Personalized Evaluation of Atrial Complexity of Patients Undergoing Atrial Fibrillation Ablation: A Clinical Computational Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Database
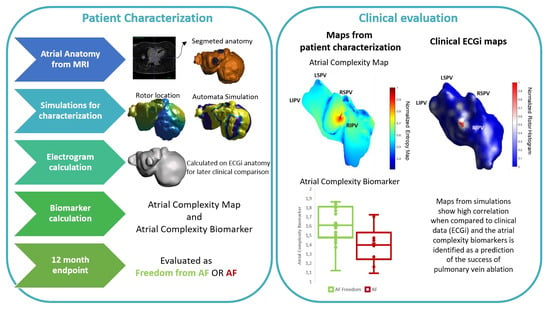
2.2. Atrial Electroanatomical Complexity Evaluation Protocol
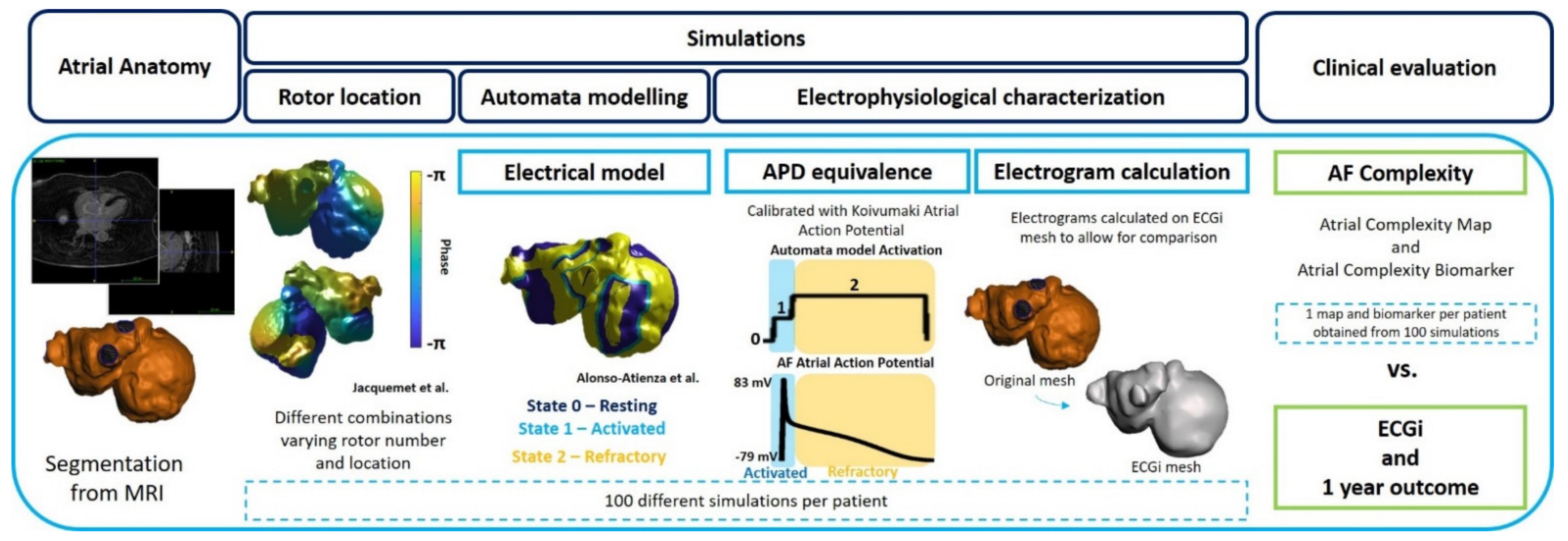
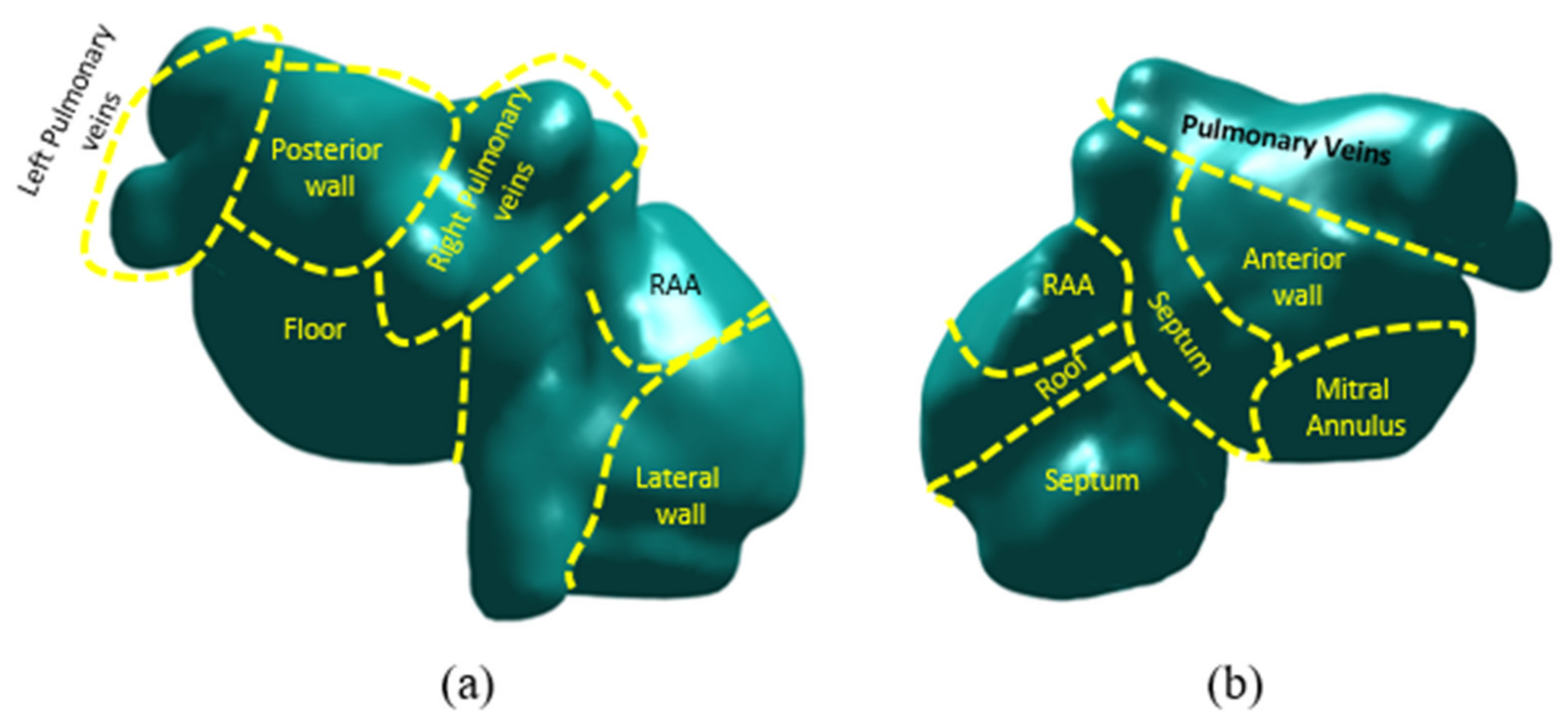
2.2.1. Atrial Anatomy
2.2.2. Computational Models of the Atria
AF Initiation: Automatic Rotor Location
Automata Model Simulations
Electrophysiological Equivalence and Characterization
2.2.3. Clinical Evaluation
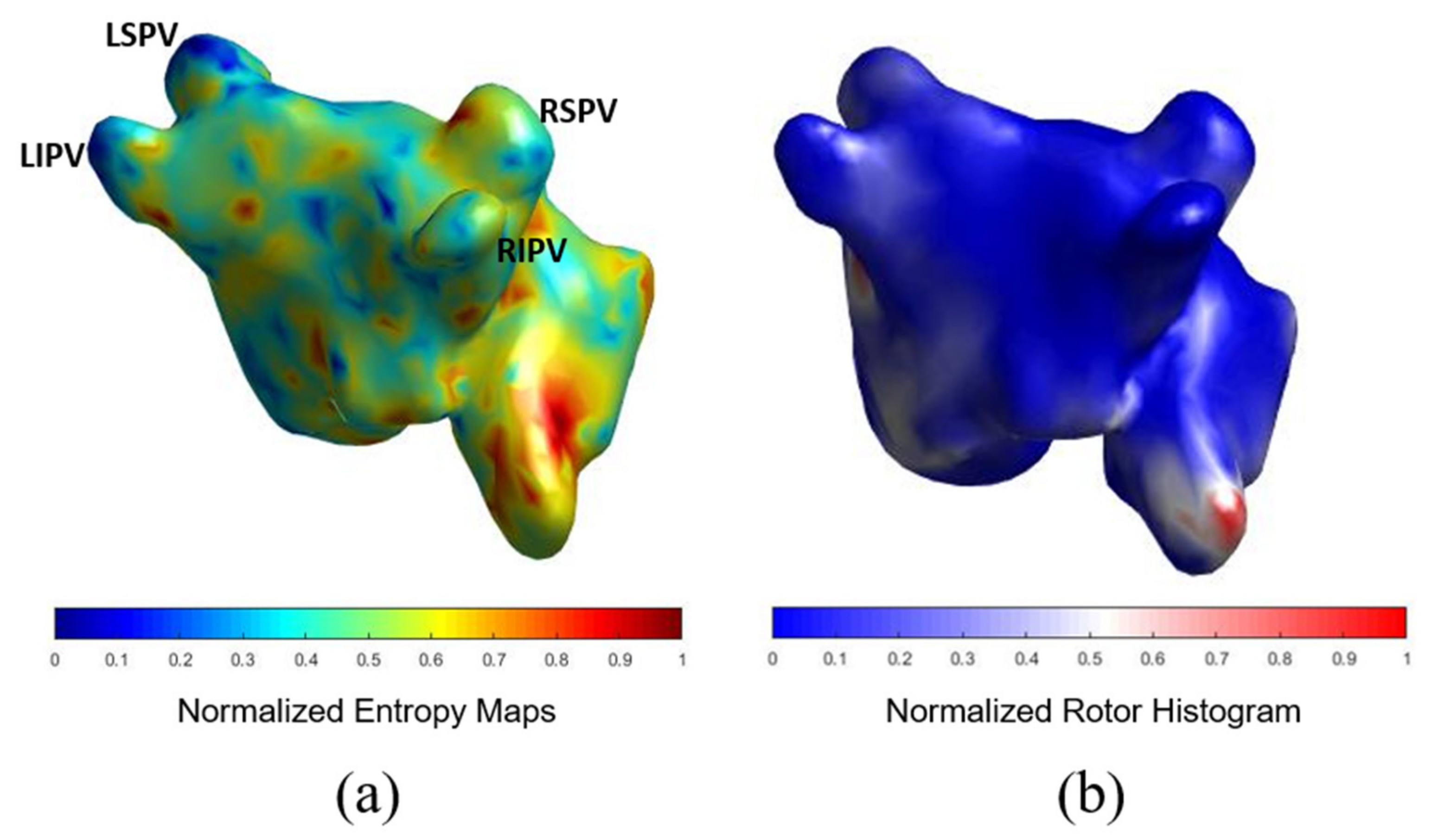
AF Complexity: Atrial Complexity Map vs. ECGi
AF Complexity and 1-Year Ablation Outcome
2.2.4. Statistical Analysis
3. Results
3.1. Cohort Description
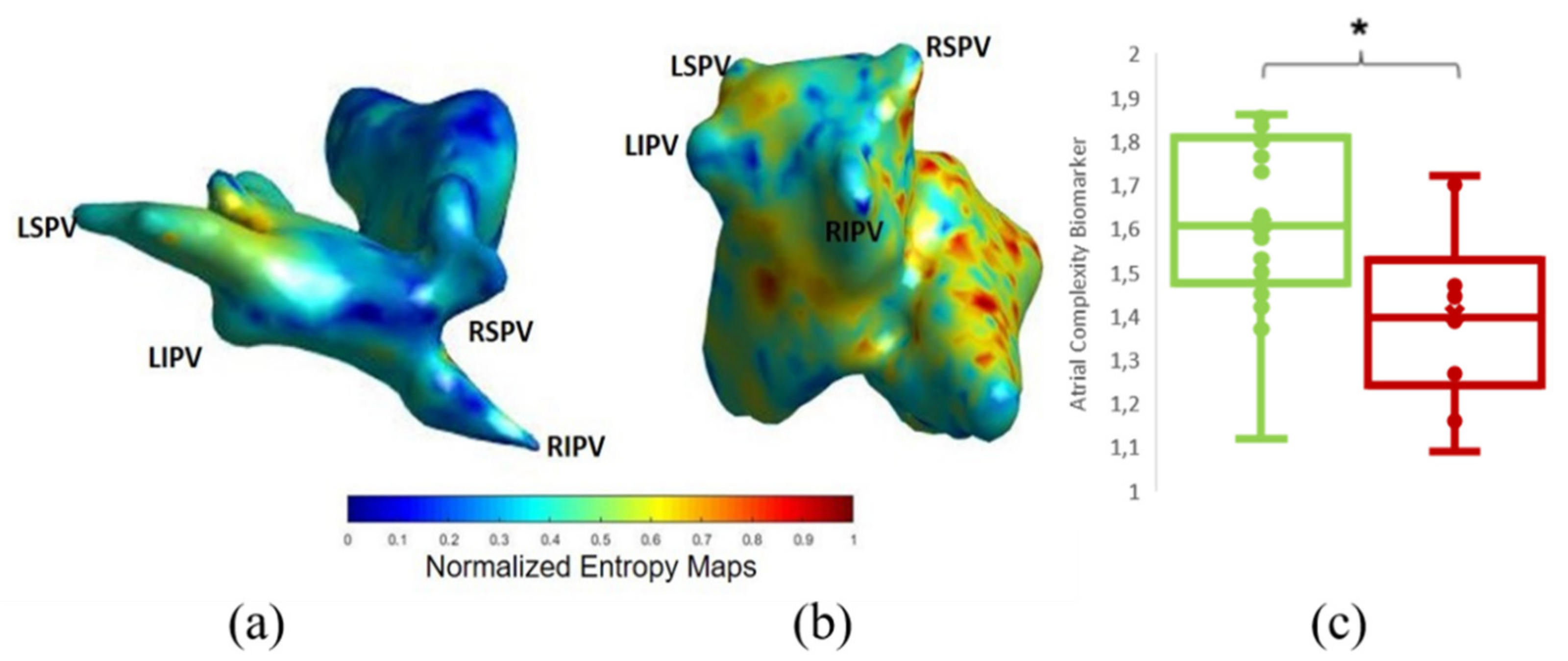
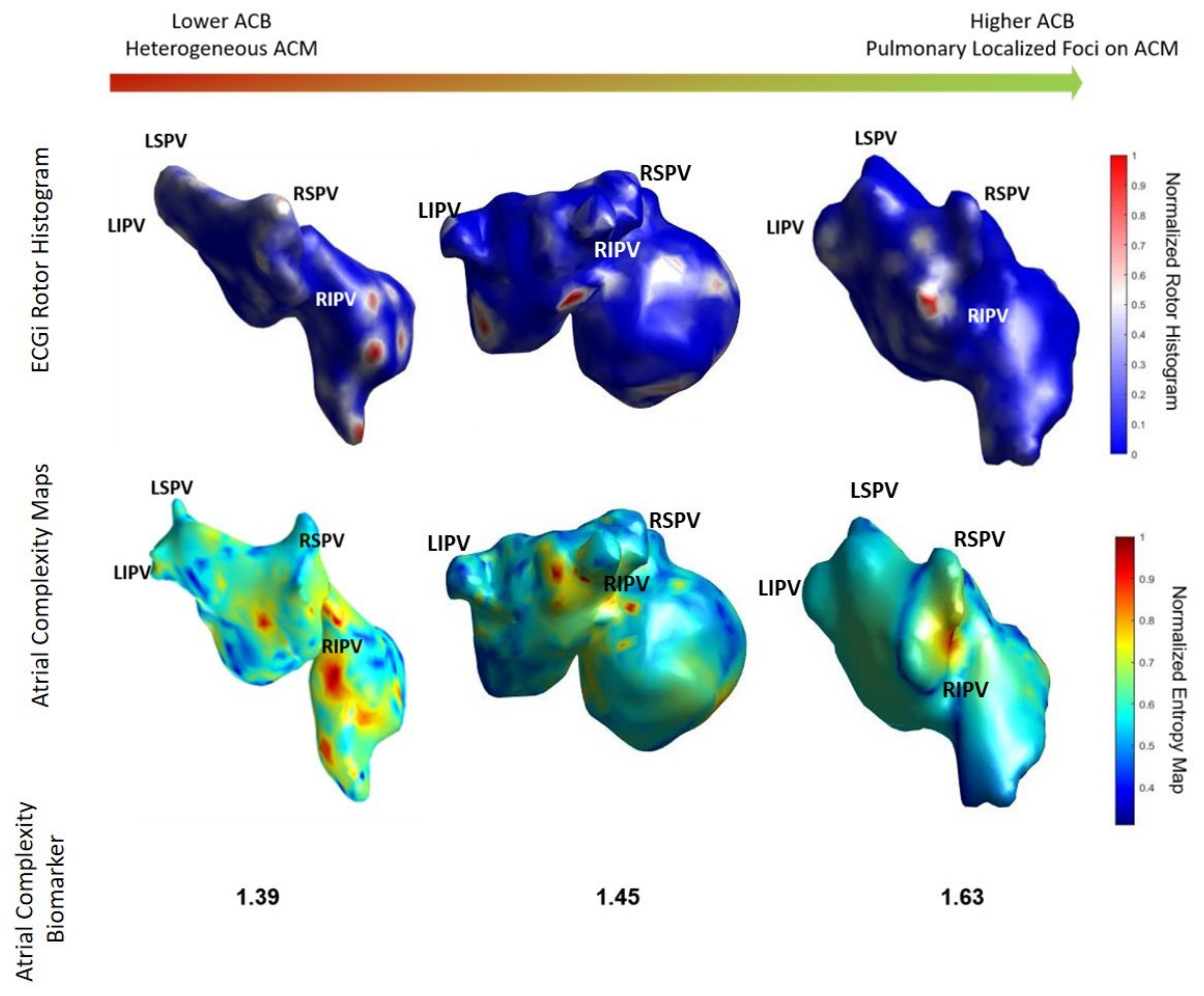
3.2. Comparison of ACM with ECGI
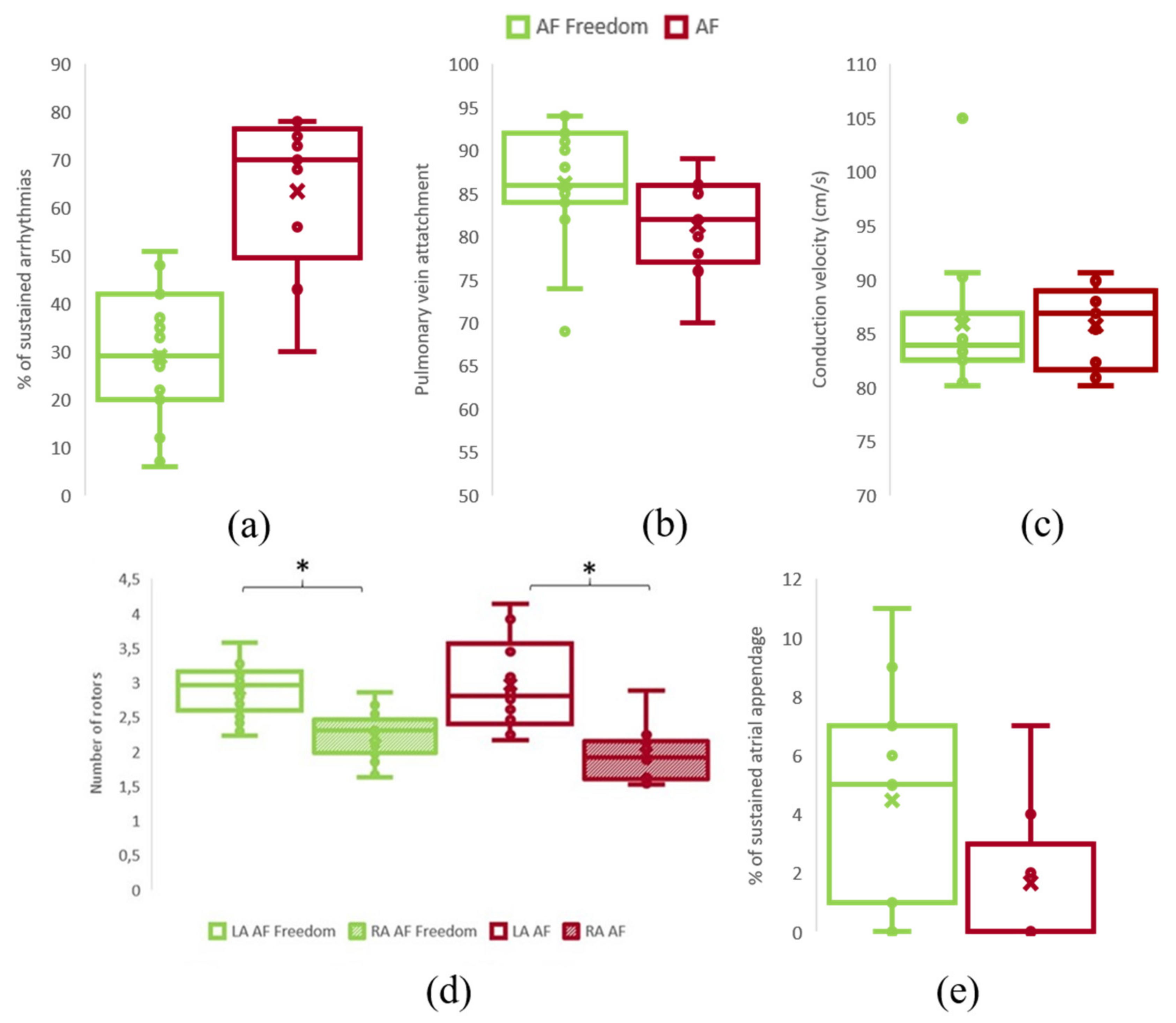
3.3. AF Complexity and 1-Year Ablation Outcome
Sustained AF Simulation Induction and Rotor Distribution
3.4. Comparison with AF Type
3.5. Applicability to Clinical Environment
4. Discussion
4.1. Simulation Models
4.2. Clinical Implications
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [Green Version]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Lim, H.S.; Hocini, M.; Dubois, R.; Denis, A.; Derval, N.; Zellerhoff, S.; Yamashita, S.; Berte, B.; Mahida, S.; Komatsu, Y.; et al. Complexity and Distribution of Drivers in Relation to Duration of Persistent Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 69, 1257–1269. [Google Scholar] [CrossRef]
