Combined Production of Astaxanthin and β-Carotene in a New Strain of the Microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) Cultivated in Photobioreactor
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Vegetative Growth Conditions
2.2. Carotenoid Synthesis Induction
2.3. Dry Mass Determination
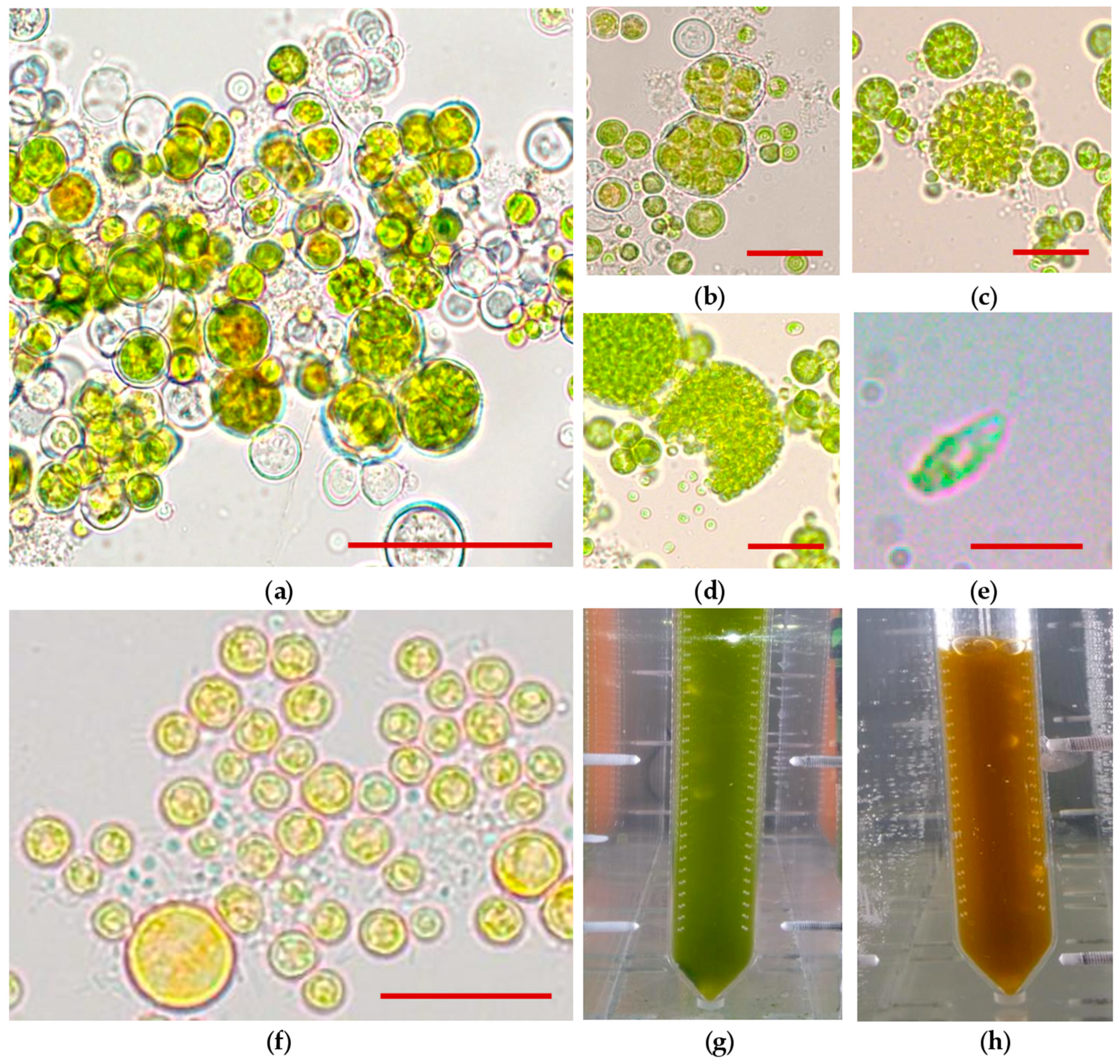
2.4. Microscopic Observations
2.5. Pigment Extraction
2.6. Chromatographic Analysis
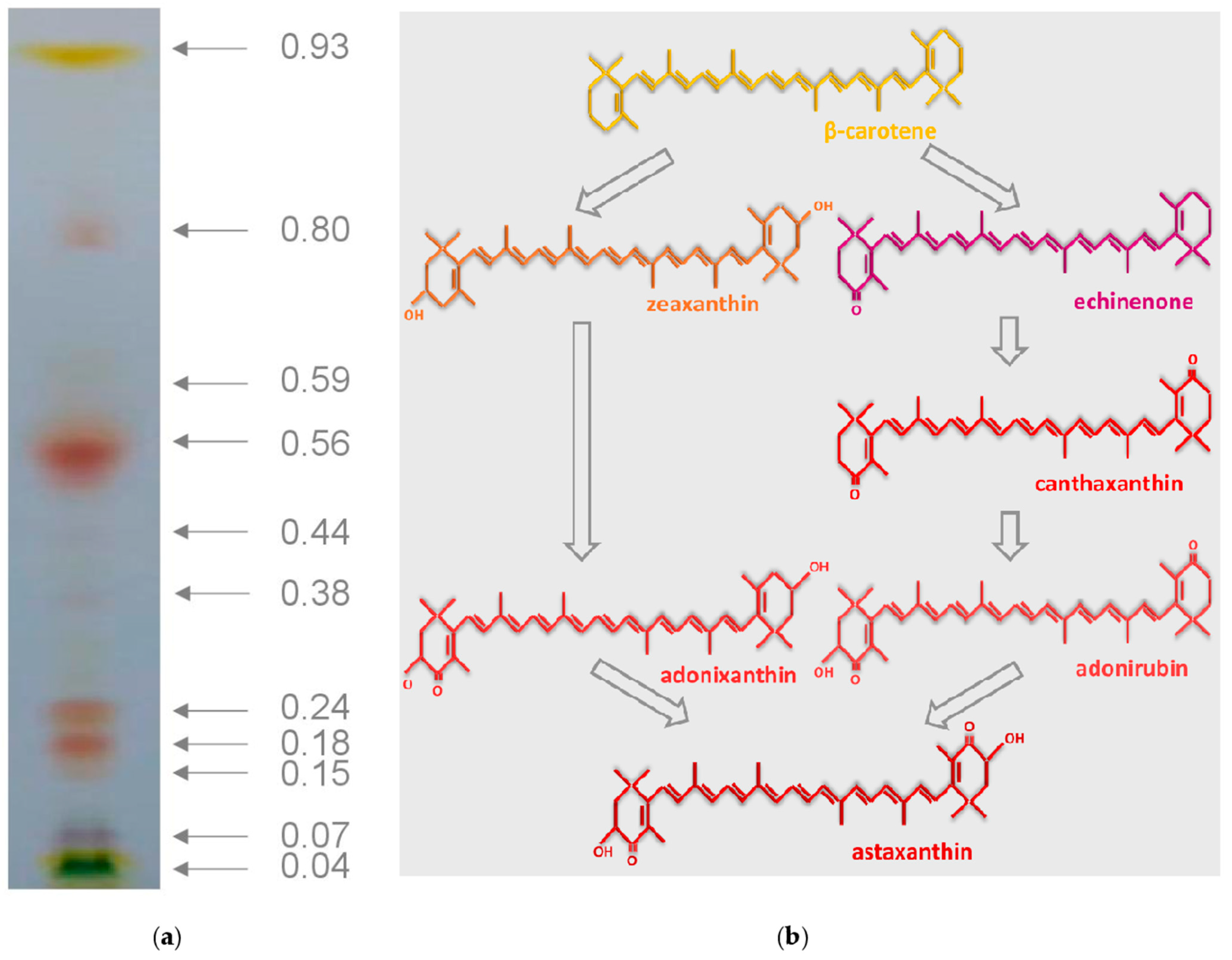
2.6.1. Thin Layer Chromatography
2.6.2. Gas Chromatography–Mass Spectrometry
2.7. Data Treatment
3. Results and Discussion
3.1. Evaluation of B. aggregatus BM5/15 Cell Morphology
3.2. Parameters of B. aggregatus BM5/15 Cultured in Photobioreactor
3.3. Carotenoid Profile in the B. aggregatus BM5/15 Biomass at the Induction Stage
3.4. Fatty Acid Profile B. aggregatus BM5/15 Biomass at the Induction Stage of Cultivation
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, L. Carotenoid metabolism: Biosynthesis, regulation, and beyond. J. Integr. Plant Biol. 2008, 50, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Khachik, F. Carotenoids in food. In Carotenoids; Britton, G., Pfanden, H., Liaaen-Jensen, S., Eds.; Birkhäuser: Basel, Switzerland, 2009; Volume 5, pp. 45–66. [Google Scholar]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A.; Schroeder, W.A. Microbial carotenoids. In Downstream Processing Biosurfactants Carotenoids; Fiechter, A., Ed.; Springer: Berlin, Germany, 1995; pp. 119–178. [Google Scholar]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Cezare-Gomes, E.A.; del Carmen Mejia-da-Silva, L.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of microalgae carotenoids for industrial application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef]
- Solovchenko, A.; Chekanov, K. Production of carotenoids using microalgae cultivated in photobioreactors. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.-Y., Murthy, H.N., Zhong, J.-J., Eds.; Springer: Dordrecht, Germany, 2014; pp. 63–92. [Google Scholar]
- Isler, O.; Rüegg, R.; Schwieter, U. Carotenoids as food colourants. Pure Appl. Chem. 1967, 14, 245–264. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009, 5, 333–342. [Google Scholar] [CrossRef]
- Fassett, R.G.; Healy, H.; Driver, R.; Robertson, I.K.; Geraghty, D.P.; Sharman, J.E.; Coombes, J.S. Astaxanthin vs placebo on arterial stiffness, oxidative stress and inflammation in renal transplant patients (Xanthin): A randomised controlled trial. BMC Nephrol. 2008, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Preuss, H.G.; Echard, B.; Yamashita, E.; Perricone, N.V. High dose astaxanthin lowers blood pressure and increases insulin sensitivity in rats: Are these effects interdependent? Int. J. Med. Sci. 2011, 8, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Gal, A.F.; Andrei, S.; Cernea, C.; Taulescu, M.; Catoi, C. Effects of astaxanthin supplementation on chemically induced tumorigenesis in Wistar rats. Acta Vet. Scand. 2012, 54, 50. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Akiba, Y.; Sato, K.; Takahashi, K.; Matsushita, K.; Komiyama, H.; Tsunekawa, H.; Nagao, H. Meat color modification in broiler chickens by feeding yeast Phaffia rhodozyma containing high concentrations of astaxanthin. J. Appl. Poult. Res. 2001, 10, 154–161. [Google Scholar] [CrossRef]
