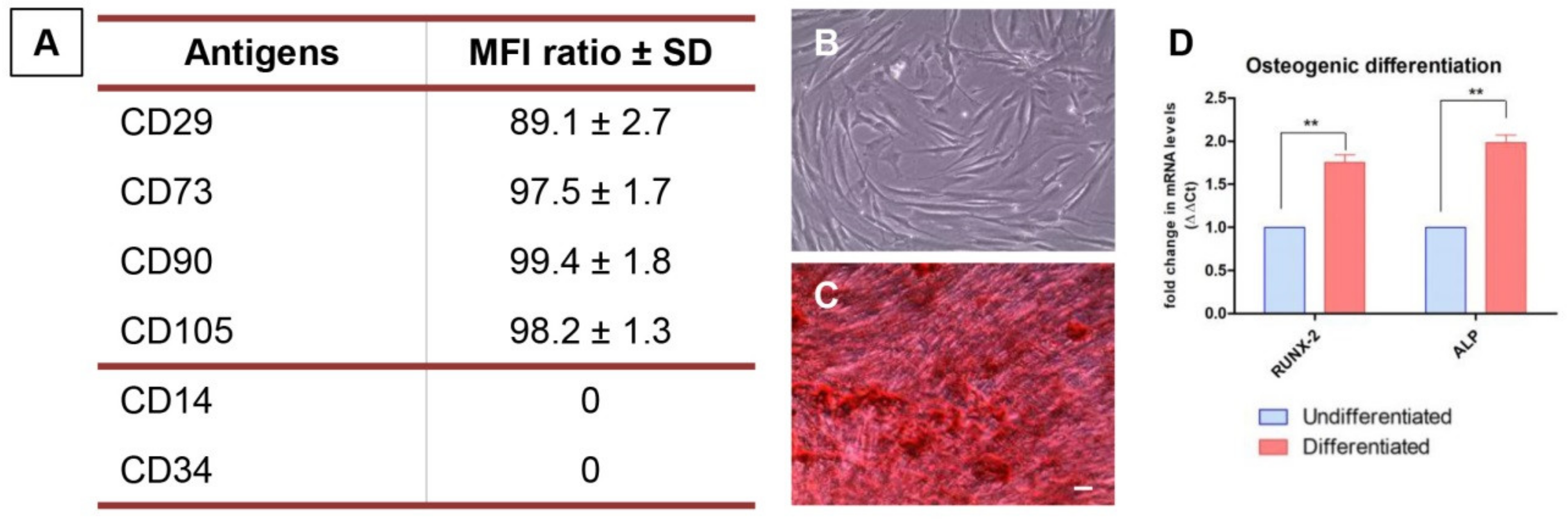
2.3. hPDLSCs Characterization
2.4. Dental Implants
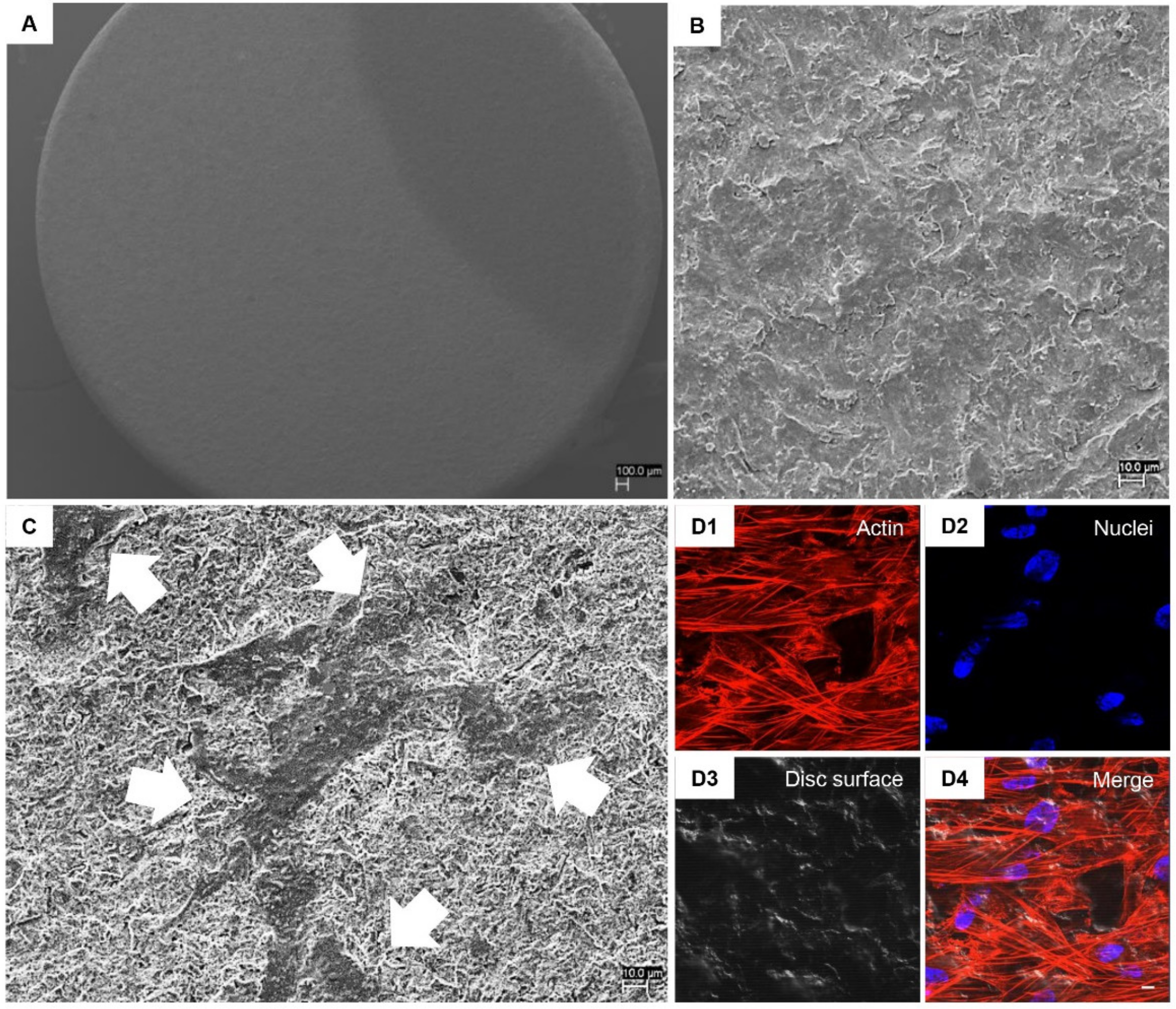
2.5. Scanning Electron Microscopy (SEM) Analysis
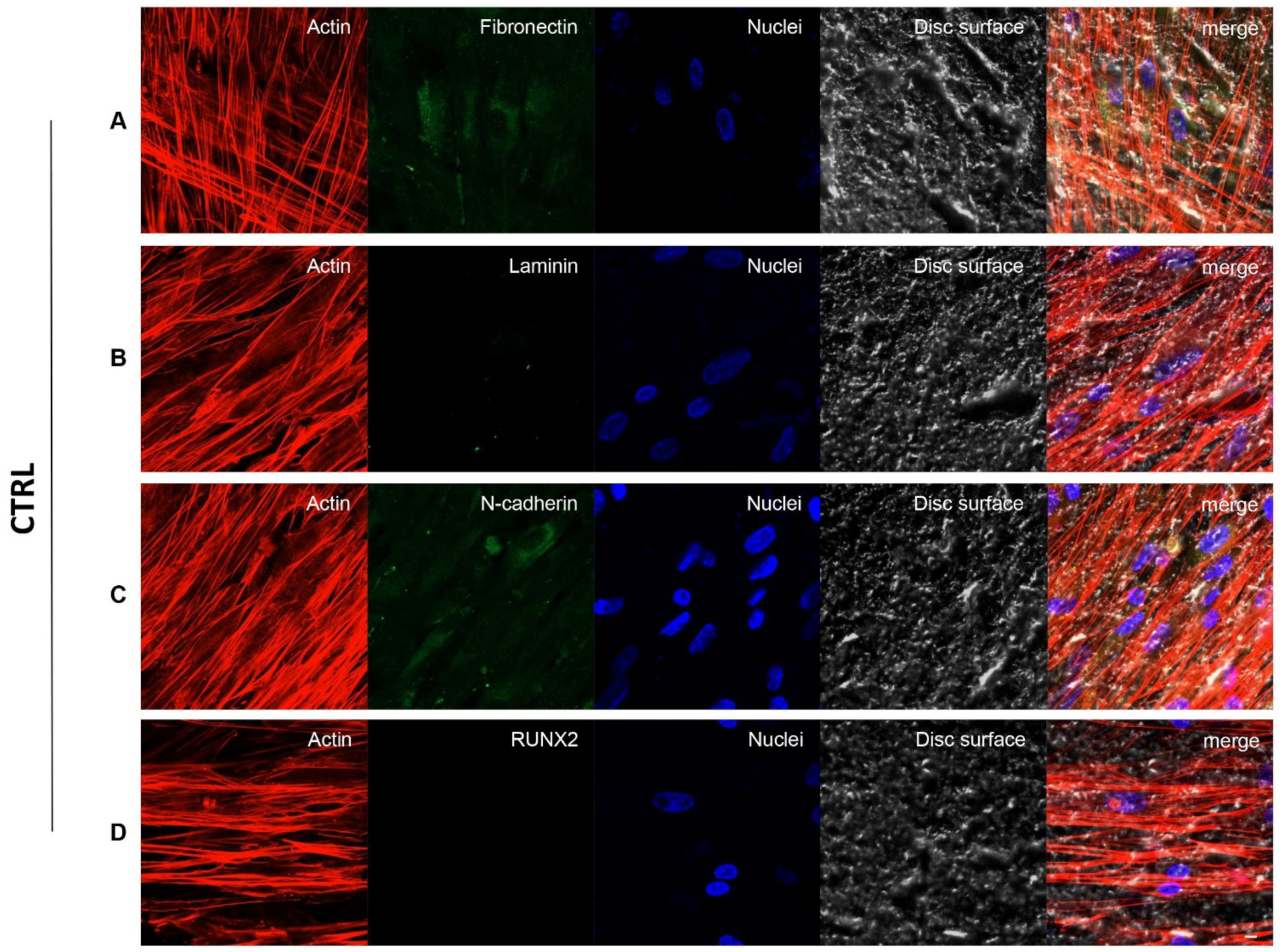
2.6. Confocal Laser Scanning Microscopy (CLSM) Analysis
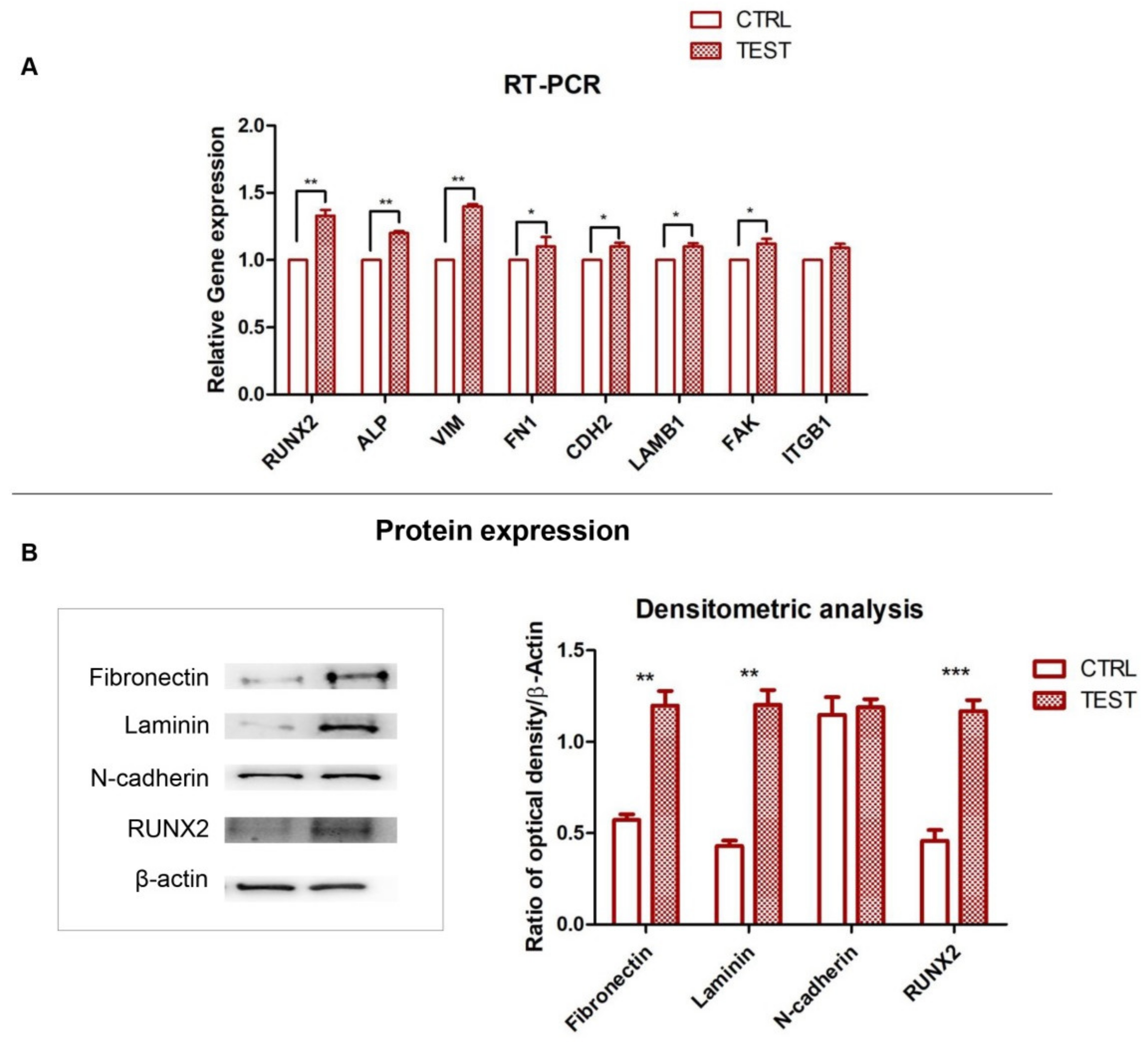
2.7. Gene Expression
2.8. Protein Expression
2.9. Statistical Analysis
3. Results
3.1. hPDLSCs Characterization
3.2. hPDLSCs Adhesion Capability on Implant Surfaces
3.3. Fibronectin, Laminin, N-Cadherin and RUNX2 Overexpression in TEST Surface Titanium Implant by CLSM
3.4. RUNX2, ALP and ECM Components: Gene and Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | extracellular matrix |
| hPDLSCs | human periodontal ligament stem cells |
| ITGB1 | integrin beta-1 |
| FAK | focal adhesion kinase |
| FN | fibronectin |
| CDH2 | N-cadherin |
| LAMB1, | laminin |
| ALP | alkaline phosphatase |
| RUNX2 | runt related transcription factor 2 |
| SEM | scanning electron microscopy |
| CLSM | confocal laser scanning microscopy |
| VEGF | vascular endothelial growth factor |
| PBS | phosphate buffered saline |
| RT-PCR | real time–polymerase chain reaction |
| MSCs | mesenchymal stem cells |
| RT | room temperature |
| ECL | chemiluminescence detection method |
| IFs | intermediate filaments |
| VIM | vimentin |
References
- Le Guehennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, F.; Antonelli, A.; Brancaccio, Y.; Averta, F.; Figliuzzi, M.M.; Fortunato, L.; Giudice, A. Primary Stability of Three Different Osteotomy Techniques in Medullary Bone: An in Vitro Study. Dent. J. 2020, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Liddell, R.S.; Ajami, E.; Li, Y.; Bajenova, E.; Yang, Y.; Davies, J.E. The influence of implant design on the kinetics of osseointegration and bone anchorage homeostasis. Acta Biomater. 2021, 121, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S.; Coulthard, P. Bone quality: A reality for the process of osseointegration. Implant. Dent. 2009, 18, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontol. 