- Rodrigo, M.; Guillem, M.S.; Climent, A.M.; Pedrón-Torrecilla, J.; Liberos, A.; Millet, J.; Fernández-Avilés, F.; Atienza, F.; Berenfeld, O. Body surface localization of left and right atrial high-frequency rotors in atrial fibrillation patients: A clinical-computational study. Heart Rhythm 2014, 11, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Roney, C.H.; Williams, S.E.; Cochet, H.; Mukherjee, R.K.; O’Neill, L.; Sim, I.; Whitaker, J.; Razeghi, O.; Klein, G.J.; Vigmond, E.J.; et al. Patient-specific simulations predict efficacy of ablation of interatrial connections for treatment of persistent atrial fibrillation. EP Eur. 2018, 20, iii55–iii68. [Google Scholar] [CrossRef]
- Ferrer, A.; Sebastián, R.; Sánchez-Quintana, D.; Rodríguez, J.F.; Godoy, E.J.; Martínez, L.; Saiz, J. Detailed Anatomical and Electrophysiological Models of Human Atria and Torso for the Simulation of Atrial Activation. PLoS ONE 2015, 10, e0141573. [Google Scholar] [CrossRef]
- Muszkiewicz, A.; Britton, O.J.; Gemmell, P.; Passini, E.; Sanchez, C.; Zhou, X.; Carusi, A.; Quinn, T.A.; Burrage, K.; Bueno-Orovio, A.; et al. Variability in cardiac electrophysiology: Using experimentally-calibrated populations of models to move beyond the single virtual physiological human paradigm. Prog. Biophys. Mol. Biol. 2016, 120, 115–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronis, K.N.; Ali, R.L.; Liang, J.A.; Zhou, S.; Trayanova, N.A. Understanding AF mechanisms through computational modelling and simulations. Arrhythmia Electrophysiol. Rev. 2019, 8, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S.; Lim, B.; Shim, J.; Hwang, M.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, S.-H.; Joung, B.; On, Y.K.; et al. Clinical Usefulness of Simulation-Guided Catheter Ablation of Atrial Fibrillation: Updated Outcome of a Multicenter Prospective Randomized Study. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Yang, P.C.; Demarco, K.R.; Aghasafari, P.; Jeng, M.T.; Dawson, J.R.D.; Bekker, S.; Noskov, S.Y.; Yarov-Yarovoy, V.; Vorobyov, I.; Clancy, C.E. A Computational Pipeline to Predict Cardiotoxicity: From the Atom to the Rhythm. Circ. Res. 2020, 126, 947–964. [Google Scholar] [CrossRef] [PubMed]
