Investigation of Anti-Tumor Effects of an MLK1 Inhibitor in Prostate and Pancreatic Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
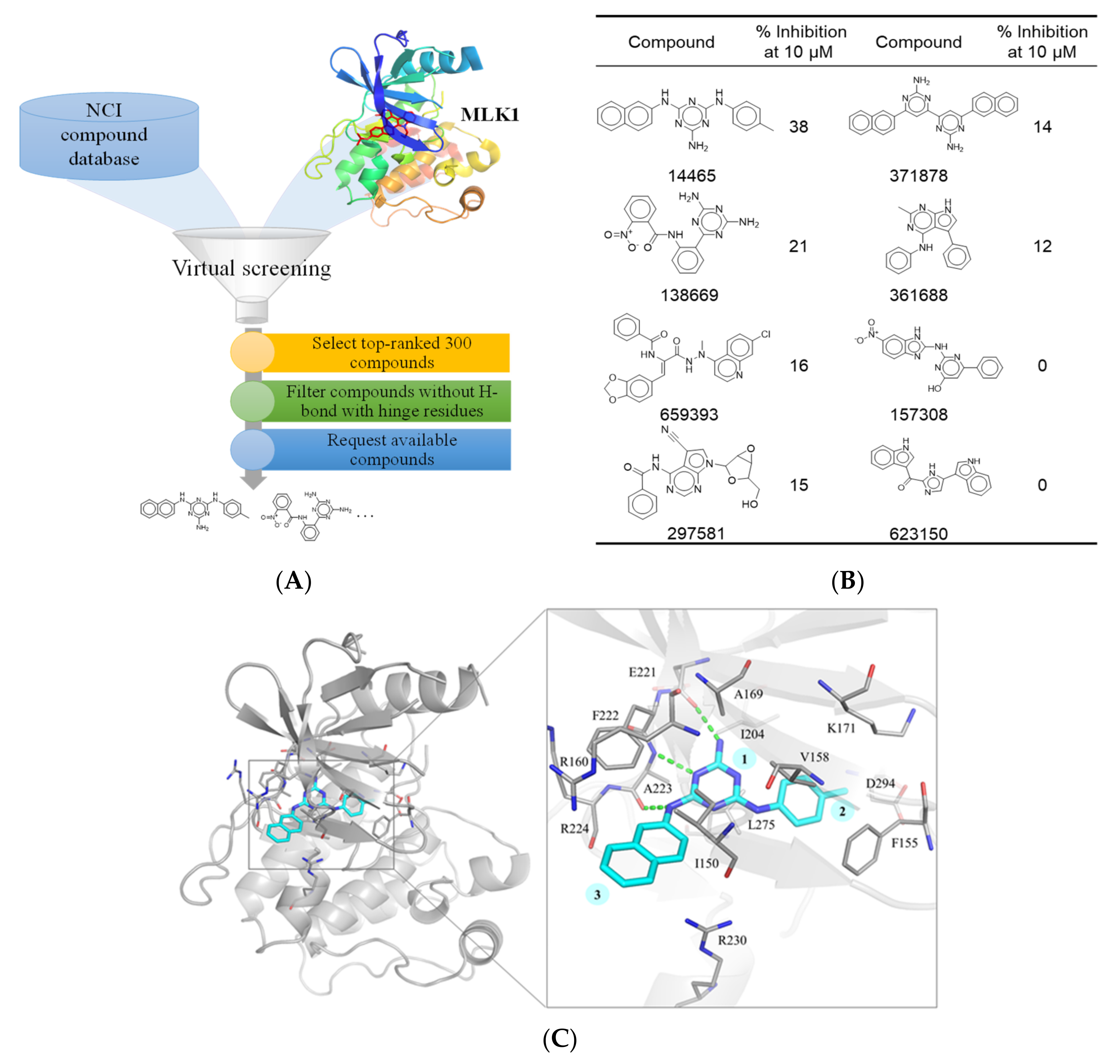
2.2. Structure-Based Virtual Screening
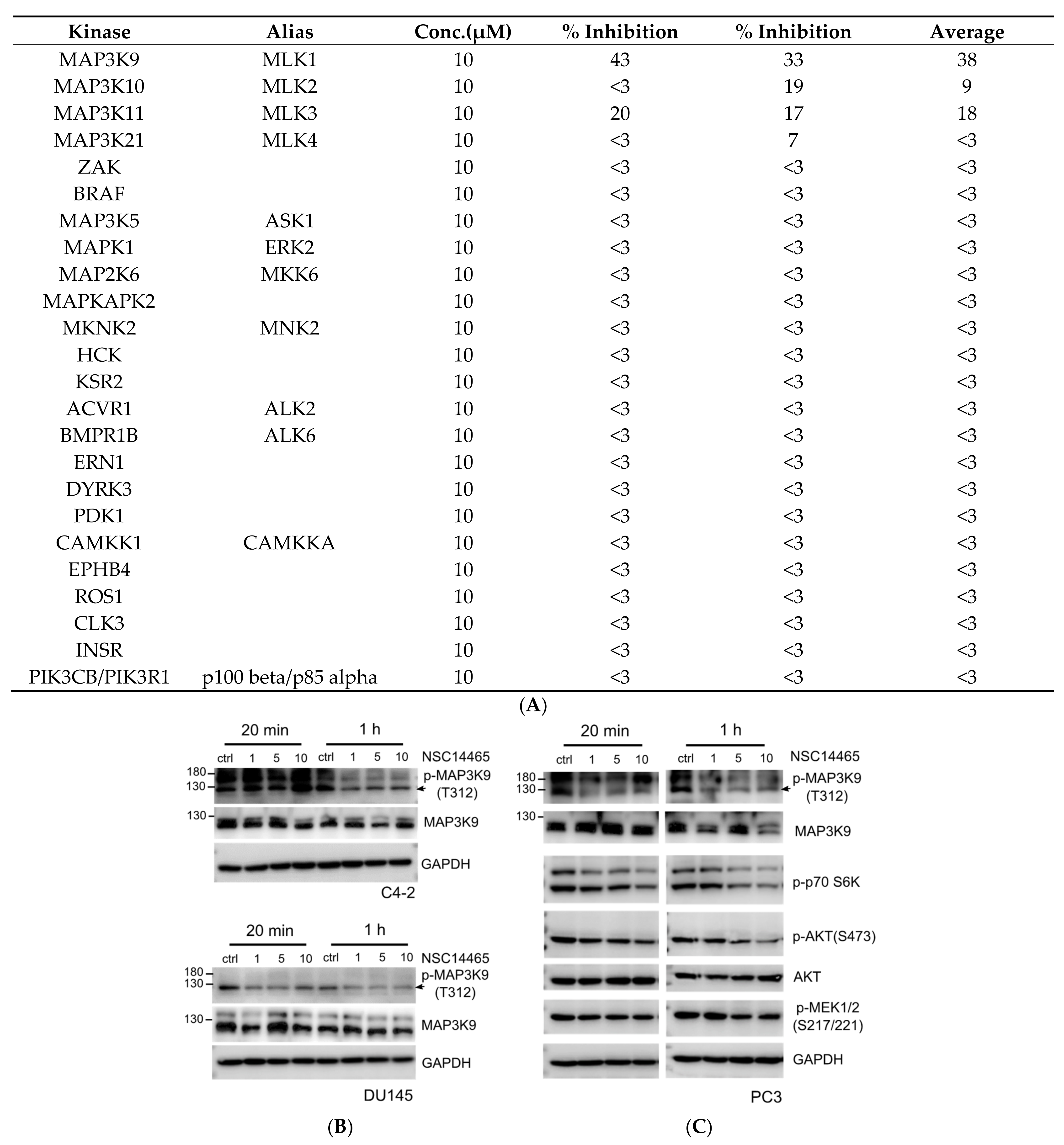
2.3. In Vitro Kinase Assay
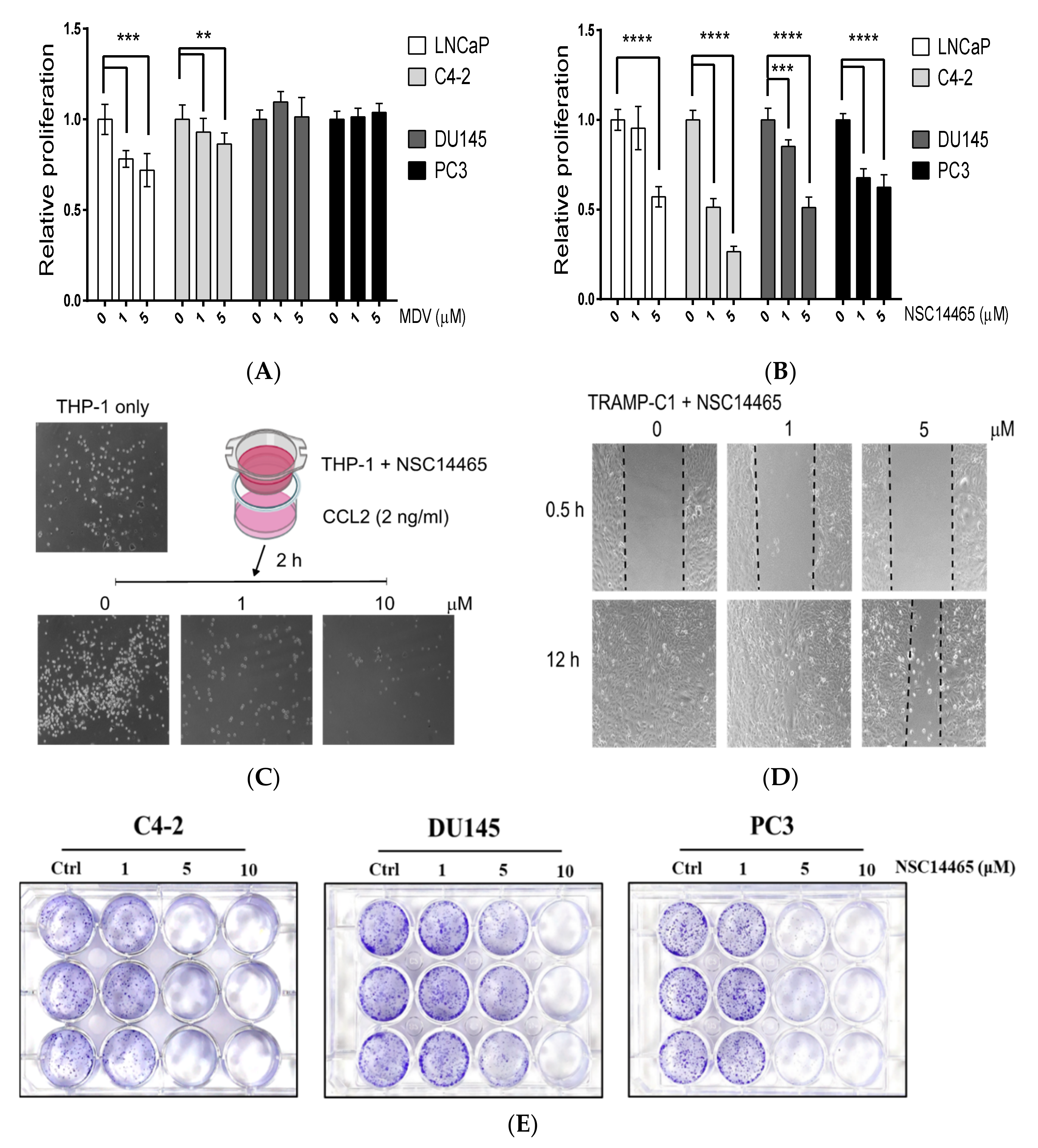
2.4. Transwell and Wound-Healing Assays
2.5. Proliferation and Colony-Formation Assays