- Johnson, E.A.; An, G.H. Astaxanthin from microbial sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Johnson, E.A.; Villa, T.G.; Lewis, M.J. Phaffia rhodozyma as an astaxanthin source in salmonid diets. Aquaculture 1981, 20, 123–134. [Google Scholar] [CrossRef]
- Schlipalius, L. The extensive commercial cultivation of Dunaliella salina. Biores. Technol. 1991, 38, 241–243. [Google Scholar] [CrossRef]
- Borowitzka, L.J.; Borowitzka, M.A. Commercial production of β-carotene by Dunaliella salina in open ponds. Bull. Mar. Sci. 1990, 47, 244–252. [Google Scholar]
- Borowitzka, M.A. Dunaliella: Biology, production, and markets. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; Blackwell Publishing Company: Oxford, UK, 2013; pp. 359–368. [Google Scholar]
- Ben-Amotz, A. Industrial production of microalgal cell-mass and secondary products-major industrial species. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Publishing Company: Oxford, UK, 2004; pp. 273–280. [Google Scholar]
- Del Campo, J.A.; Rodriguez, H.; Moreno, J.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef]
- García-Malea, M.C.; Acién, F.G.; Del Río, E.; Fernández, J.M.; Cerón, M.C.; Guerrero, M.G.; Molina-Grima, E. Production of astaxanthin by Haematococcus pluvialis: Taking the one-step system outdoors. Biotechnol. Bioeng. 2009, 102, 651–657. [Google Scholar] [CrossRef]
- Minyuk, G.S.; Chelebieva, E.S.; Chubchikova, I.N. Secondary carotenogenesis of the green microalga Bracteacoccus minor (Chodat) Petrova (Chlorophyta) in a two-stage culture. Internat. J. Algae 2014, 16, 21–34. [Google Scholar] [CrossRef]
- Borovkov, A.B.; Gudvilovich, I.N.; Avsiyan, A.L. Scale-up of Dunaliella salina cultivation: From strain selection to open ponds. J. Appl. Phycol. 2020, 32, 1545–1558. [Google Scholar] [CrossRef]
- Weissman, J.C.; Goebel, R.P.; Benemann, J.R. Photobioreactor design: Mixing, carbon utilization, and oxygen accumulation. Biotechnol. Bioeng. 1988, 31, 336–344. [Google Scholar] [CrossRef]
- Singh, R.N.; Sharma, S. Development of suitable photobioreactor for algae production—A review. Renew. Sustain. Energy Rev. 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Chekanov, K.; Zaytseva, A.; Mamedov, I.; Solovchenko, A.; Lobakova, E. The dynamics of the bacterial community of the photobioreactor-cultivated green microalga Haematococcus lacustris during stress-Induced astaxanthin accumulation. Biology 2021, 10, 115. [Google Scholar] [CrossRef]
