Bitter Taste Disrupts Spatial Discrimination of Piperine-Evoked Burning Sensations: A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Psychophysical Olfactory and Gustatory Testing
2.3. Stimuli
2.4. Procedures
2.4.1. Experiment 1: Psychophysiological Responses to PTS
2.4.2. Experiment 2: Spatial Discrimination between Gustatory and Piperine-Evoked Burning Sensations
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. Olfactory and Gustatory Test Results Correspond to the Self-Perceived Functions
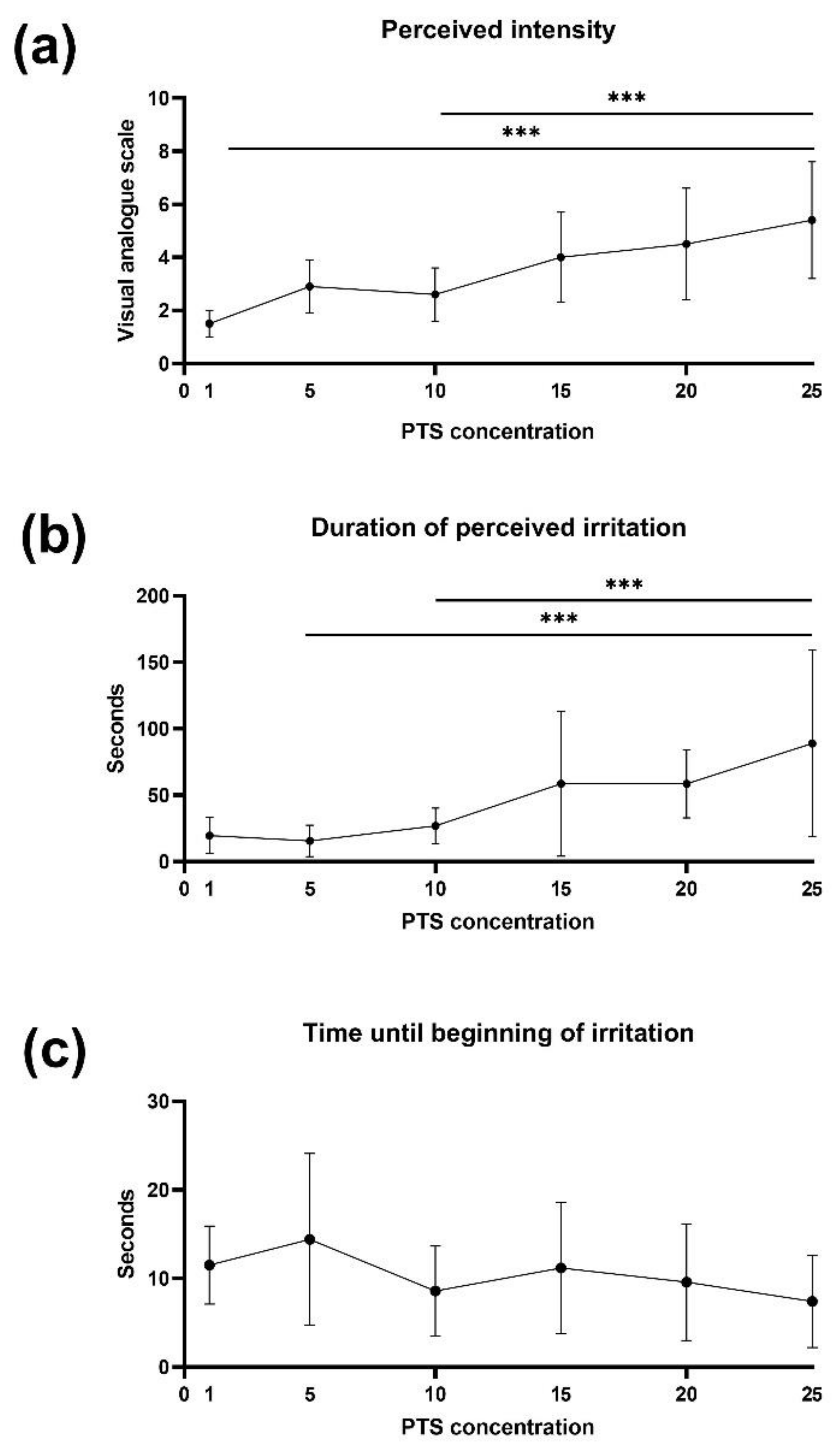
3.3. Increasing PTS Concentrations Lead to Higher Perceived Intensity and Longer Duration of Irritation
3.4. Bitter Taste Disrupts Spatial Discrimination of Piperine-Evoked Burning Sensations
3.5. Spatial-Discrimination between Piperine-Evoked Burning Sensations and Gustatory Stimuli Is Reliable
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green, B.G. Chemesthesis and the chemical senses as components of a “chemofensor complex”. Chem. Senses 2012, 37, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Scott, T.R.; Mark, G.P. The taste system encodes stimulus toxicity. Brain Res. 1987, 414, 197–203. [Google Scholar] [CrossRef]
- Dalton, P.H.; Jaén, C. Responses to odors in occupational environments. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Cain, W.S.; Lee, N.S.; Wise, P.M.; Schmidt, R.; Ahn, B.H.; Cometto-Muñiz, J.E.; Abraham, M.H. Chemesthesis from volatile organic compounds: Psychophysical and neural responses. Physiol. Behav. 2006, 88, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, C.T.; Carstens, E. Oral Chemesthesis and Taste. Senses A Compr. Ref. 2008, 4, 345–369. [Google Scholar] [CrossRef]
- Lundström, J.N.; Boesveldt, S.; Albrecht, J. Central processing of the chemical senses: An overview. ACS Chem. Neurosci. 2011, 2, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Lawless, H.; Stevens, D.A. Effects of oral chemical irritation on taste. Physiol. Behav. 1984, 32, 995–998. [Google Scholar] [CrossRef]
- Lawless, H.T.; Stevens, D.A. Responses by humans to oral chemical irritants as a function of locus of stimulation. Percept. Psychophys. 1988, 43, 72–78. [Google Scholar] [CrossRef]
- Stevens, D.A.; Lawless, H.T. Putting out the fire: Effects of tastants on oral chemical irritation. Percept. Psychophys. 1986, 39, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Lawless, H. Oral chemical irritation: Psychophysical properties. Chem. Senses 1984, 9, 143–155. [Google Scholar] [CrossRef]
- Green, B.G.; Schullery, M.T. Stimulation of bitterness by capsaicin and menthol: Differences between lingual areas innervated by the glossopharyngeal and chorda tympani nerves. Chem. Senses 2003, 28, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, B.G.; Hayes, J.E. Capsaicin as a probe of the relationship between bitter taste and chemesthesis. Physiol. Behav. 2003, 79, 811–821. [Google Scholar] [CrossRef]
- Green, B.G.; Hayes, J.E. Individual differences in perception of bitterness from capsaicin, piperine and zingerone. Chem. Senses 2004, 29, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.; Green, B.G. The psychophysical relationship between bitter taste and burning sensation: Evidence of qualitative similarity. Chem. Senses 2007, 32, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Nolden, A.A.; McGeary, J.E.; Hayes, J.E. Differential bitterness in capsaicin, piperine, and ethanol associates with polymorphisms in multiple bitter taste receptor genes. Physiol. Behav. 