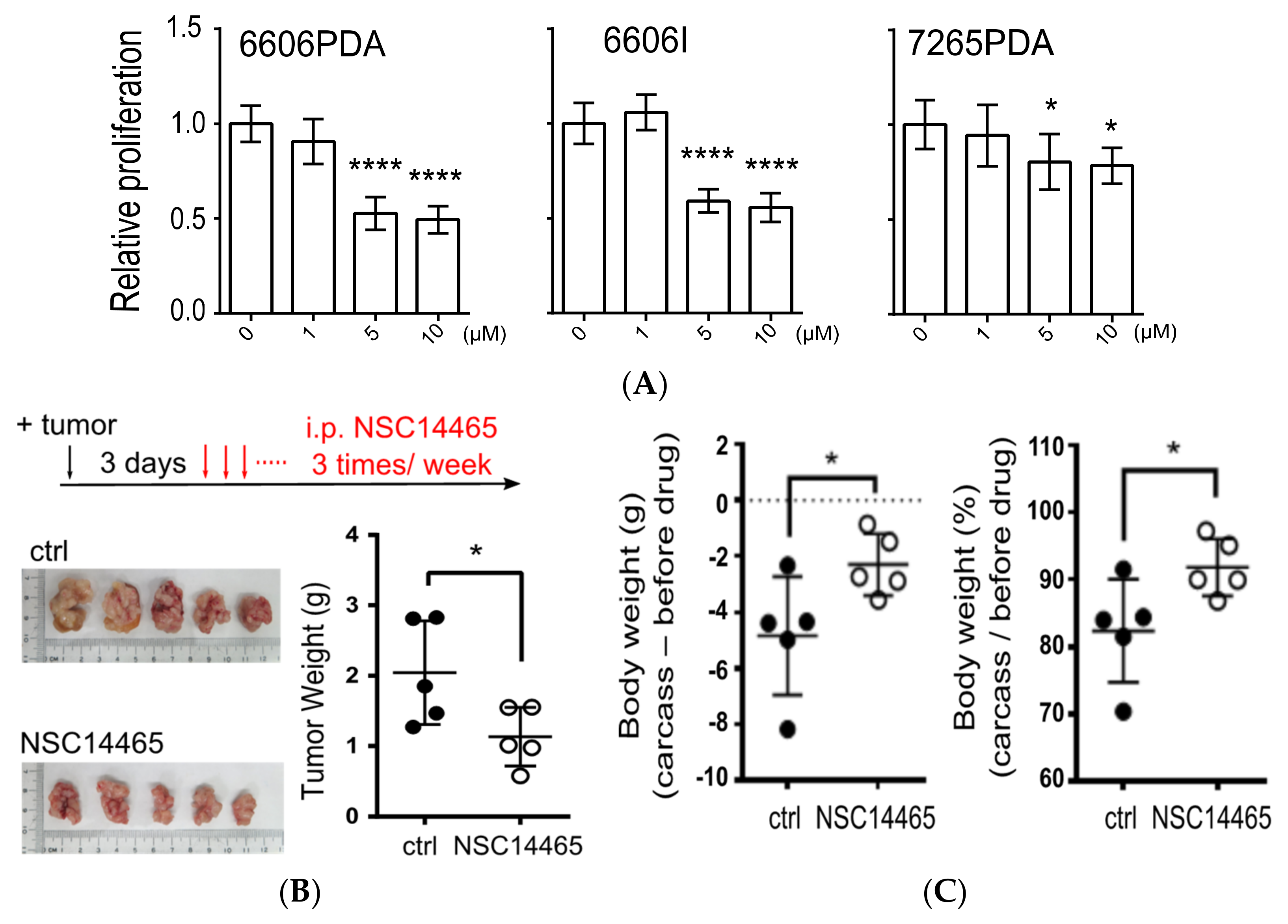
2.6. Animals and the Syngeneic Orthotopic Mouse Model of Pancreatic Cancer
2.7. Western Blot Analysis
2.8. Bioinformatics and Statistical Analyses
3. Results
3.1. Association of Increased MLK1 Messenger Ribonucleic Acid (mRNA) with Tumors and Poor Survival Rate in Clinical Datasets of Prostate Cancers
3.2. Identification of an MLK1 Inhibitor, NSC14465
3.3. Validation of Inhibitory Effects of NSC14465 on MLK1
3.4. Characterization of the Anti-Proliferation and Anti-Migration Effects of NSC14465
3.5. Demonstration of Anti-Tumor Effects of NSC14465 in a Syngeneic Pancreatic Cancer Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef] [PubMed]
- Ferraldeschi, R.; Welti, J.; Luo, J.; Attard, G.; de Bono, J.S. Targeting the androgen receptor pathway in castration-resistant prostate cancer: Progresses and prospects. Oncogene 2015, 34, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H. Cancer Death Rates Are Falling; Five-Year Survival Rates Are Rising. 2019. Available online: https://ourworldindata.org/cancer-death-rates-are-falling-five-year-survival-rates-are-rising (accessed on 6 February 2021).