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Antonelli, A.; Bennardo, F.; Brancaccio, Y.; Barone, S.; Femiano, F.; Nucci, L.; Minervini, G.; Fortunato, L.; Attanasio, F.; Giudice, A. Can Bone Compaction Improve Primary Implant Stability? An In Vitro Comparative Study with Osseodensification Technique. Appl. Sci. 2020, 10, 8623. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.S.; Marconi, G.D.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef]
- Soundara Rajan, T.; Giacoppo, S.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Human periodontal ligament stem cells secretome from multiple sclerosis patients suppresses NALP3 inflammasome activation in experimental autoimmune encephalomyelitis. Int. J. Immunopathol. Pharmacol. 2017, 30, 238–252. [Google Scholar] [CrossRef]
- Orbay, H.; Tobita, M.; Mizuno, H. Mesenchymal stem cells isolated from adipose and other tissues: Basic biological properties and clinical applications. Stem. Cells Int. 2012, 2012, 461718. [Google Scholar] [CrossRef]
- Giudice, A.; Antonelli, A.; Chiarella, E.; Baudi, F.; Barni, T.; Di Vito, A. The Case of Medication-Related Osteonecrosis of the Jaw Addressed from a Pathogenic Point of View. Innovative Therapeutic Strategies: Focus on the Most Recent Discoveries on Oral Mesenchymal Stem Cell-Derived Exosomes. Pharmaceuticals 2020, 13, 423. [Google Scholar] [CrossRef]
- Vazquez, A.; Fernandez-Sevilla, L.M.; Jimenez, E.; Perez-Cabrera, D.; Yanez, R.; Subiza, J.L.; Varas, A.; Valencia, J.; Vicente, A. Involvement of Mesenchymal Stem Cells in Oral Mucosal Bacterial Immunotherapy. Front. Immunol. 2020, 11, 567391. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lozano, F.J.; Bueno, C.; Insausti, C.L.; Meseguer, L.; Ramirez, M.C.; Blanquer, M.; Marin, N.; Martinez, S.; Moraleda, J.M. Mesenchymal stem cells derived from dental tissues. Int. Endod. J. 2011, 44, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; D’Aurora, M.; Gugliandolo, A.; Merciaro, I.; Orsini, T.; Gatta, V.; Piattelli, A.; Trubiani, O.; Mazzon, E. Biofunctionalized Scaffold in Bone Tissue Repair. Int. J. Mol. Sci. 2018, 19, 1022. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Diomede, F.; Pizzicannella, J.; Fonticoli, L.; Merciaro, I.; Pierdomenico, S.D.; Mazzon, E.; Piattelli, A.; Trubiani, O. Enhanced VEGF/VEGF-R and RUNX2 Expression in Human Periodontal Ligament Stem Cells Cultured on Sandblasted/Etched Titanium Disk. Front. Cell Dev. Biol. 2020, 8, 315. [Google Scholar] [CrossRef]
- Chu, L.; Jiang, G.; Hu, X.L.; James, T.D.; He, X.P.; Li, Y.; Tang, T. Osteogenesis, vascularization and osseointegration of a bioactive multiphase macroporous scaffold in the treatment of large bone defects. J. Mater. Chem. B 2018, 6, 4197–4204. [Google Scholar] [CrossRef]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; Del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Investig. Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef]
- Lin, Z.; Rios, H.F.; Volk, S.L.; Sugai, J.V.; Jin, Q.; Giannobile, W.V. Gene expression dynamics during bone healing and osseointegration. J. Periodontol. 2011, 82, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Niessen, C.M.; Leckband, D.; Yap, A.S. Tissue organization by cadherin adhesion molecules: Dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol. Rev. 2011, 91, 691–731. [Google Scholar] [CrossRef] [PubMed]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Feller, L.; Jadwat, Y.; Khammissa, R.A.; Meyerov, R.; Schechter, I.; Lemmer, J. Cellular responses evoked by different surface characteristics of intraosseous titanium implants. BioMed Res. Int. 2015, 2015, 171945. [Google Scholar] [CrossRef]
- Sverzut, A.T.; de Albuquerque, G.C.; Crippa, G.E.; Chiesa, R.; Della Valle, C.; de Oliveira, P.T.; Beloti, M.M.; Rosa, A.L. Bone tissue, cellular, and molecular responses to titanium implants treated by anodic spark deposition. J. Biomed. Mater. Res. Part A 2012, 100, 3092–3098. [Google Scholar] [CrossRef]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, A.; Chiarella, E.; Baudi, F.; Scardamaglia, P.; Antonelli, A.; Giudice, D.; Barni, T.; Fortunato, L.; Giudice, A. Dose-Dependent Effects of Zoledronic Acid on Human Periodontal Ligament Stem Cells: An In Vitro Pilot Study. Cell Transplant. 