Carbonic Anhydrase IX and Survivin in Colorectal Adenocarcinoma Cells: Slovakian Population Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemical Detection of CAIX and Survivin, Scoring Method and Statistical Analysis
3. Results
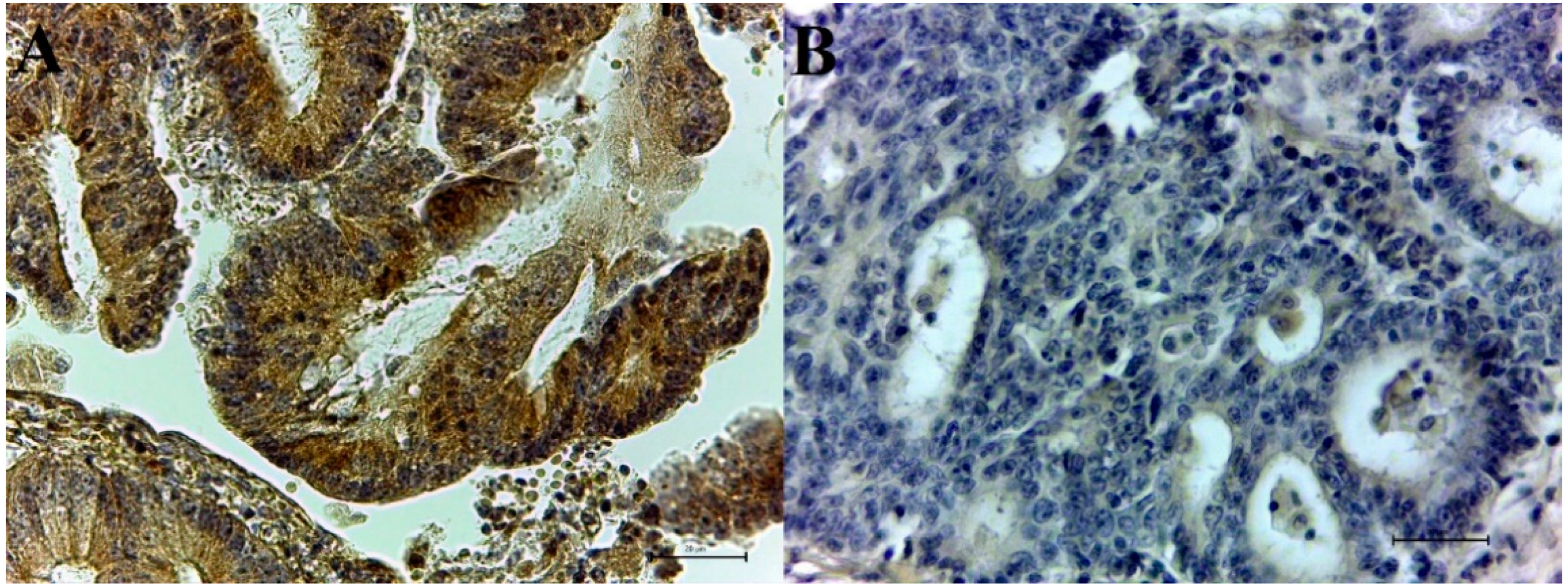
3.1. Immunohistochemical CAIX and Survivin Expression in Colorectal Adenocarcinoma Samples
3.2. Co-Expression of CAIX and Survivin
3.3. Immunohistochemical CAIX and Survivin Expression in Normal Colorectal Tissue
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019, 14, 2. [Google Scholar] [CrossRef]
- Hilvo, M.; Baranauskiene, L.; Salzano, A.M.; Scaloni, A.; Matulis, D.; Innocenti, A.; Scozzafava, A.; Monti, S.M.; Di Fiore, A.; De Simone, G.; et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J. Biol. Chem. 2008, 283, 27799–27809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedlakova, O.; Svastova, E.; Takacova, M.; Kopacek, J.; Pastorek, J.; Pastorekova, S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front. Physiol. 2014, 4, 400. [Google Scholar] [CrossRef] [Green Version]
- Mucaj, V.; Shay, J.E.; Simon, M.C. Effects of hypoxia and HIFs on cancer metabolism. Int. J. Hematol. 2012, 95, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Chiche, J.; Ilc, K.; Laferriere, J.; Trottier, E.; Dayan, F.; Mazure, N.M.; Brahimi-Horn, M.C.; Pouyssegur, J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009, 69, 358–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svastova, E.; Zilka, N.; Zat’ovicova, M.; Gibadulinova, A.; Ciampor, F.; Pastorek, J.; Pastorekova, S. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with beta-catenin. Exp. Cell Res. 2003, 290, 332–345. [Google Scholar] [CrossRef]
- Horie, K.; Kawakami, K.; Fujita, Y.; Sugaya, M.; Kameyama, K.; Mizutani, K.; Deguchi, T.; Ito, M. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem. Biophys. Res. Commun. 2017, 492, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Monaco, S.; Sparano, V.; Gioia, M.; Sbardella, D.; Di Pierro, D.; Marini, S.; Coletta, M. Enzymatic processing of collagen IV by MMP-2 (gelatinase A) affects neutrophil migration and it is modulated by extracatalytic domains. Protein Sci. 2006, 15, 2805–2815. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, A.; Patiar, S.; Wigfield, S.; Li, J.L.; Ledaki, I.; Turley, H.; Leek, R.; Snell, C.; Gatter, K.; Sly, W.S.; et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin. Cancer Res. 2012, 18, 3100–3111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, J.M.; Farma, J.M.; Coppola, D.; Hakam, A.; Fulp, W.J.; Chen, D.T.; Siegel, E.M.; Yeatman, T.J.; Shibata, D. Expression of the antiapoptotic protein survivin in colon cancer. Clin. Colorectal. Cancer 2011, 10, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Mita, A.C.; Mita, M.M.; Nawrocki, S.T.; Giles, F.J. Survivin: Key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin. Cancer Res. 2008, 14, 5000–5005. [Google Scholar] [CrossRef] [Green Version]