2016, 156, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Rentmeister-Bryant, H.; Green, B.G. Perceived irritation during ingestion of capsaicin or piperine: Comparison of trigeminal and non-trigeminal areas. Chem. Senses 1997, 22, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Affeltranger, M.A.; McBurney, D.H.; Balaban, C.D. Temporal interactions between oral irritants: Piperine, zingerone, and capsaicin. Chem. Senses 2007, 32, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Simon, S.A.; De Araujo, I.E.; Stapleton, J.R.; Nicolelis, M.A.L. Multisensory processing of gustatory stimuli. Chemosens. Percept. 2008, 1, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Moran, M.M.; Szallasi, A. Targeting nociceptive transient receptor potential channels to treat chronic pain: Current state of the field. Br. J. Pharmacol. 2018, 175, 2185–2203. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Montell, C. TRP Channels TRP: Transient Receptor Potential. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Okumura, Y.; Narukawa, M.; Iwasaki, Y.; Ishikawa, A.; Matsuda, H.; Yoshikawa, M.; Watanabe, T. Activation of TRPV1 and TRPA1 by black pepper components. Biosci. Biotechnol. Biochem. 2010, 74, 1068–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renner, B.; Mueller, C.A.; Shephard, A. Environmental and non-infectious factors in the aetiology of pharyngitis (sore throat). Inflamm. Res. 2012, 61, 1041–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Just, T.; Steiner, S.; Pau, H.W. Oral pain perception and taste in Burning Mouth Syndrome. J. Oral Pathol. Med. 2010, 39, 22–27. [Google Scholar] [CrossRef]
- Green, B.G. Rapid recovery from capsaicin desensitization during recurrent stimulation. Pain 1996, 68, 245–253. [Google Scholar] [CrossRef]
- Nolden, A.A.; Hayes, J.E. Perceptual and Affective Responses to Sampled Capsaicin Differ by Reported Intake. Food Qual Prefer. 2017, 55, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Nolden, A.A.; Lenart, G.; Hayes, J.E. Putting out the fire—Efficacy of common beverages in reducing oral burn from capsaicin. Physiol. Behav. 2019, 208, 112557. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.N.; Simons, C.T. Assessing Regional Sensitivity and Desensitization to Capsaicin Among Oral Cavity Mucosae. Chem. Senses 2020, XX, 1–10. [Google Scholar] [CrossRef]
- Yamasaki, M.; Ebihara, S.; Ebihara, T.; Freeman, S.; Yamanda, S.; Asada, M.; Yoshida, M.; Arai, H. Cough reflex and oral chemesthesis induced by capsaicin and capsiate in healthy never-smokers. Cough 2007, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Bennett, S.M.; Hayes, J.E. Differences in the chemesthetic subqualities of capsaicin, ibuprofen, and olive oil. Chem. Senses 2012, 37, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.D.; McBurney, D.H.; Affeltranger, M.A. Three distinct categories of time course of pain produced by oral capsaicin. J. Pain 2005, 6, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.D.; McBurney, D.H.; Stoulis, M. Time course of burn to repeated applications of capsaicin. Physiol. Behav. 1999, 66, 109–112. [Google Scholar] [CrossRef]
- McBurney, D.H.; Balaban, C.D.; Popp, J.R.; Rosenkranz, J.E. Adaptation to capsaicin burn: Effects of concentration and individual differences. Physiol. Behav. 2001, 72, 205–216. [Google Scholar] [CrossRef]
- McBurney, D.H.; Balaban, C.D.; Christopher, D.E.; Harvey, C. Adaptation to capsaicin within and across days. Physiol. Behav. 1997, 61, 181–190. [Google Scholar] [CrossRef]