- Passini, E.; Britton, O.J.; Lu, H.R.; Rohrbacher, J.; Hermans, A.N.; Gallacher, D.J.; Greig, R.J.H.; Bueno-Orovio, A.; Rodriguez, B. Human In Silico Drug Trials Demonstrate Higher Accuracy than Animal Models in Predicting Clinical Pro-Arrhythmic Cardiotoxicity. Front. Physiol. 2017, 8, 668. [Google Scholar] [CrossRef] [Green Version]
- Boyle, P.M.; Zghaib, T.; Zahid, S.; Ali, R.L.; Deng, D.; Franceschi, W.H.; Hakim, J.B.; Murphy, M.J.; Prakosa, A.; Zimmerman, S.L.; et al. Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat. Biomed. Eng. 2019, 3, 870–879. [Google Scholar] [CrossRef]
- Varela, M.; Bisbal, F.; Zacur, E.; Berruezo, A.; Aslanidi, O.V.; Mont, L.; Lamata, P. Novel Computational Analysis of Left Atrial Anatomy Improves Prediction of Atrial Fibrillation Recurrence after Ablation. Front. Physiol. 2017, 0, 68. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Atienza, F.; Carrión, J.R.; Alberola, A.G.; Álvarez, J.L.R.; Muñoz, J.J.S.; Sánchez, J.M.; Chávarri, M.V. A Probabilistic Model of Cardiac Electrical Activity Based on a Cellular Automata System. Rev. Española Cardiol. 2005, 58, 41–47. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herlin, A.; Jacquemet, V. Eikonal-based initiation of fibrillatory activity in thin-walled cardiac propagation models. Chaos 2011, 21, 043136. [Google Scholar] [CrossRef]
- Skibsbye, L.; Jespersen, T.; Christ, T.; Maleckar, M.M.; van den Brink, J.; Tavi, P.; Koivumaki, J.T. Refractoriness in human atria: Time and voltage dependence of sodium channel availability. J. Mol. Cell. Cardiol. 