2020, 29, 1–12. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Diomede, F.; Gugliandolo, A.; Chiricosta, L.; Bramanti, P.; Merciaro, I.; Orsini, T.; Mazzon, E.; Trubiani, O. 3D Printing PLA/Gingival Stem Cells/ EVs Upregulate miR-2861 and -210 during Osteoangiogenesis Commitment. Int. J. Mol. Sci. 2019, 20, 3256. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Merciaro, I.; Martinotti, S.; Cavalcanti, M.F.; Caputi, S.; Mazzon, E.; Trubiani, O. miR-2861 is involved in osteogenic commitment of human periodontal ligament stem cells grown onto 3D scaffold. J. Biol. Regul. Homeost. Agents 2016, 30, 1009–1018. [Google Scholar] [PubMed]
- Pistilli, R.; Genova, T.; Canullo, L.; Faga, M.G.; Terlizzi, M.E.; Gribaudo, G.; Mussano, F. Effect of Bioactivation on Traditional Surfaces and Zirconium Nitride: Adhesion and Proliferation of Preosteoblastic Cells and Bacteria. Int. J. Oral Maxillofac. Implant. 2018, 33, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Zini, N.; Pizzicannella, J.; Merciaro, I.; Pizzicannella, G.; D’Orazio, M.; Piattelli, A.; Trubiani, O. 5-Aza Exposure Improves Reprogramming Process Through Embryoid Body Formation in Human Gingival Stem Cells. Front. Genet. 2018, 9, 419. [Google Scholar] [CrossRef]
- Zara, S.; De Colli, M.; Rapino, M.; Di Valerio, V.; Marconi, G.D.; Cataldi, A.; Macchi, V.; De Caro, R.; Porzionato, A. NF-kappaB involvement in hyperoxia-induced myocardial damage in newborn rat hearts. Histochem. Cell Biol. 2013, 140, 575–583. [Google Scholar] [CrossRef]
- Mammana, S.; Gugliandolo, A.; Cavalli, E.; Diomede, F.; Iori, R.; Zappacosta, R.; Bramanti, P.; Conti, P.; Fontana, A.; Pizzicannella, J.; et al. Human gingival mesenchymal stem cells pretreated with vesicular moringin nanostructures as a new therapeutic approach in a mouse model of spinal cord injury. J. Tissue Eng. Regen. Med. 2019, 13, 1109–1121. [Google Scholar] [CrossRef]