- Fábregas, J.; Otero, A.; Maseda, A.; Domínguez, A. Two-stage cultures for the production of astaxanthin from Haematococcus pluvialis. J. Biotechnol. 2001, 89, 65–71. [Google Scholar] [CrossRef]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the relative efficiency of two-vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A. New mode of Dunaliella biotechnology: Two-phase growth for β-carotene production. J. Appl. Phycol. 1995, 7, 65–68. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Neverov, K. Carotenogenic response in photosynthetic organisms: A colorful story. Photosynth. Res. 2017, 133, 31–47. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. On the factors which determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol. 1983, 72, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plant. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Olaizola, M.; Huntley, M.E. Recent advances in commercial production of astaxanthin from microalgae. Biomater. Bioprocess. 2003, 9, 143–164. [Google Scholar]
- Fábregas, J.; Dominguez, A.; Maseda, A.; Otero, A. Interactions between irradiance and nutrient availability during astaxanthin accumulation and degradation in Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2003, 61, 545–551. [Google Scholar] [CrossRef]
- Minyuk, G.S.; Chelebieva, E.S.; Chubchikova, I.N.; Dantsyuk, N.V.; Drobetskaya, I.V.; Sakhon, E.G.; Chivkunova, O.B.; Chekanov, K.A.; Lobakova, E.S.; Sidorov, R.A.; et al. pH and CO2 effects on Coelastrella (Scotiellopsis) rubescens growth and metabolism. Russ. J. Plant Physiol. 2016, 63, 566–574. [Google Scholar] [CrossRef]
- Minyuk, G.; Chelebieva, E.; Chubchikova, I.; Dantsyuk, N.; Drobetskaya, I.; Sakhon, E.; Chekanov, K.; Solovchenko, A. Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae 2017, 32, 245–259. [Google Scholar] [CrossRef]
- Mulders, K.J.; Janssen, J.H.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Effect of biomass concentration on secondary carotenoids and triacylglycerol (TAG) accumulation in nitrogen-depleted Chlorella zofingiensis. Algal Res. 2014, 6, 8–16. [Google Scholar] [CrossRef]
- Mulders, K.J.; Lamers, P.P.; Wijffels, R.H.; Martens, D.E. Dynamics of biomass composition and growth during recovery of nitrogen-starved Chromochloris zofingiensis. Appl. Microbiol. Biotechnol. 2015, 99, 1873–1884. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Y.; Ding, W.; Mao, X.; Li, Y.; Gerken, H.; Liu, J. Astaxanthin is ketolated from zeaxanthin independent of fatty acid synthesis in Chromochloris zofingiensis. Plant Physiol. 2020, 183, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Minyuk, G.; Sidorov, R.; Solovchenko, A. Effect of nitrogen source on the growth, lipid, and valuable carotenoid production in the green microalga Chromochloris zofingiensis. J. Appl. Phycol. 2020, 923–935. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Zhang, Y.; Chen, F. Glucose triggers cell structure changes and regulates astaxanthin biosynthesis in Chromochloris zofingiensis. Algal Res. 2019, 39, 101455. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Sun, Z.; Li, T.; Zhou, Z.G.; Jiang, Y. Microalgae as a source of lutein: Chemistry, biosynthesis, and carotenogenesis. In Microalgae Biotechnology; Posten, C., Cheng, S.F., Eds.; Springer: Cham, Switzerland, 2015; Volume 153, pp. 37–58. [Google Scholar]