2016, 101, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, M.; Climent, A.M.; Liberos, A.; Calvo, D.; Fernández-Avilés, F.; Berenfeld, O.; Atienza, F.; Guillem, M.S. Identification of Dominant Excitation Patterns and Sources of Atrial Fibrillation by Causality Analysis. Ann. Biomed. Eng. 2016, 44, 2364–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydın, S.; Melih, H.; Saraoğlu, H.M.; Kara, S. Log Energy Entropy-Based EEG Classification with Multilayer Neural Networks in Seizure. Ann. Biomed. Eng. 2009, 37, 2626. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhou, M. ECG classification usingwavelet packet entropy and random forests. Entropy 2016, 18, 285. [Google Scholar] [CrossRef]
- Rodrigo, M.; Climent, A.M.; Hernández-Romero, I.; Liberos, A.; Baykaner, T.; Rogers, A.J.; Alhusseini, M.; Wang, P.J.; Fernández-Avilés, F.; Guillem, M.S.; et al. Noninvasive Assessment of Complexity of Atrial Fibrillation: Correlation With Contact Mapping and Impact of Ablation. Circ. Arrhythm. Electrophysiol. 2020, 13, e007700. [Google Scholar] [CrossRef]
- Guillem, M.S.; Climent, A.M.; Rodrigo, M.; Fernández-Avilés, F.; Atienza, F.; Berenfeld, O. Presence and stability of rotors in atrial fibrillation: Evidence and therapeutic implications. Cardiovasc. Res. 2016, 109, 480–492. [Google Scholar] [CrossRef] [Green Version]
- McAlpine, W.A. Heart and Coronary Arteries, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1975; ISBN 978-3-642-65985-0. [Google Scholar]
- Atienza, F.; Almendral, J.; Jalife, J.; Zlochiver, S.; Ploutz-Snyder, R.; Torrecilla, E.G.; Arenal, Á.; Kalifa, J.; Fernández-Avilés, F.; Berenfeld, O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm 2009, 6, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Atienza, F.; Almendral, J.; Ormaetxe, J.M.; Moya, Á.; Martínez-Alday, J.D.; Hernández-Madrid, A.; Castellanos, E.; Arribas, F.; Arias, M.Á.; Tercedor, L.; et al. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: A noninferiority randomized multicenter RADAR-AF trial. J. Am. Coll. Cardiol. 