Effect of Electrical Stimulation on Diabetic Human Skin Fibroblast Growth and the Secretion of Cytokines and Growth Factors Involved in Wound Healing
Abstract
Simple Summary
Abstract
1. Introduction
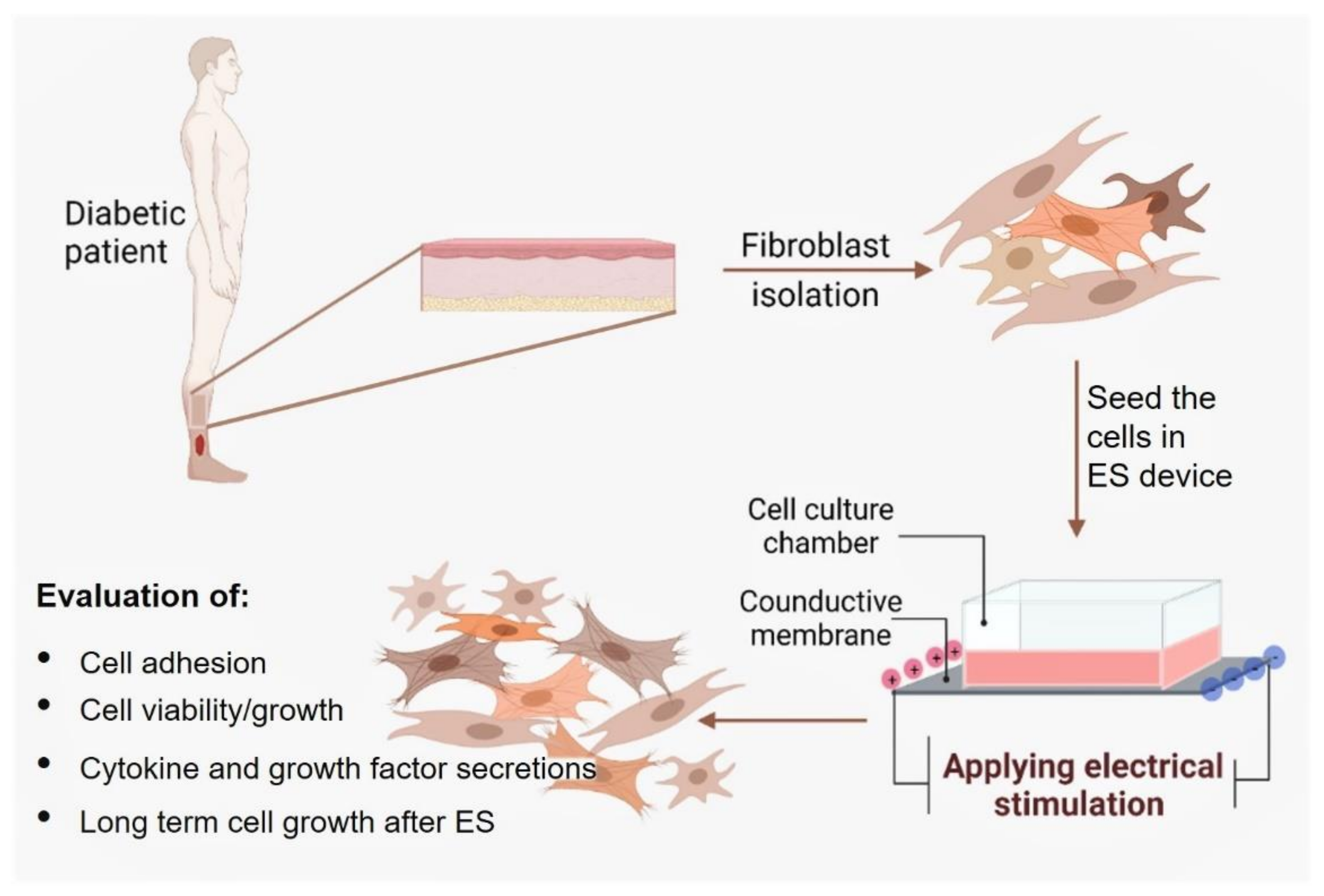
2. Materials and Methods
2.1. Preparation of Conductive PPy/HE/PLLA Membranes
2.2. Primary Diabetic Human Skin Fibroblast Culture
2.3. Biocompatibility of the Conductive Membranes
2.4. Safe ES Intensities for the DHSF
2.5. MTT Assay
2.6. LDH Activity
2.7. Cell Adhesion Following ES
2.8. Fibroblast Growth after Exposure to the Safe ES Regime