- Diomede, F.; Thangavelu, S.R.; Merciaro, I.; D’Orazio, M.; Bramanti, P.; Mazzon, E.; Trubiani, O. Porphyromonas gingivalis lipopolysaccharide stimulation in human periodontal ligament stem cells: Role of epigenetic modifications to the inflammation. Eur. J. Histochem. 2017, 61, 2826. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Cavalcanti, M.F.X.B.; Pizzicannella, J.; Pierdomenico, S.D.; Fonticoli, L.; Piattelli, A.; Trubiani, O. VEGF/VEGF-R/RUNX2 Upregulation in Human Periodontal Ligament Stem Cells Seeded on Dual Acid Etched Titanium Disk. Materials 2020, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, Y.; Zuo, J.; Li, J.; Wei, Q.; Yu, Z.; Tang, Z. Cell responses to titanium treated by a sandblast-free method for implant applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1187–1194. [Google Scholar] [CrossRef]
- Plekhova, N.G.; Lyapun, I.N.; Drobot, E.I.; Shevchuk, D.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Functional State of Mesenchymal Stem Cells upon Exposure to Bioactive Coatings on Titanium Alloys. Bull. Exp. Biol. Med. 2020, 169, 147–156. [Google Scholar] [CrossRef]
- Zizzari, V.L.; Marconi, G.D.; De Colli, M.; Zara, S.; Zavan, B.; Salini, V.; Fontana, A.; Cataldi, A.; Piattelli, A. In Vitro Behavior of Primary Human Osteoblasts Onto Microrough Titanium Surface. Implant. Dent. 2015, 24, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Gentili, C.; Cancedda, R. Cartilage and Bone Extracellular Matrix. Curr. Pharm. Des. 2009, 15, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, S.; George, A. Multifunctional ECM proteins in bone and teeth. Exp. Cell Res. 2014, 325, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, Z.; Zhang, Y.; Miao, X.; Yu, Q.; Xie, Z.; Yang, G. Stem-cell-derived ECM sheet-implant complexes for enhancing osseointegration. Biomater. Sci. 2020, 8, 6647–6656. [Google Scholar] [CrossRef]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Bio. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Cabodi, S.; Di Stefano, P.; Leal, M.D.C.; Tinnirello, A.; Bisaro, B.; Morello, V.; Damiano, L.; Aramu, S.; Repetto, D.; Tornillo, G.; et al. Integrins and Signal Transduction. Integrins Ion. Channels Mol. Complexes Signal. 2010, 674, 43–54. [Google Scholar] [CrossRef]
- Li, Q.F.; Wang, Z.L. Involvement of FAK/P38 Signaling Pathways in Mediating the Enhanced Osteogenesis Induced by Nano-Graphene Oxide Modification on Titanium Implant Surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Rodil, S.E.; Hyzy, S.L.; Dunn, G.R.; Almaguer-Flores, A.; Schwartz, Z.; Boyan, B.D. Role of integrin subunits in mesenchymal stem cell differentiation and osteoblast maturation on graphitic carbon-coated microstructured surfaces. Biomaterials 2015, 51, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Xu, S.W.; Blumbach, K.; Eastwood, M.; Denton, C.P.; Eckes, B.; Krieg, T.; Abraham, D.J.; Leask, A. Expression of integrin beta 1 by fibroblasts is required for tissue repair in vivo. J. Cell Sci. 2010, 123, 3674–3682. [Google Scholar] [CrossRef] [PubMed]
- Lopes, H.B.; Souza, A.T.P.; Freitas, G.P.; Elias, C.N.; Rosa, A.L.; Beloti, M.M. Effect of focal adhesion kinase inhibition on osteoblastic cells grown on titanium with different topographies. J. Appl. Oral Sci. Rev. FOB 2020, 28, e20190156. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Bar, H.; Kreplak, L.; Strelkov, S.V.; Aebi, U. Intermediate filaments: From cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 2007, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, T.; Nakagawa, T. Mesenchymal stem cell-based tissue regeneration therapies for periodontitis. Regen. Ther. 2020, 14, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Rajan, T.S.; D’Aurora, M.; Bramanti, P.; Merciaro, I.; Marchisio, M.; Gatta, V.; Mazzon, E.; Trubiani, O. Stemness Characteristics of Periodontal Ligament Stem Cells from Donors and Multiple Sclerosis Patients: A Comparative Study. Stem Cells Int. 2017, 2017, 1606125. [Google Scholar] [CrossRef]