- Simon, S.A.; De Araujo, I.E. The salty and burning taste of capsaicin. J. Gen. Physiol. 2005, 125, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Lötsch, J.; Hummel, T. Clinical usefulness of self-rated olfactory performance—A data science-based assessment of 6000 patients. Chem. Senses 2019, 44, 357–364. [Google Scholar] [CrossRef]
- Landis, B.N.; Hummel, T.; Hugentobler, M.; Giger, R.; Lacroix, J.S. Ratings of overall olfactory function. Chem. Senses 2003, 28, 691–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welge-Luessen, A.; Hummel, T.; Stojan, T.; Wolfensberger, M. What is the correlation between ratings and measures of olfactory function in patients with olfactory loss? Am. J. Rhinol. 2005, 19, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Kobal, G.; Hummel, T.; Sekinger, B.; Barz, S.; Roscher, S.; Wolf, S. “Sniffin’’ sticks: Screening of olfactory performance. Rhinology 1996, 34, 222–226. [Google Scholar]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. “Sniffin” sticks. Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Mueller, C.; Kallert, S.; Renner, B.; Stiassny, K.; Temmel, A.F.P.; Hummel, T.; Kobal, G. Quantitative assessment of gustatory function in a clinical context using impregnated “taste strips”. Rhinology 2003, 41, 2–6. [Google Scholar] [PubMed]
- Kobal, G.; Van Toller, S.; Hummel, T. Is there directional smelling? Experientia 1989, 14, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.A.; Khatib, S.; Landis, B.N.; Temmel, A.F.P.; Hummel, T. Gustatory function after tonsillectomy. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Soter, A.; Kim, J.; Jackman, A.; Tourbier, I.; Kaul, A.; Doty, R.L. Accuracy of self-report in detecting taste dysfunction. Laryngoscope 2008, 118, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Rombaux, P.; Mouraux, A.; Keller, T.; Hummel, T. Trigeminal event-related potentials in patients with olfactory dysfunction. Rhinology 2008, 46, 170–174. [Google Scholar] [PubMed]
- De Araujo, I.E.; Simon, S.A. The gustatory cortex and multisensory integration. Int. J. Obes. 2009, 33, S34–S43. [Google Scholar] [CrossRef] [Green Version]
- Rhyu, M.R.; Kim, Y.; Lyall, V. Interactions between chemesthesis and taste: Role of TRPA1 and TRPV1. Int. J. Mol. Sci. 2021, 22, 3360. [Google Scholar] [CrossRef]
- Braud, A.; Boucher, Y. Intra-oral trigeminal-mediated sensations influencing taste perception: A systematic review. J. Oral Rehabil. 2020, 47, 258–269. [Google Scholar] [CrossRef]
- Kapaun, C.L.; Dando, R. Deconvoluting physical and chemical heat: Temperature and spiciness influence flavor differently. Physiol. Behav. 2017, 170, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Smutzer, G.; Devassy, R.K. Integrating TRPV1 Receptor Function with Capsaicin Psychophysics. Adv. Pharmacol. Sci. 2016, 2016, 1512457. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Gabitto, M.; Peng, Y.; Ryba, N.J.P.; Zuker, C.S. A gustotopic map of taste qualities in the mammalian brain. Science 2011, 333, 1262–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikazoe, J.; Lee, D.H.; Kriegeskorte, N.; Anderson, A.K. Distinct representations of basic taste qualities in human gustatory cortex. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Boughter, J.D., Jr.; Fletcher, M. Rethinking the role of taste processing in insular cortex and forebrain circuits. Curr. Opin. Physiol. 2021, 20, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.L.; Ogg, M.C.; Lu, L.; Ogg, R.J.; Boughter, J.D. Overlapping Representation of Primary Tastes in a Defined Region of the Gustatory Cortex. J. Neurosci. 2017, 37, 7595–7605. [Google Scholar] [CrossRef] [Green Version]