2.9. Cytokine Array and ELISA Assay
2.10. Cell Behaviors after Exposure to ES and Subculture
2.11. Statistical Analysis
3. Results
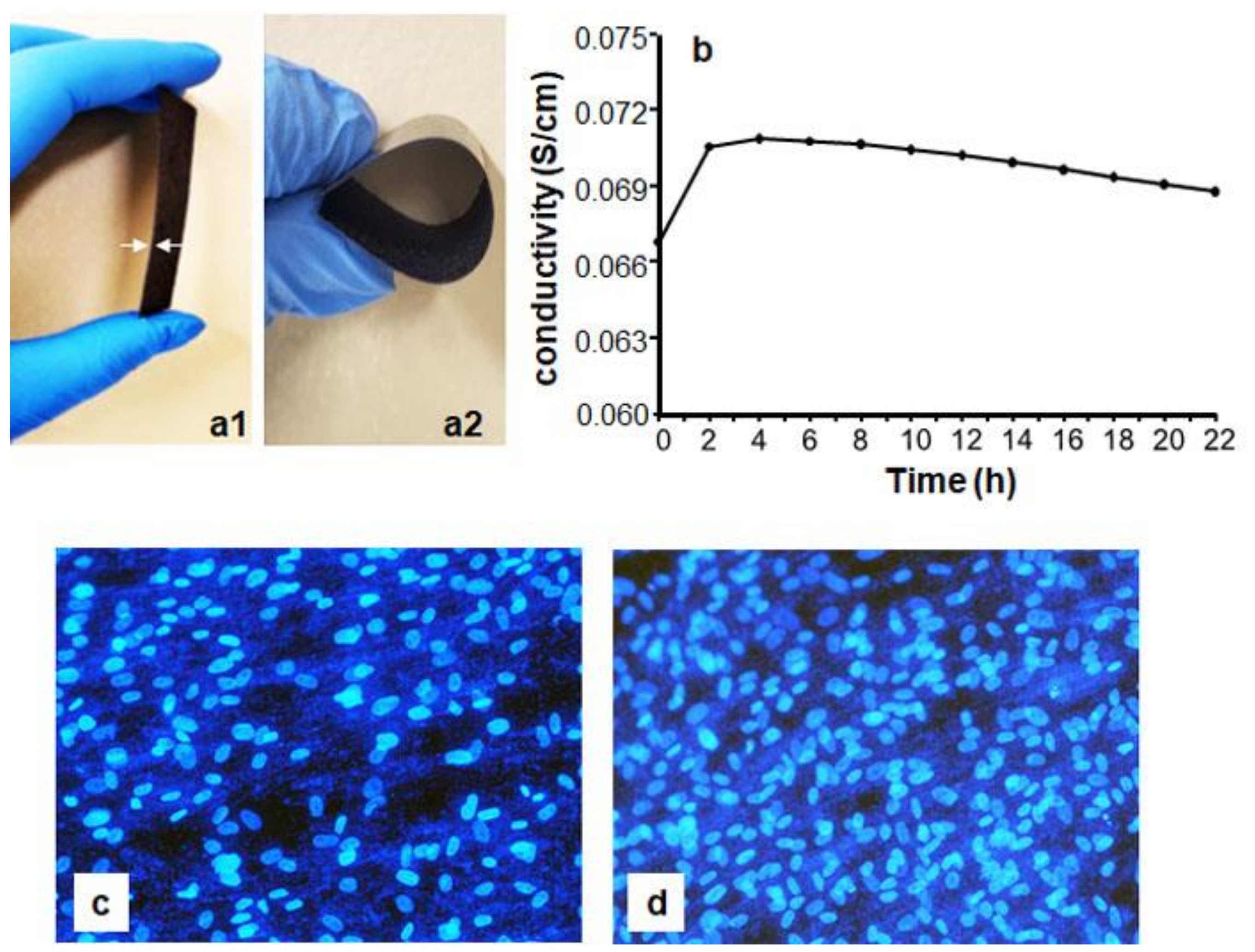
3.1. PPy/HE/PLLA Membrane Characteristics
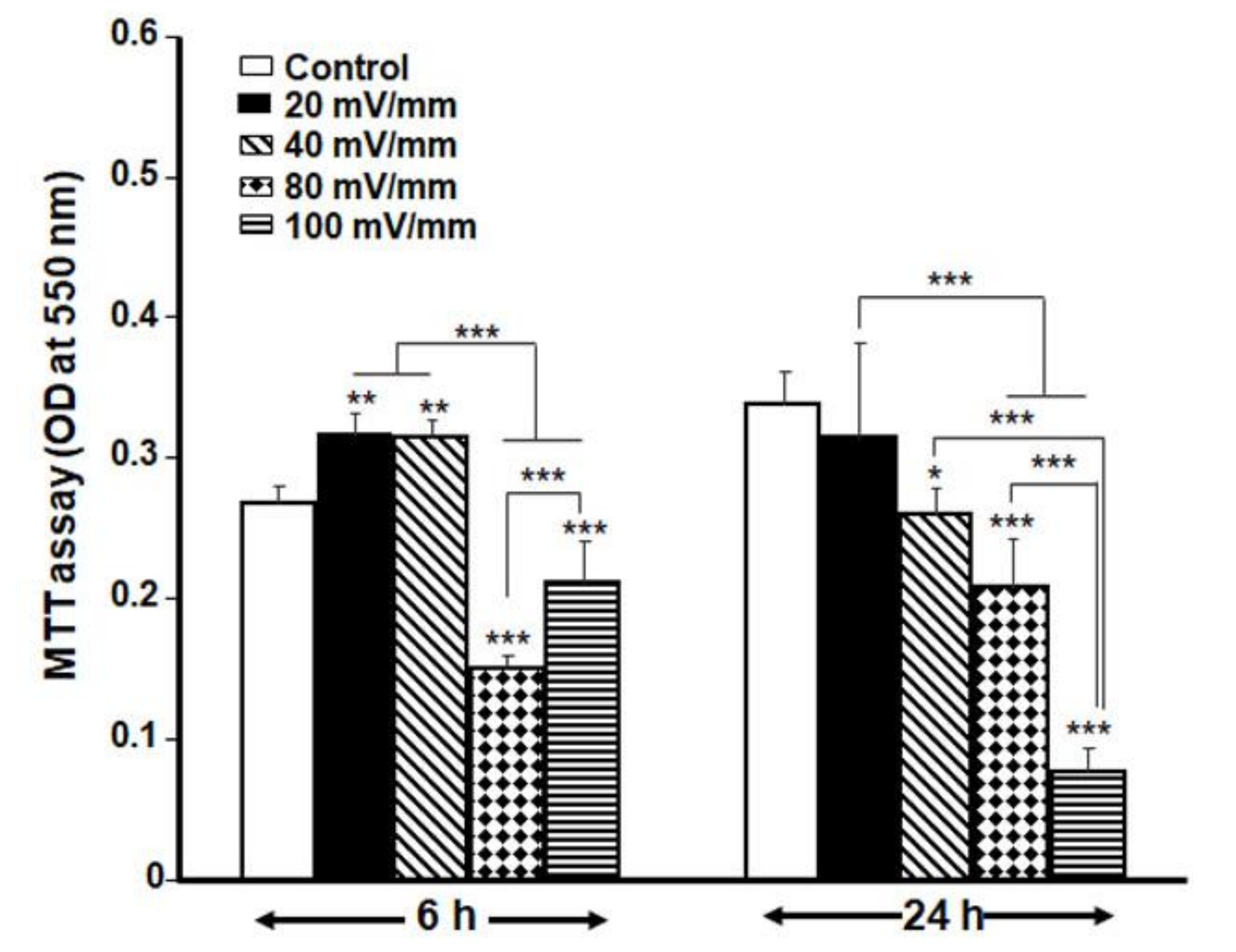
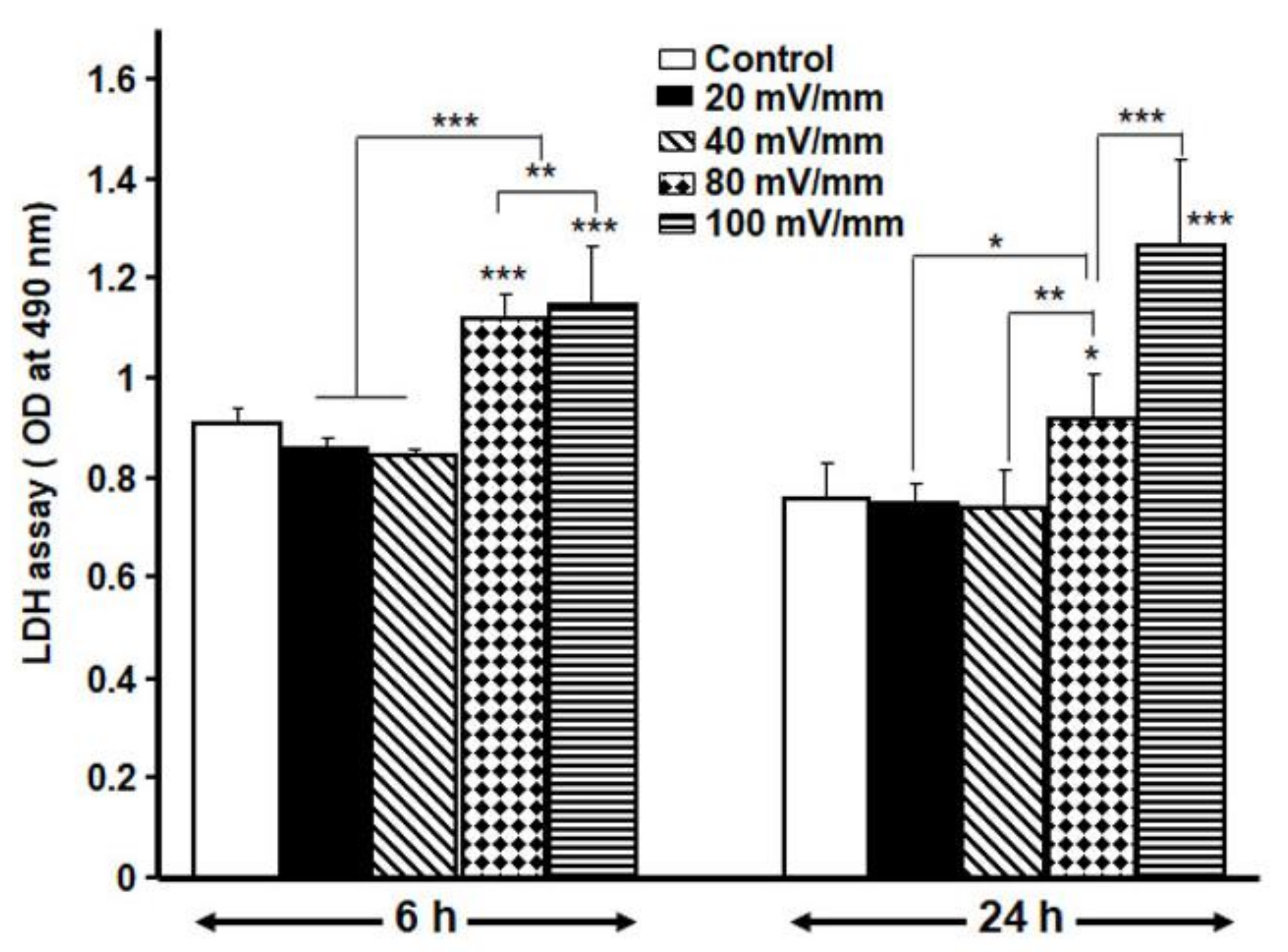
3.2. Optimal ES Parameters for the DHSF
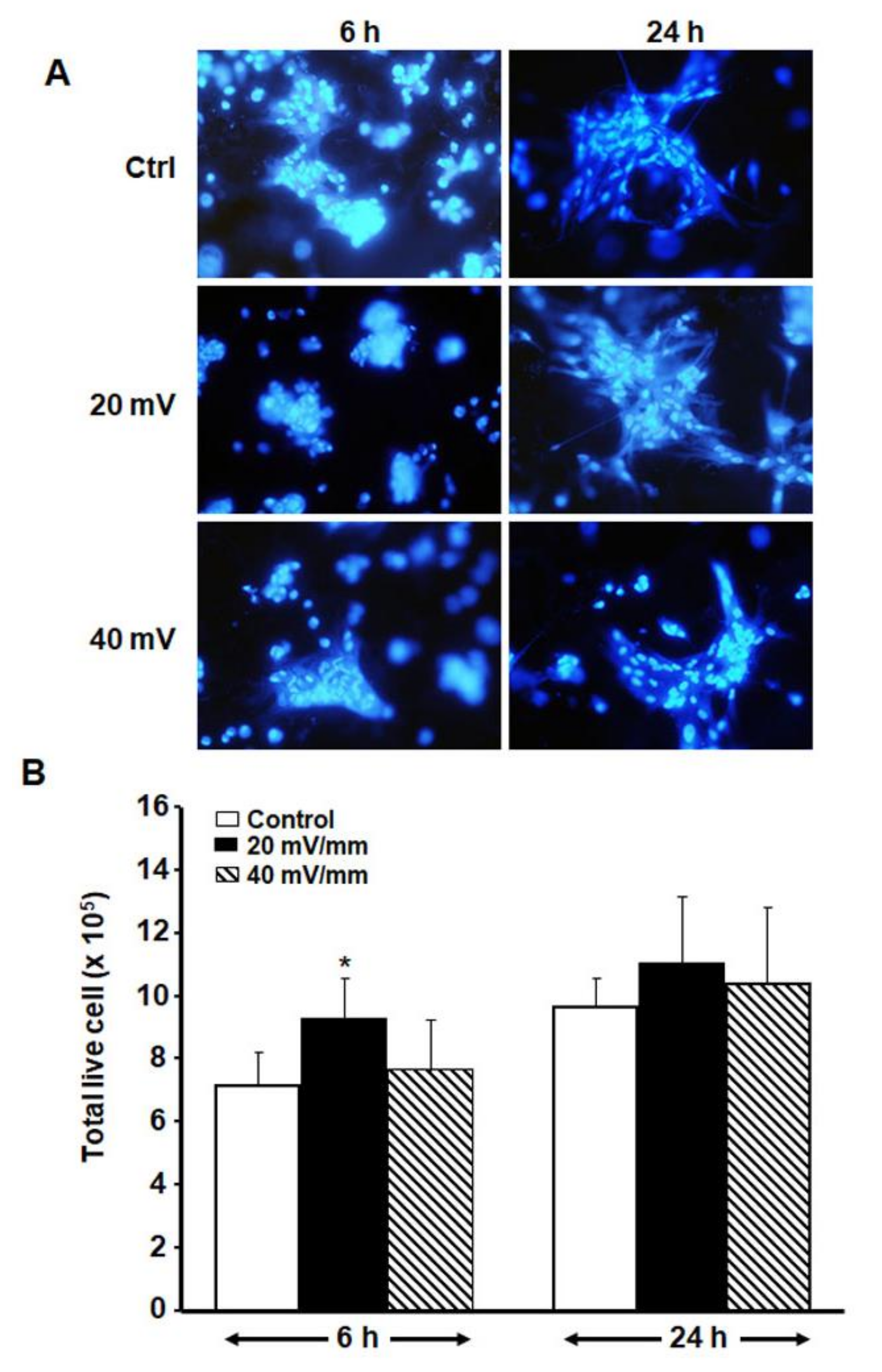
3.3. Low ES Intensities Promoted DHSF Adhesion, Viability and Growth
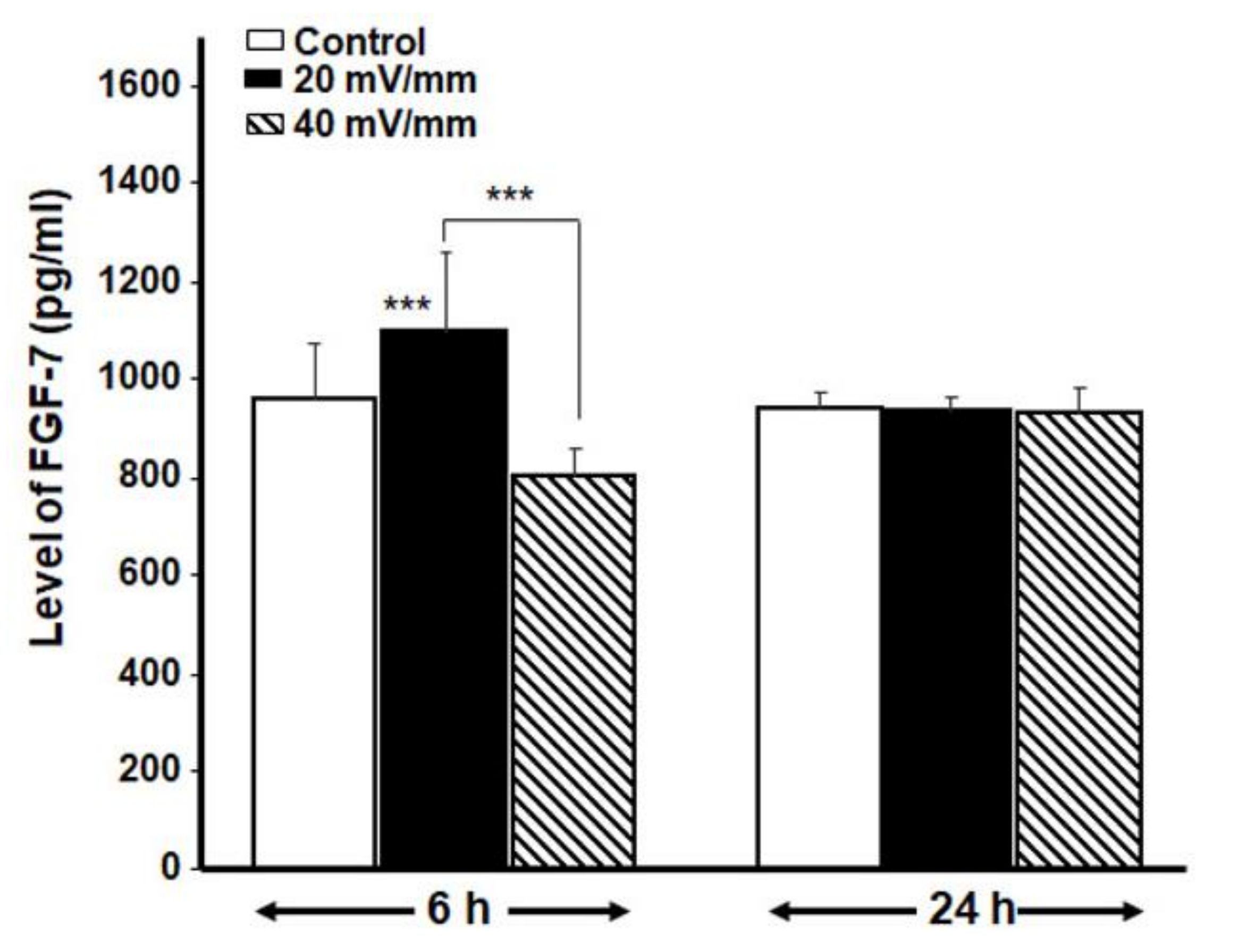
3.4. ES Promoted the Secretion of Various Cytokines and FGF-7 by the DHSF
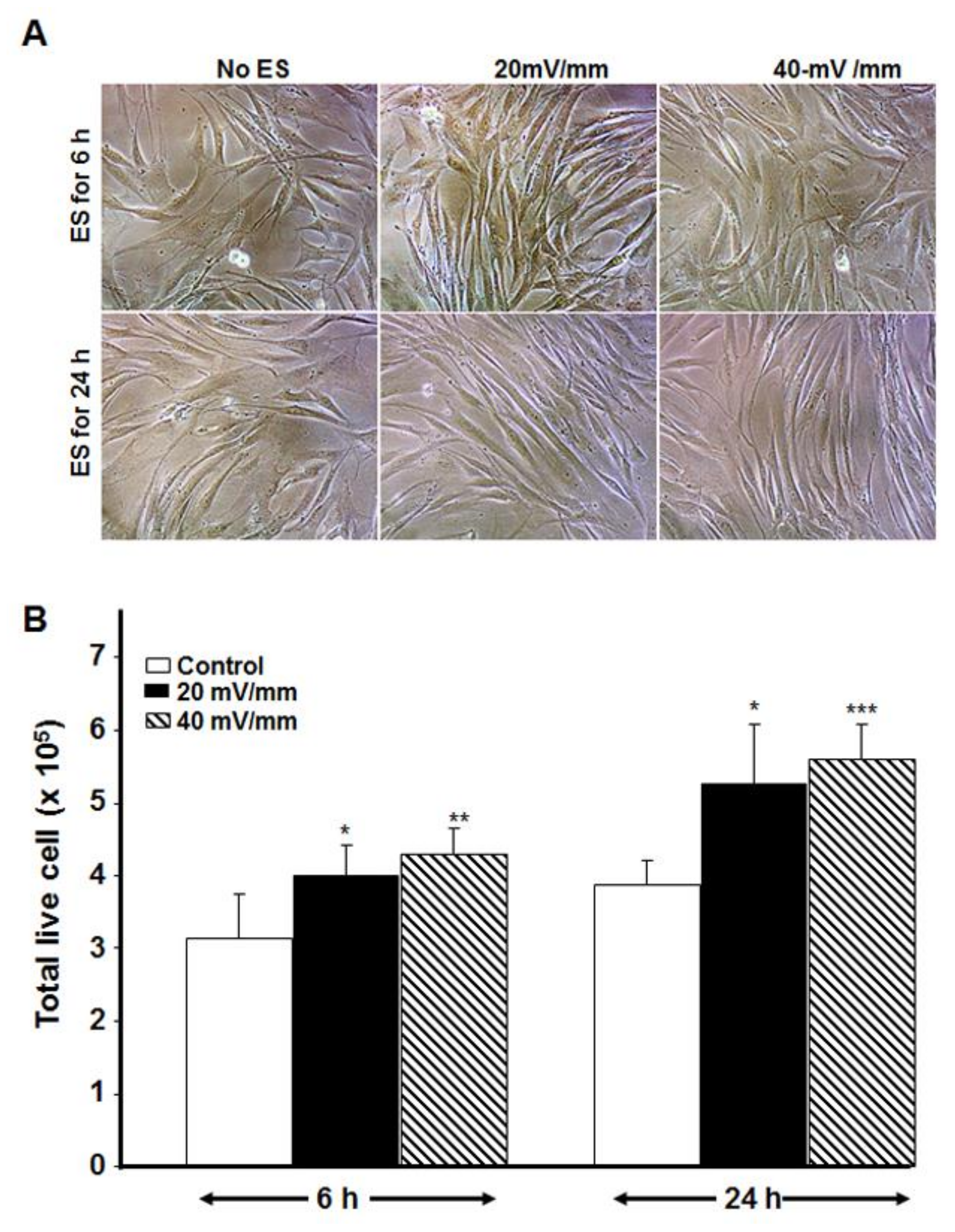