2014, 64, 2455–2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arevalo, H.J.; Vadakkumpadan, F.; Guallar, E.; Jebb, A.; Malamas, P.; Wu, K.C.; Trayanova, N.A. Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Haissaguerre, M.; Lim, K.T.; Jacquemet, V.; Rotter, M.; Dang, L.; Hocini, M.; Matsuo, S.; Knecht, S.; Jaïs, P.; Virag, N. Atrial fibrillatory cycle length: Computer simulation and potential clinical importance. Europace 2007, 9, vi64–vi70. [Google Scholar] [CrossRef] [PubMed]
- Gharaviri, A.; Pezzuto, S.; Potse, M.; Verheule, S.; Conte, G.; Krause, R.; Schotten, U.; Auricchio, A. Left Atrial Appendage Electrical Isolation Reduces Atrial Fibrillation Recurrences. Circ. Arrhythmia Electrophysiol. 2021, 14, e009230. [Google Scholar] [CrossRef] [PubMed]
- Sachetto Oliveira, R.; Martins Rocha, B.; Burgarelli, D.; Meira, W.; Constantinides, C.; Weber dos Santos, R. Performance evaluation of GPU parallelization, space-time adaptive algorithms, and their combination for simulating cardiac electrophysiology. Int. J. Numer. Method. Biomed. Eng. 2018, 34, e2913. [Google Scholar] [CrossRef]
- Clayton, R.H.; Bernus, O.; Cherry, E.M.; Dierckx, H.; Fenton, F.H.; Mirabella, L.; Pan, A.V.; Sachse, F.B.; Seemann, G.; Zhang, H. Models of cardiac tissue electrophysiology: Progress, challenges and open questions. Prog. Biophys. Mol. Biol. 2011, 104, 22–48. [Google Scholar] [CrossRef]
- Fastl, T.E.; Tobon-Gomez, C.; Crozier, A.; Whitaker, J.; Rajani, R.; McCarthy, K.P.; Sanchez-Quintana, D.; Ho, S.Y.; O’Neill, M.D.; Plank, G.; et al. Personalized computational modeling of left atrial geometry and transmural myofiber architecture. Med. Image Anal. 2018, 47, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.M.; Krummen, D.E.; Shivkumar, K.; Clopton, P.; Rappel, W.-J.; Miller, J.M. Treatment of Atrial Fibrillation by the Ablation of Localized Sources. J. Am. Coll. Cardiol. 