- Avery, J.A. Against gustotopic representation in the human brain: There is no Cartesian restaurant. Curr. Opin. Physiol. 2021, 20, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yuyama, N.; Kato, T.; Kawamura, Y. Gustatory responses of cortical neurons in rats. II. Information processing of taste quality. J. Neurophysiol. 1985, 53, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Accolla, R.; Bathellier, B.; Petersen, C.C.H.; Carleton, A. Differential spatial representation of taste modalities in the rat gustatory cortex. J. Neurosci. 2007, 27, 1396–1404. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Kurita, K.; Ito, T.; Arao, M. Pain threshold and pain recovery after experimental stimulation in patients with burning mouth syndrome. Psychiatry Clin. Neurosci. 2002, 56, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Forssell, H.; Jääskeläinen, S.; Tenovuo, O.; Hinkka, S. Sensory dysfunction in burning mouth syndrome. Pain 2002, 99, 41–47. [Google Scholar] [CrossRef]

| Order | Strips | Concentration |
|---|---|---|
| 1 | Piperine | 1 mg/dL |
| 2 | Piperine | 5 mg/dL |
| 3 | Piperine | 10 mg/dL |
| 4 | Piperine | 15 mg/dL |
| 5 | Blank | - |
| 6 | Piperine | 20 mg/dL |
| 7 | Blank | - |
| 8 | Piperine | 25 mg/dL |
| Trial | Left Anterior Tongue | Right Anterior Tongue |
|---|---|---|
| 1 | Sweet | Piperine |
| 2 | Bitter | Piperine |
| 3 | Piperine | Blank |
| 4 | Sweet | Piperine |
| 5 | Piperine | Bitter |
| 6 | Piperine | Blank |
| 7 | Sweet | Piperine |
| 8 | Piperine | Bitter |
| 9 | Blank | Piperine |
| PTS | Irritation Perceived, N | Delay in Seconds, Mean/SD | Duration in Seconds, Mean/SD | Intensity in Estimation Units, Mean/SD | Burning, N | Stinging, N |
|---|---|---|---|---|---|---|
| PTS1 | 4 | 4.2/6.4 | 6.5/12.6 | 0.5/0.8 | 1 | 3 |
| PTS5 | 7 | 14.4/10.1 | 9.1/12.4 | 1.7/1.7 | 4 | 3 |
| PTS10 | 10 | 8.6/5.4 | 22.4/16.4 | 2.2/1.4 | 6 | 4 |
| PTS15 | 12 | 11.2/7.7 | 58.5/56.9 | 3.8/2.1 | 8 | 4 |
| Blank 1 | 6 | 5.3/7.4 | 13.7/21.3 | 1.3/1.8 | 4 | 2 |
| PTS20 | 11 | 9.4/6.9 | 52.8/30.6 | 4.1/2.4 | 9 | 2 |
| Blank 2 | 6 | 10.0/10.2 | 12.9/19.8 | 1.2/1.3 | 3 | 3 |
| PTS25 | 11 | 7.4/5.4 | 80.1/74.6 | 4.9/2.7 | 9 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.T.; Besser, G.; Bayer, K.; Prem, B.; Mueller, C.A.; Renner, B. Bitter Taste Disrupts Spatial Discrimination of Piperine-Evoked Burning Sensations: A Pilot Study. Biology 2021, 10, 886. https://doi.org/10.3390/biology10090886
Liu DT, Besser G, Bayer K, Prem B, Mueller CA, Renner B. Bitter Taste Disrupts Spatial Discrimination of Piperine-Evoked Burning Sensations: A Pilot Study. Biology. 2021; 10(9):886. https://doi.org/10.3390/biology10090886
Chicago/Turabian StyleLiu, David T., Gerold Besser, Karina Bayer, Bernhard Prem, Christian A. Mueller, and Bertold Renner. 2021. "Bitter Taste Disrupts Spatial Discrimination of Piperine-Evoked Burning Sensations: A Pilot Study" Biology 10, no. 9: 886. https://doi.org/10.3390/biology10090886
APA StyleLiu, D. T., Besser, G., Bayer, K., Prem, B., Mueller, C. A., & Renner, B. (2021). Bitter Taste Disrupts Spatial Discrimination of Piperine-Evoked Burning Sensations: A Pilot Study. Biology, 10(9), 886. https://doi.org/10.3390/biology10090886