3.5. Electrically Stimulated DHSF Maintained Their Growth Capacity Even after Subculture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oryan, A.; Mohammadalipour, A.; Moshiri, A.; Tabandeh, M.R. Topical Application of Aloe vera Accelerated Wound Healing, Modeling, and Remodeling. Ann. Plast. Surg. 2016, 77, 37–46. [Google Scholar] [CrossRef]
- Fukuba, M.; Uozaki, H.; Komuro, Y. Effectiveness of the combination of fat grafting and injection on radiation ulcer healing. J. Plast. Surg. Hand Surg. 2019, 54, 24–28. [Google Scholar] [CrossRef]
- Schmidt, B.A.; Horsley, V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 2013, 140, 1517–1527. [Google Scholar] [CrossRef]
- Amin, Z.A.; Ali, H.M.; Alshawsh, M.A.; Darvish, P.H.; Abdulla, M.A. Application of Antrodia camphorata promotes rat’s wound healing in vivo and facilitates fibroblast cell proliferation in vitro. Evid. Based Complement. Altern. Med. 2015, 2015, 317693. [Google Scholar] [CrossRef]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef]
- Xie, C.; Shi, K.; Zhang, X.; Zhao, J.; Yu, J. MiR-1908 promotes scar formation post-burn wound healing by suppressing Ski-mediated inflammation and fibroblast proliferation. Cell Tissue Res. 