- Sampathkumar, S.J.; Gothandam, K.M. Sodium bicarbonate augmentation enhances lutein biosynthesis in green microalgae Chlorella pyrenoidosa. Biocatal. Agric. Biotechnol. 2019, 22, 101406. [Google Scholar] [CrossRef]
- Přibyl, P.; Pilný, J.; Cepák, V.; Kaštánek, P. The role of light and nitrogen in growth and carotenoid accumulation in Scenedesmus sp. Algal Res. 2016, 16, 69–75. [Google Scholar] [CrossRef]
- Cohen, Z. The chemicals of Spirulina. In Spirulina Platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology; Vonshak, A., Ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 175–204. [Google Scholar]
- Allewaert, C.C.; Vanormelingen, P.; Pröschold, T.; Gomez, P.I.; Gonzalez, M.A.; Bilcke, G.; D’Hondt, S.; Vyverman, W. Species diversity in European Haematococcus pluvialis (Chlorophyceae, Volvocales). Phycologia 2015, 54, 583–598. [Google Scholar] [CrossRef]
- Allewaert, C.C.; Vanormelingen, P.; Daveloose, I.; Verstraete, T.; Vyverman, W. Intraspecific trait variation affecting astaxanthin productivity in two Haematococcus (Chlorophyceae) species. Algal Res. 2017, 21, 191–202. [Google Scholar] [CrossRef]
- Chekanov, K.; Fedorenko, T.; Kublanovskaya, A.; Litvinov, D.; Lobakova, E. Diversity of carotenogenic microalgae in the White Sea polar region. FEMS Microbiol. Ecol. 2020, 96, fiz183. [Google Scholar] [CrossRef]
- Mazumdar, N.; Gopalakrishnan, K.K.; Visnovsky, G.; Novis, P.M. A novel alpine species of Haematococcus (Chlamydomonadales: Chlorophyta) from New Zealand. N. Z. J. Bot. 2018, 56, 216–226. [Google Scholar] [CrossRef]
- Procházková, L.; Leya, T.; Křížková, H.; Nedbalová, L. Sanguina nivaloides and Sanguina aurantia gen. et spp. nov. (Chlorophyta): The taxonomy, phylogeny, biogeography and ecology of two newly recognised algae causing red and orange snow. FEMS Microbiol. Ecol. 2019, 95, fiz064. [Google Scholar] [CrossRef] [Green Version]
- Klochkova, T.A.; Kwak, M.S.; Han, J.W.; Motomura, T.; Nagasato, C.; Kim, G.H. Cold-tolerant strain of Haematococcus pluvialis (Haematococcaceae, Chlorophyta) from Blomstrandhalvøya (Svalbard). Algae 2013, 28, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Chekanov, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Sidorov, R.; Solovchenko, A. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the White Sea coastal rocks (Russia). Mar. Drugs 2014, 12, 4504–4520. [Google Scholar] [CrossRef] [Green Version]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Chekanov, K.; Schastnaya, E.; Solovchenko, A.; Lobakova, E. Effects of CO2 enrichment on primary photochemistry, growth and astaxanthin accumulation in the chlorophyte Haematococcus pluvialis. J. Photochem. Photobiol. B Biol. 2017, 171, 58–66. [Google Scholar] [CrossRef]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Rasa, E.; Tantayotai, P.; Scow, K.M.; Yuan, H.; Hristova, K.R. Mathematical model of Chlorella minutissima UTEX2341 growth and lipid production under photoheterotrophic fermentation conditions. Biores. Technol. 2011, 102, 3077–3082. [Google Scholar] [CrossRef] [Green Version]