- Garg, H.; Suri, P.; Gupta, J.C.; Talwar, G.P.; Dubey, S. Survivin: A unique target for tumor therapy. Cancer Cell Int. 2016, 16, 49. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Zhang, Y.; Yang, F.; Wang, P.; Wang, W.; Su, Y.; Luo, W. Survivin promotes the invasion of human colon carcinoma cells by regulating the expression of MMP7. Mol. Med. Rep. 2014, 9, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ren, W.; Zeng, Q.; Chen, S.; Zhang, M.; Zhao, Y.; Cheng, J.; Wang, X. Effects of survivin on angiogenesis in vivo and in vitro. Am. J. Transl. Res. 2016, 8, 270–283. [Google Scholar] [PubMed]
- Salz, W.; Eisenberg, D.; Plescia, J.; Garlick, D.S.; Weiss, R.M.; Wu, X.R.; Sun, T.T.; Altieri, D.C. A survivin gene signature predicts aggressive tumor behavior. Cancer Res. 2005, 65, 3531–3534. [Google Scholar] [CrossRef] [Green Version]
- Santarelli, A.; Mascitti, M.; Lo Russo, L.; Sartini, D.; Troiano, G.; Emanuelli, M.; Lo Muzio, L. Survivin-Based Treatment Strategies for Squamous Cell Carcinoma. Int. J. Mol. Sci. 2018, 19, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaffaroni, N.; Daidone, M.G. Survivin expression and resistance to anticancer treatments: Perspectives for new therapeutic interventions. Drug Resist. Update 2002, 5, 65–72. [Google Scholar] [CrossRef]
- van Diest, P.J. A scoring system for immunohistochemical staining: Consensus report of the task force for basic research of the EORTC-GCCG. European Organization for Research and Treatment of Cancer-Gynaecological Cancer Cooperative Group. J. Clin. Pathol. 1997, 50, 801–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. Carcinoma of the colon and rectum. In WHO Classification of Tumours of the Digestive System, 4th ed.; WHO Press: Geneva, Switzerland, 2010; pp. 134–146. [Google Scholar]
- Fukuda, S.; Pelus, L.M. Survivin, a cancer target with an emerging role in normal adult tissues. Mol. Cancer Ther. 2006, 5, 1087–1098. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, P.K.; Goel, A.; Mittal, R.D. Survivin: A molecular biomarker in cancer. Indian J. Med. Res. 2015, 141, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Saarnio, J.; Parkkila, S.; Parkkila, A.-K.; Haukipuro, K.; Pastoreková, S.; Pastorek, J.; Kairaluoma, M.I.; Karttunen, T.J. Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am. J. Pathol. 1998, 153, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Korkeila, E.; Talvinen, K.; Jaakkola, P.M.; Minn, H.; Syrjanen, K.; Sundstrom, J.; Pyrhonen, S. Expression of carbonic anhydrase IX suggests poor outcome in rectal cancer. Br. J. Cancer 2009, 100, 874–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemelä, A.M.; Hynninen, P.; Mecklin, J.