2016, 366, 371–380. [Google Scholar] [CrossRef]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Khawandanah, J. Double or hybrid diabetes: A systematic review on disease prevalence, characteristics and risk factors. Nutr. Diabetes 2019, 9, 33. [Google Scholar] [CrossRef]
- World Health Organization. Facts and Figures about Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes#:~:text=Key%20facts,in%20premature%20mortality%20from%20diabetes (accessed on 2 June 2021).
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Kotha, V.S.; Fan, K.L.; Schwitzer, J.A.; Youn, R.; Black, C.K.; Attinger, C.E.; Evans, K.K. Amputation versus Free Flap: Long-Term Outcomes of Microsurgical Limb Salvage and Risk Factors for Amputation in the Diabetic Population. Plast. Reconstr. Surg. 2021, 147, 742–750. [Google Scholar] [CrossRef]
- Lotfollahi, Z.; Dawson, J.; Fitridge, R.; Bursill, C. The Anti-inflammatory and Proangiogenic Properties of High-Density Lipoproteins: An Emerging Role in Diabetic Wound Healing. Adv. Wound Care 2021, 10, 370–380. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Smith, P.C.; Martínez, C.; Martínez, J.; McCulloch, C.A. Role of Fibroblast Populations in Periodontal Wound Healing and Tissue Remodeling. Front. Physiol. 