- Mazumdar, N.; Novis, P.M.; Visnovsky, G.; Gostomski, P.A. Effect of nutrients on the growth of a new alpine strain of Haematococcus (Chlorophyceae) from New Zealand. Phycol. Res. 2019, 67, 21–27. [Google Scholar] [CrossRef]
- Chekanov, K.A.; Solovchenko, A.E. Possibilities and limitations of non-destructive monitoring of the unicellular green microalgae (Chlorophyta) in the course of balanced growth. Russ. J. Plant Physiol. 2015, 62, 270–278. [Google Scholar] [CrossRef]
- Solovchenko, A.; Merzlyak, M.N.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. Coordinated carotenoid and lipid syntheses induced in Parietochloris incisa (Chlorophyta, Trebouxiophyceae) mutant deficient in δ5 desaturase by nitrogen starvation and high light. J. Phycol. 2010, 46, 763–772. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Britton, G. General carotenoid methods. Methods Enzymol. 1985, 111, 113–149. [Google Scholar] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kates, M. Techniques of Lipidology: Isolation, Analysis and Identification of Lipids, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1986; p. 464. [Google Scholar]
- Minyuk, G.S.; Solovchenko, A.E. Express Analysis of Microalgal Secondary Carotenoids by TLC and UV-Vis Spectroscopy. In Microbial Carotenoids; Barreiro, C., Barredo, J.-L., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1825, pp. 73–95. [Google Scholar]
- Fuíková, K.; Flechtner, V.R.; Lewis, L.A. Revision of the genus Bracteacoccus Tereg (Chlorophyceae, Chlorophyta) based on a phylogenetic approach. Nova Hedwig. 2012, 96, 15–59. [Google Scholar] [CrossRef]
- Chelebieva, E.S.; Dantsyuk, N.V.; Chekanov, K.A.; Chubchikova, I.N.; Drobetskaya, I.V.; Minyuk, G.S.; Lobakova, E.S.; Solovchenko, A.E. Identification and morphological-physiological characterization of astaxanthin producer strains of Haematococcus pluvialis from the Black Sea Region. Appl. Biochem. Microbiol. 2018, 54, 639–648. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Z.; Hu, Q.; Sommerfeld, M.; Lu, Y.; Han, D. Cellular capacities for high-light acclimation and changing lipid profiles across life cycle stages of the green alga Haematococcus pluvialis. PLoS ONE 2014, 9, e106679. [Google Scholar]
- Kublanovskaya, A.; Baulina, O.; Chekanov, K.; Lobakova, E. The microalga Haematococcus lacustris (Chlorophyceae) forms natural biofilms in supralittoral White Sea coastal rock ponds. Planta 2020, 252, 37. [Google Scholar] [CrossRef]
- Kublanovskaya, A.; Solovchenko, A.; Fedorenko, T.; Chekanov, K.; Lobakova, E. Natural communities of carotenogenic chlorophyte Haematococcus lacustris and bacteria from the White Sea coastal rock ponds. Microb. Ecol. 2020, 79, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Jeon, M.S.; Kim, J.Y.; Lee, S.H.; Kim, D.G.; Roh, S.W.; Choi, Y.E. Effects of an auxin-producing symbiotic bacterium on cell growth of the microalga Haematococcus pluvialis: Elevation of cell density and prolongation of exponential stage. Algal Res. 2019, 41, 101547. [Google Scholar] [CrossRef]