-P.; Kuopio, T.; Kokko, A.; Aaltonen, L.; Parkkila, A.-K.; Pastorekova, S.; Pastorek, J.; Waheed, A. Carbonic anhydrase IX is highly expressed in hereditary nonpolyposis colorectal cancer. Cancer Epidemiol. Prev. Biomark. 2007, 16, 1760–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubowska, K.; Pryczynicz, A.; Dymicka-Piekarska, V.; Famulski, W.; Guzinska-Ustymowicz, K. Immunohistochemical expression and serum level of survivin protein in colorectal cancer patients. Oncol. Lett. 2016, 12, 3591–3597. [Google Scholar] [CrossRef] [Green Version]
- Ponnelle, T.; Chapusot, C.; Martin, L.; Bouvier, A.M.; Plenchette, S.; Faivre, J.; Solary, E.; Piard, F. Cellular localisation of survivin: Impact on the prognosis in colorectal cancer. J. Cancer Res. Clin. Oncol. 2005, 131, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Sagheb, H.M.; Hakimi, A.; Moudi, B. Evaluation of immunohistochemical expression of survivin and its correlation with -31G/C gene polymorphism in colorectal cancer. Med. Mol. Morphol 2019, 52, 82–89. [Google Scholar] [CrossRef]
- Lin, L.J.; Zheng, C.Q.; Jin, Y.; Ma, Y.; Jiang, W.G.; Ma, T. Expression of survivin protein in human colorectal carcinogenesis. World J. Gastroenterol. 2003, 9, 974–977. [Google Scholar] [CrossRef]
- van Kuijk, S.J.; Yaromina, A.; Houben, R.; Niemans, R.; Lambin, P.; Dubois, L.J. Prognostic Significance of Carbonic Anhydrase IX Expression in Cancer Patients: A Meta-Analysis. Front. Oncol. 2016, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Sun, Z.; Bai, C.; Luo, F. The expression of pAKT/survivin and their role in colorectal cancer. J. Clin. Oncol. 2018, 36, 595. [Google Scholar] [CrossRef]
- Dubois, L.; Peeters, S.G.; van Kuijk, S.J.; Yaromina, A.; Lieuwes, N.G.; Saraya, R.; Biemans, R.; Rami, R.; Parvathaneni, N.K.; Vullo, D.; et al. Targeting carbonic anhydrase IX by nitroimidazole based sulfamides enhances the therapeutic effect of tumor irradiation: A new concept of dual targeting drugs. Radiother. Oncol. 2013, 108, 523–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, G.; Tuncel, H.; Aoki, E.; Tanaka, S.; Oka, S.; Kaneko, I.; Okamoto, M.; Tatsuka, M.; Nakai, S.; Shimamoto, F. Intracellular localization of survivin determines biological behavior in colorectal cancer. Oncol. Rep. 2009, 22, 557–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| No. of Patients | n = 74 | 100% |
|---|---|---|
| Sex | female | 30 (41%) |
| male | 44 (59%) | |
| Age | ≤50 | 2 (3%) |
| >50 | 72 (97%) | |
| Histological grade | G1 | 52 (70%) |
| G2 | 13 (18%) | |
| G3 | 9 (12%) | |
| Invasion to lymph node (LN) | positive LN | 23 (31%) |
| negative LN | 51 (69%) | |
| Metastasis | present metastasis | 17 (23%) |
| absent metastasis | 57 (77%) |