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef]
- Uhlik, M.T.; Abell, A.N.; Cuevas, B.D.; Nakamura, K.; Johnson, G.L. Wiring diagrams of MAPK regulation by MEKK1, 2, and 3. Biochem. Cell Biol. 2004, 82, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Cao, T.; Ma, C.; Shi, Y.; Sun, Y.; Wang, Z.P.; Ma, J. miR-7 Suppresses Tumor Progression by Directly Targeting MAP3K9 in Pancreatic Cancer. Mol. Nucleic Acids 2018, 13, 121–132. [Google Scholar] [CrossRef]
- Cuevas, B.D.; Abell, A.N.; Johnson, G.L. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene 2007, 26, 3159–3171. [Google Scholar] [CrossRef]
- Marusiak, A.A.; Edwards, Z.C.; Hugo, W.; Trotter, E.W.; Girotti, M.R.; Stephenson, N.L.; Kong, X.; Gartside, M.G.; Fawdar, S.; Hudson, A.; et al. Mixed lineage kinases activate MEK independently of RAF to mediate resistance to RAF inhibitors. Nat. Commun. 2014, 5, 3901. [Google Scholar] [CrossRef]
- Zechner, D.; Burtin, F.; Amme, J.; Lindner, T.; Radecke, T.; Hadlich, S.; Kuhn, J.P.; Vollmar, B. Characterization of novel carcinoma cell lines for the analysis of therapeutical strategies fighting pancreatic cancer. Cell Biosci. 2015, 5, 51. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- BIOVIA Pipeline Pilot, Release; Dassault Systèmes: Vélizy-Villacoublay, France, 2017.
- Rarey, M.; Kramer, B.; Lengauer, T.; Klebe, G. A fast flexible docking method using an incremental construction algorithm. J. Mol. Biol. 1996, 261, 470–489. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Klug-Mcleod, J.; Rai, B.; Lunney, E.A. Kinase hinge binding scaffolds and their hydrogen bond patterns. Bioorg. Med. Chem. 2015, 23, 6520–6527. [Google Scholar] [CrossRef]
- Wallace, T.A.; Prueitt, R.L.; Yi, M.; Howe, T.M.; Gillespie, J.W.; Yfantis, H.G.; Stephens, R.M.; Caporaso, N.E.; Loffredo, C.A.; Ambs, S. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008, 68, 927–936. [Google Scholar] [CrossRef]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef]
- Arredouani, M.S.; Lu, B.; Bhasin, M.; Eljanne, M.; Yue, W.; Mosquera, J.M.; Bubley, G.J.; Li, V.; Rubin, M.A.; Libermann, T.A.; et al. Identification of the transcription factor single-minded homologue 2 as a potential biomarker and immunotherapy target in prostate cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 5794–5802. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Mehra, R.; Rhodes, D.R.; Cao, X.; Wang, L.; Dhanasekaran, S.M.; Kalyana-Sundaram, S.; Wei, J.T.; Rubin, M.A.; Pienta, K.J.; et al. Integrative molecular concept modeling of prostate cancer progression. Nat. Genet. 2007, 39, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.R.; Kalyana-Sundaram, S.; Mahavisno, V.; Varambally, R.; Yu, J.; Briggs, B.B.; Barrette, T.R.; Anstet, M.J.; Kincead-Beal, C.; Kulkarni, P.; et al. Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 2007, 9, 166–180. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Bjorling, E.; Agaton, C.; Szigyarto, C.A.; Amini, B.; Andersen, E.; Andersson, A.C.; Angelidou, P.; Asplund, A.; Asplund, C.; et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteom. 2005, 4, 1920–1932. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Durkin, J.T.; Holskin, B.P.; Kopec, K.K.; Reed, M.S.; Spais, C.M.; Steffy, B.M.; Gessner, G.; Angeles, T.S.; Pohl, J.; Ator, M.A.; et al. Phosphoregulation of mixed-lineage kinase 1 activity by multiple phosphorylation in the activation loop. Biochemistry 2004, 43, 16348–16355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, J.; Zhang, M.; Seleh-Zo, E.; Wang, J.; Cao, B.; An, X. circ-016910 sponges miR-574-5p to regulate cell physiology and milk synthesis via MAPK and PI3K/AKT-mTOR pathways in GMECs. J. Cell Physiol. 2020, 235, 4198–4216. [Google Scholar] [CrossRef]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Siu, M.K.; Chen, W.Y.; Tsai, H.Y.; Chen, H.Y.; Yin, J.J.; Chen, C.L.; Tsai, Y.C.; Liu, Y.N. TCF7 is suppressed by the androgen receptor via microRNA-1-mediated downregulation and is involved in the development of resistance to androgen deprivation in prostate cancer. Prostate Cancer Prostatic Dis. 2017. [Google Scholar] [CrossRef]
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.K.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef]
- Gao, J.; Isaacs, J.T. Mixed lineage kinase (MLK) family members are not involved in androgen regulation of prostatic proliferation or apoptosis. Prostate 2001, 48, 67–70. [Google Scholar] [CrossRef]
- Kohrt, S.E.; Awadallah, W.N.; Phillips, R.A., 3rd; Case, T.C.; Jin, R.; Nanda, J.S.; Yu, X.; Clark, P.E.; Yi, Y.; Matusik, R.J.; et al. Identification of Genes Required for Enzalutamide Resistance in Castration-Resistant Prostate Cancer Cells In Vitro. Mol. Cancer Ther. 2021, 20, 398–409. [Google Scholar] [CrossRef]
- Kordes, M.; Larsson, L.; Engstrand, L.; Lohr, J.M. Pancreatic cancer cachexia: Three dimensions of a complex syndrome. Br. J. Cancer 2021, 124, 1623–1636. [Google Scholar] [CrossRef] [PubMed]
- Lerner, L.; Tao, J.; Liu, Q.; Nicoletti, R.; Feng, B.; Krieger, B.; Mazsa, E.; Siddiquee, Z.; Wang, R.; Huang, L.; et al. MAP3K11/GDF15 axis is a critical driver of cancer cachexia. J. Cachexia Sarcopenia Muscle 2016, 7, 467–482. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.-C.; Hsu, K.-C.; Lin, T.-E.; Zechner, D.; Hsu, S.-P.; Tsai, Y.-C. Investigation of Anti-Tumor Effects of an MLK1 Inhibitor in Prostate and Pancreatic Cancers. Biology 2021, 10, 742. https://doi.org/10.3390/biology10080742
Fan Y-C, Hsu K-C, Lin T-E, Zechner D, Hsu S-P, Tsai Y-C. Investigation of Anti-Tumor Effects of an MLK1 Inhibitor in Prostate and Pancreatic Cancers. Biology. 2021; 10(8):742. https://doi.org/10.3390/biology10080742
Chicago/Turabian StyleFan, Yu-Ching, Kai-Cheng Hsu, Tony-Eight Lin, Dietmar Zechner, Sung-Po Hsu, and Yuan-Chin Tsai. 2021. "Investigation of Anti-Tumor Effects of an MLK1 Inhibitor in Prostate and Pancreatic Cancers" Biology 10, no. 8: 742. https://doi.org/10.3390/biology10080742
APA StyleFan, Y.-C., Hsu, K.-C., Lin, T.-E., Zechner, D., Hsu, S.-P., & Tsai, Y.-C. (2021). Investigation of Anti-Tumor Effects of an MLK1 Inhibitor in Prostate and Pancreatic Cancers. Biology, 10(8), 742. https://doi.org/10.3390/biology10080742