- Novoseletskaya, E.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Eremichev, R.; Milovskaya, I.; Kulebyakin, K.; Kulebyakina, M.; Rodionov, S.; Omelyanenko, N.; et al. Mesenchymal Stromal Cell-Produced Components of Extracellular Matrix Potentiate Multipotent Stem Cell Response to Differentiation Stimuli. Front. Cell Dev. Biol. 2020, 8, 555378. [Google Scholar] [CrossRef]
- Marie, P.J.; Hay, E.; Saidak, Z. Integrin and cadherin signaling in bone: Role and potential therapeutic targets. Trends Endocrinol. Metab. TEM 2014, 25, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, Z.; Yang, G. Laminins in osteogenic differentiation and pluripotency maintenance. Differentiation 2020, 114, 13–19. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marconi, G.D.; Fonticoli, L.; Della Rocca, Y.; Rajan, T.S.; Piattelli, A.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Human Periodontal Ligament Stem Cells Response to Titanium Implant Surface: Extracellular Matrix Deposition. Biology 2021, 10, 931. https://doi.org/10.3390/biology10090931
Marconi GD, Fonticoli L, Della Rocca Y, Rajan TS, Piattelli A, Trubiani O, Pizzicannella J, Diomede F. Human Periodontal Ligament Stem Cells Response to Titanium Implant Surface: Extracellular Matrix Deposition. Biology. 2021; 10(9):931. https://doi.org/10.3390/biology10090931
Chicago/Turabian StyleMarconi, Guya Diletta, Luigia Fonticoli, Ylenia Della Rocca, Thangavelu Soundara Rajan, Adriano Piattelli, Oriana Trubiani, Jacopo Pizzicannella, and Francesca Diomede. 2021. "Human Periodontal Ligament Stem Cells Response to Titanium Implant Surface: Extracellular Matrix Deposition" Biology 10, no. 9: 931. https://doi.org/10.3390/biology10090931
APA StyleMarconi, G. D., Fonticoli, L., Della Rocca, Y., Rajan, T. S., Piattelli, A., Trubiani, O., Pizzicannella, J., & Diomede, F. (2021). Human Periodontal Ligament Stem Cells Response to Titanium Implant Surface: Extracellular Matrix Deposition. Biology, 10(9), 931. https://doi.org/10.3390/biology10090931