2012, 60, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Pandit, S.V.; Jalife, J. Rotors and the dynamics of cardiac fibrillation. Circ. Res. 2013, 112, 849–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, T.E.; Kalman, J.M. Human Persistent Atrial Fibrillation Is Maintained by Rotors. Circ. Arrhythmia Electrophysiol. 2015, 8, 517–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaman, J.A.B.; Rogers, A.J.; Narayan, S.M. Rotational Drivers in Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2017, 10, 6022. [Google Scholar] [CrossRef]
- Atienza, F.; Martins, R.P.; Jalife, J. Translational research in atrial fibrillation: A quest for mechanistically based diagnosis and therapy. Circ. Arrhythmia Electrophysiol. 2012, 5, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Haissaguerre, M.; Hocini, M.; Denis, A.; Shah, A.J.; Komatsu, Y.; Yamashita, S.; Daly, M.; Amraoui, S.; Zellerhoff, S.; Picat, M.Q.; et al. Driver domains in persistent atrial fibrillation. Circulation 2014, 130, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo, C.J.; Deo, M.; Zlochiver, S.; Millet, J.; Berenfeld, O. Attraction of Rotors to the Pulmonary Veins in Paroxysmal Atrial Fibrillation: A Modeling Study. Biophys. J. 2014, 106, 1811–1821. [Google Scholar] [CrossRef] [Green Version]
- Gosai, J.; Purva, M.; Gunn, J. Simulation in cardiology: State of the art. Eur. Heart J. 2015, 36, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Di Biase, L.; Natale, A.; Romero, J. Thrombogenic and Arrhythmogenic Roles of the Left Atrial Appendage in Atrial Fibrillation. Circulation 2018, 138, 2036–2050. [Google Scholar] [CrossRef]

| Characteristics | Complete Cohort | AF-Freedom Group | AF Group | p-Value |
|---|---|---|---|---|
| Anthropometrics | 30 patients | 18 patients | 12 patients | |