- Zheng, L.L.; Zhang, Q.; Li, T.L.; SONG, L.R. Axenation of Haematococcus pluvialis and the effects of axenic cultivation on the growth and physiology of the strain. J. Fujian Norm. Univ. (Nat. Sci. Ed.) 2017, 33, 44–50. [Google Scholar]
- Padmaperuma, G.; Kapoore, R.V.; Gilmour, D.J.; Vaidyanathan, S. Microbial consortia: A critical look at microalgae co-cultures for enhanced biomanufacturing. Crit. Rev. Biotechnol. 2018, 38, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Joo, H.N.; Lee, C.G. Antibiotics addition as an alternative sterilization method for axenic cultures in Haematococcus pluvialis. J. Ind. Eng. Chem. 2007, 13, 110–115. [Google Scholar]
- Sensen, C.W.; Heimann, K.; Melkonian, M. The production of clonal and axenic cultures of microalgae using fluorescence-activated cell sorting. Eur. J. Phycol. 1993, 28, 93–97. [Google Scholar] [CrossRef]
- Mamaeva, A.; Namsaraev, Z.; Maltsev, Y.; Gusev, E.; Kulikovskiy, M.; Petrushkina, M.; Filimonova, A.; Sorokin, B.; Zotko, N.; Vinokurov, C.; et al. Simultaneous increase in cellular content and volumetric concentration of lipids in Bracteacoccus bullatus cultivated at reduced nitrogen and phosphorus concentrations. J. Appl. Phycol. 2018, 30, 2237–2246. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Yamaguchi, K.; Nishio, N.; Nagai, S. Growth and astaxanthin formation of Haematococcus pluvialis in heterotrophic and mixotrophic conditions. J. Ferment. Bioeng. 1992, 74, 17–20. [Google Scholar] [CrossRef]
- Kaewpintong, K.; Shotipruk, A.; Powtongsook, S.; Pavasant, P. Photoautotrophic high-density cultivation of vegetative cells of Haematococcus pluvialis in airlift bioreactor. Biores. Technol. 2007, 98, 288–295. [Google Scholar] [CrossRef]
- Karuppan, R.; Javee, A.; Gopidas, S.K.; Subramani, N. Influence of agriculture fertilizer for the enhanced growth and astaxanthin production from Haematococcus lacustris RRGK isolated from Himachal Pradesh, India. SN Appl. Sci. 2019, 1, 532. [Google Scholar] [CrossRef] [Green Version]
- Imaizumi, Y.; Nagao, N.; Yusoff, F.M.; Taguchi, S.; Toda, T. Estimation of optimum specific light intensity per cell on a high-cell-density continuous culture of Chlorella zofingiensis not limited by nutrients or CO2. Biores. Technol. 2014, 162, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hata, N.; Ogbonna, J.C.; Hasegawa, Y.; Taroda, H.; Tanaka, H. Production of astaxanthin by Haematococcus pluvialis in a sequential heterotrophic-photoautotrophic culture. J. Appl. Phycol. 2001, 13, 395–402. [Google Scholar] [CrossRef]
- Chekanov, K.; Lukyanov, A.; Boussiba, S.; Aflalo, C.; Solovchenko, A. Modulation of photosynthetic activity and photoprotection in Haematococcus pluvialis cells during their conversion into haematocysts and back. Photosynth. Res. 2016, 128, 313–323. [Google Scholar] [CrossRef]
- Wu, K.; Ying, K.; Liu, L.; Zhou, J.; Cai, Z. High irradiance compensated with CO2 enhances the efficiency of Haematococcus lacustris growth. Biotechnol. Rep. 2020, 26, e00444. [Google Scholar] [CrossRef]