| Quantity of CAIX Expression No./% | No./% of Negative Samples | No./% of Positive Samples | ||||
|---|---|---|---|---|---|---|
| Protein | [−] | [+] | [++] | [+++] | ||
| CAIX | 27 (36%) | 16 (22%) | 21 (28%) | 10 (14%) | 43 (58%) | 31 (42%) |
| Survivin | 42 (57%) | 17 (23%) | 12 (16%) | 3 (4%) | 59 (80%) | 15 (20%) |
| Clinicopathological Parameter | Quantity of CAIX Expression (No.) | No. of CAIX-Negative Samples | No. of CAIX-Positive Samples | Squared-Test | |||
|---|---|---|---|---|---|---|---|
| [−] | [+] | [++] | [+++] | ||||
| Sex | p = 0.938 | ||||||
| Female (n = 30) | 10 | 8 | 7 | 5 | 18 | 12 | |
| Male (n = 44) | 17 | 9 | 13 | 5 | 26 | 18 | |
| Grade of tumor | |||||||
| G1 (n = 52) | 16 | 10 | 18 | 8 | 26 | 26 | p = 0.182 |
| G2 (n = 13) | 4 | 5 | 3 | 1 | 9 | 4 | |
| G3 (n = 9) | 7 | 0 | 1 | 1 | 7 | 2 | |
| Lymph nodes (LN) | |||||||
| positive LN (n = 23) | 9 | 5 | 5 | 4 | 14 | 9 | p = 0.746 |
| negative LN (n = 51) | 18 | 11 | 16 | 6 | 29 | 22 | |
| Metastasis (MTS) | |||||||
| present MTS (n = 17) | 8 | 2 | 6 | 1 | 10 | 7 | p = 0.946 |
| absent MTS (n = 57) | 19 | 14 | 15 | 9 | 33 | 24 | |
| Clinicopathological Parameter | Quantity of Survivin Expression (No.) | No. of Survivin-Negative Samples | No. of Survivin-Positive Samples | Chi Squared-Test | |||
|---|---|---|---|---|---|---|---|
| [−] | [+] | [++] | [+++] | ||||
| Sex | p = 0.588 | ||||||
| Female (n = 30) | 15 | 8 | 7 | 0 | 23 | 7 | |
| Male (n = 44) | 27 | 9 | 5 | 3 | 36 | 8 | |
| Grade of tumor | |||||||
| G1 (n = 52) | 30 | 13 | 8 | 1 | 43 | 9 | p = 0.155 |
| G2 (n = 13) | 7 | 4 | 0 | 2 | 11 | 2 | |
| G3 (n = 9) | 5 | 0 | 4 | 0 | 5 | 4 | |
| Lymph nodes (LN) | |||||||
| positive LN (n = 23) | 14 | 3 | 5 | 1 | 17 | 6 | p = 0.403 |
| negative LN (n = 51) | 28 | 14 | 7 | 2 | 42 | 9 | |
| Metastasis (MTS) | |||||||
| present MTS (n = 17) | 10 | 3 | 4 | 0 | 13 | 4 | p = 0.703 |
| absent MTS (n = 57) | 32 | 14 | 8 | 3 | 46 | 11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kováčová, Z.; Hodorová, I. Carbonic Anhydrase IX and Survivin in Colorectal Adenocarcinoma Cells: Slovakian Population Study. Biology 2021, 10, 872. https://doi.org/10.3390/biology10090872
Kováčová Z, Hodorová I. Carbonic Anhydrase IX and Survivin in Colorectal Adenocarcinoma Cells: Slovakian Population Study. Biology. 2021; 10(9):872. https://doi.org/10.3390/biology10090872
Chicago/Turabian StyleKováčová, Zuzana, and Ingrid Hodorová. 2021. "Carbonic Anhydrase IX and Survivin in Colorectal Adenocarcinoma Cells: Slovakian Population Study" Biology 10, no. 9: 872. https://doi.org/10.3390/biology10090872
APA StyleKováčová, Z., & Hodorová, I. (2021). Carbonic Anhydrase IX and Survivin in Colorectal Adenocarcinoma Cells: Slovakian Population Study. Biology, 10(9), 872. https://doi.org/10.3390/biology10090872