| Persistent AF | 16 (53.3%) | 7 (38.89%) | 9 (75%) | <0.001 |
| Age, yrs | 59 ± 14 | 57 ± 15 | 62 ± 12 | 0.38 |
| Female | 17 (56.67%) | 9 (50%) | 8 (66.67%) | <0.001 |
| Height (cm) | 164.15 ± 9.38 | 164.87 ± 8.85 | 163.25 ± 10.33 | 0.67 |
| Weight (kg) | 76.03 ± 16.24 | 77.80 ± 14.51 | 73.67 ± 18.68 | 0.52 |
| Blood samples | ||||
| Potassium | 4.07 ± 0.40 | 4.04 ± 0.44 | 4.12 ± 0.35 | 0.60 |
| Creatinine | 0.91 ± 0.18 | 0.91 ± 0.17 | 0.90 ± 0.20 | 0.90 |
| Hemoglobin | 13.83 ± 1.67 | 13.57 ± 1.93 | 14.21 ± 1.18 | 0.32 |
| Leucocytes | 7.24 ± 2.29 | 7.65 ± 2.73 | 6.65 ± 1.34 | 0.25 |
| Platelets | 206.83 ± 47.90 | 212.94 ± 54.50 | 198.17 ± 37.16 | 0.42 |
| INR | 1.25 ± 0.55 | 1.22 ± 0.55 | 1.28 ± 0.57 | 0.80 |
| LVEF | 54.42 ± 9.67 | 53.00 ± 11.21 | 56.78 ± 6.24 | 0.37 |
| Atria Size (cm2) | 31.49 ± 7.88 | 30.71 ± 8.83 | 32.87 ± 6.05 | 0.52 |
| Previous diagnostics | ||||
| Mitral insufficiency | 11 (36.67%) | 6 (33.33%) | 5 (41.67%) | 0.64 |
| Tricuspid Insufficiency | 11 (36.67%) | 8 (44.44%) | 3 (25%) | 0.28 |
| Mitral stenosis | 6 (20%) | 4 (22.22%) | 2 (16.67%) | 0.71 |
| Medical therapy | ||||
| Beta-blockers | 18 (60%) | 11 (61.11%) | 7 (58.33%) | 0.88 |
| Flecainide | 9 (30%) | 7 (38.89%) | 2 (16.67%) | 0.19 |
| Amiodarone | 4 (13.33%) | 3 (16.67%) | 1 (8.33%) | 0.51 |
| Complete Cohort | AF-Freedom Group | AF Group | p-Value | |
|---|---|---|---|---|
| Simulation characterization | 30 patients | 18 patients | 12 patients | |
| Sustained simulations (%) | 33.70 ± 17.73 | 33.00 ± 17.82 | 34.90 ± 17.63 | 0.79 |
| PV attachment (%) | 84.67 ± 6.83 | 86.20 ± 7.06 | 81.33 ± 5.97 | 0.10 |
| Simulations presenting high entropy values in PV (%) | 81.25 | 93.75 | 62.50 | p < 0.001 |
| Rotor distribution | ||||
| Right Atrium rotors | 2.13 ± 0.38 | 2.22 ± 0.34 | 1.97 ± 0.40 | 0.09 |
| Left Atrium rotors | 2.97 ± 0.48 | 2.96 ± 0.35 | 2.97 ± 0.68 | 0.99 |