- Tan, S.; Cunningham, F.X., Jr.; Youmans, M.; Grabowski, B.; Sun, Z.; Gantt, E. Cytochrome f loss in astaxanthin-accumulating red cells of Haematococcus pluvialis (Chlorophyceae): Comparison of photosynthetic activity, photosynthetic enzymes, and thylakoid membrane polypeptides in red and green cells. J. Phycol. 1995, 31, 897–905. [Google Scholar] [CrossRef]
- Chekanov, K.; Schastnaya, E.; Neverov, K.; Leu, S.; Boussiba, S.; Zarka, A.; Solovchenko, A. Non-photochemical quenching in the cells of the carotenogenic chlorophyte Haematococcus lacustris under favorable conditions and under stress. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1429–1442. [Google Scholar] [CrossRef]
- Zhekisheva, M.; Boussiba, S.; Khozin-Goldberg, I.; Zarka, A.; Cohen, Z. Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters1. J. Phycol. 2002, 38, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Lamers, P.P.; van de Laak, C.C.; Kaasenbrood, P.S.; Lorier, J.; Janssen, M.; De Vos, R.C.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol. Bioeng. 2010, 106, 638–648. [Google Scholar] [CrossRef]
- Recht, L.; Zarka, A.; Boussiba, S. Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2012, 94, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.X., Jr.; Gantt, E. Genes and enzymes of carotenoid biosynthesis in plants. Annu. Rev. Plant Biol. 1998, 49, 557–583. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in microalgae: Pathways, functions and biotechnological implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Zhekisheva, M.; Zarka, A.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. Inhibition of astaxanthin synthesis under high irradiance does not abolish triacylglycerol accumulation in the green alga Haematococcus pluvialis (Chlorophyceae) 1. J. Phycol. 2005, 41, 819–826. [Google Scholar] [CrossRef]
- Orosa, M.; Torres, E.; Fidalgo, P.; Abalde, J. Production and analysis of secondary carotenoids in green algae. J. Appl. Phycol. 2000, 12, 553–556. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Cheng, K.M.; Craft, N.E.; Hamberger, B.; Douglas, C.J. Over-expression of Arabidopsis thaliana carotenoid hydroxylases individually and in combination with a β-carotene ketolase provides insight into in vivo functions. Phytochemistry 2010, 71, 168–178. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Deshmukh, S.; Kumar, R.; Bala, K. Microalgae biodiesel: A review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process. Technol. 2019, 191, 232–247. [Google Scholar] [CrossRef]
- Soares, A.T.; da Costa, D.C.; Vieira, A.A.H.; Filho, N.R.A. Analysis of major carotenoids and fatty acid composition of freshwater microalgae. Heliyon 2019, 5, e01529. [Google Scholar] [CrossRef] [Green Version]
- Damiani, M.C.; Popovich, C.A.; Constenla, D.; Leonardi, P.I. Lipid analysis in Haematococcus pluvialis to asses its potential use as a biodiesel feedstock. Biores. Technol. 2010, 101, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.W. Effects of nitrogen and phosphorus limitations on fatty acid methyl esters and fuel properties of Dunaliella salina. Environ. Sci. Pollut. Res. 2020, 27, 32296–32303. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.I.; Maltseva, I.A.; Maltseva, S.Y.; Kulikovskiy, M.S. Biotechnological Potential of a New Strain of Bracteacoccus bullatus (Sphaeropleales, Chlorophyta) as a Promising Producer of Omega-6 Polyunsaturated Fatty Acids. Russ. J. Plant. Physiol. 2020, 67, 185–193. [Google Scholar] [CrossRef]