| Left atrial appendage rotors | 2.78 ± 3.45 | 3.52 ± 3.81 | 1.50 ± 2.37 | 0.14 |
| Biomarkers from simulations | ||||
| ACM presenting high entropy areas in RA (%) | 81.25 | 68.75 | 100 | p < 0.001 |
| Atrial Complexity Biomarker | 1.53 ± 0.23 | 1.61 ± 0.21 | 1.40 ± 0.20 | 0.018 |
| ACM presenting high entropy areas in RA (%) | 81.25 | 68.75 | 100 | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez de la Nava, A.M.; González Mansilla, A.; González-Torrecilla, E.; Ávila, P.; Datino, T.; Bermejo, J.; Arenal, Á.; Fernández-Avilés, F.; Atienza, F. Personalized Evaluation of Atrial Complexity of Patients Undergoing Atrial Fibrillation Ablation: A Clinical Computational Study. Biology 2021, 10, 838. https://doi.org/10.3390/biology10090838
Sánchez de la Nava AM, González Mansilla A, González-Torrecilla E, Ávila P, Datino T, Bermejo J, Arenal Á, Fernández-Avilés F, Atienza F. Personalized Evaluation of Atrial Complexity of Patients Undergoing Atrial Fibrillation Ablation: A Clinical Computational Study. Biology. 2021; 10(9):838. https://doi.org/10.3390/biology10090838
Chicago/Turabian StyleSánchez de la Nava, Ana María, Ana González Mansilla, Esteban González-Torrecilla, Pablo Ávila, Tomás Datino, Javier Bermejo, Ángel Arenal, Francisco Fernández-Avilés, and Felipe Atienza. 2021. "Personalized Evaluation of Atrial Complexity of Patients Undergoing Atrial Fibrillation Ablation: A Clinical Computational Study" Biology 10, no. 9: 838. https://doi.org/10.3390/biology10090838
APA StyleSánchez de la Nava, A. M., González Mansilla, A., González-Torrecilla, E., Ávila, P., Datino, T., Bermejo, J., Arenal, Á., Fernández-Avilés, F., & Atienza, F. (2021). Personalized Evaluation of Atrial Complexity of Patients Undergoing Atrial Fibrillation Ablation: A Clinical Computational Study. Biology, 10(9), 838. https://doi.org/10.3390/biology10090838