| Culture Parameter | Value |
|---|---|
| Maximum specific growth rate (μ), day−1 | 0.33 |
| Maximum biomass at the vegetative stage (DMmax), mg∙L−1 | 1.2–1.6 |
| Maximal carotenoid content at the inductive stage, mg∙L−1 | 24.5–27.1 |
| Maximal carotenoid content at the inductive stage, % of dry biomass | 2.2–2.4 |
| Average productivity, mg∙L−1∙day−1 | 100–200 |
| Carotenoid yield, mg∙L−1∙day−1 | 3.6–3.8 |
| Rf | Pigment | Carotenoid Content (Mas.-% of Other Carotenoids) |
|---|---|---|
| 0.93 | β-carotene | 13.1 |
| 0.80 | Adonixanthin diesters | 2.4 |
| 0.59 | Echinenone | 1.2 |
| 0.56 | Astaxanthin diesters | 31.1 |
| 0.44 | Adonirubin esters | 8.0 |
| 0.38 | Canthaxanthin | 2.1 |
| 0.24 | Astaxanthin monoesters | 16.8 |
| 0.18 | Adonirubin monoesters | 20.5 |
| 0.15 | Free ketocarotenoids 1 | 1.4 |
| 0.04 | Primary xanthophylls 2 | 3.4 |
| FA, Mas.-% | Vegetative Stage | Inductive Stage |
|---|---|---|
| C12:0 | 0.1 | 0.1 |
| C14:0 | 2.4 | 1.5 |
| C14:1Δ7 | 0.2 | - |
| C16:0 | 24.8 | 26.4 |
| C16:1Δ7 | 3.7 | 2.4 |
| C16:1Δ9 | 1.6 | 1.9 |
| C16:2Δ7,10 | 7.3 | 2.1 |
| C16:3Δ7,10,13 | 0.9 | 1.9 |
| C16:4Δ4,7,10,13 | 1.2 | 1.9 |
| C18:0 | 4.8 | 2.0 |
| C18:1Δ9 | 17.0 | 18.8 |
| C18:1Δ11 | 5.0 | 4.6 |
| C18:2Δ9,12 | 20.4 | 18.2 |
| C18:3Δ6,9,12 | 0.3 | 0.2 |
| C18:3Δ9,12,15 | 8.5 | 17.3 |
| C18:4Δ6,9,12,15 | 0.4 | 0.4 |
| C20:0 | 0.7 | 0.2 |
| C20:1Δ11 | 0.2 | 0.1 |
| C22:0 | 0.6 | 0.2 |
| UI | 1.184 | 1.354 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chekanov, K.; Litvinov, D.; Fedorenko, T.; Chivkunova, O.; Lobakova, E. Combined Production of Astaxanthin and β-Carotene in a New Strain of the Microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) Cultivated in Photobioreactor. Biology 2021, 10, 643. https://doi.org/10.3390/biology10070643
Chekanov K, Litvinov D, Fedorenko T, Chivkunova O, Lobakova E. Combined Production of Astaxanthin and β-Carotene in a New Strain of the Microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) Cultivated in Photobioreactor. Biology. 2021; 10(7):643. https://doi.org/10.3390/biology10070643
Chicago/Turabian StyleChekanov, Konstantin, Daniil Litvinov, Tatiana Fedorenko, Olga Chivkunova, and Elena Lobakova. 2021. "Combined Production of Astaxanthin and β-Carotene in a New Strain of the Microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) Cultivated in Photobioreactor" Biology 10, no. 7: 643. https://doi.org/10.3390/biology10070643
APA StyleChekanov, K., Litvinov, D., Fedorenko, T., Chivkunova, O., & Lobakova, E. (2021). Combined Production of Astaxanthin and β-Carotene in a New Strain of the Microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) Cultivated in Photobioreactor. Biology, 10(7), 643. https://doi.org/10.3390/biology10070643