2019, 10, 270. [Google Scholar] [CrossRef]
- Wall, I.B.; Moseley, R.; Baird, D.; Kipling, D.; Giles, P.; Laffafian, I.; Price, P.E.; Thomas, D.; Stephens, P. Fibroblast Dysfunction Is a Key Factor in the Non-Healing of Chronic Venous Leg Ulcers. J. Investig. Dermatol. 2008, 128, 2526–2540. [Google Scholar] [CrossRef]
- Cook, H.; Stephens, P.; Davies, K.J.; Thomas, D.; Harding, K. Defective Extracellular Matrix Reorganization by Chronic Wound Fibroblasts is Associated with Alterations in TIMP-1, TIMP-2, and MMP-2 Activity. J. Investig. Dermatol. 2000, 115, 225–233. [Google Scholar] [CrossRef]
- Phillips, T.J.; Manzoor, J.; Rojas, A.; Isaacs, C.; Carson, P.; Sabolinski, M.; Young, J.; Falanga, V. The Longevity of a Bilayered Skin Substitute after Application to Venous Ulcers. Arch. Dermatol. 2002, 138, 1079–1081. [Google Scholar] [CrossRef]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical Stimulation Promotes Wound Healing by Enhancing Dermal Fibroblast Activity and Promoting Myofibroblast Transdifferentiation. PLoS ONE 2013, 8, e71660. [Google Scholar] [CrossRef]
- Urabe, H.; Akimoto, R.; Kamiya, S.; Hosoki, K.; Ichikawa, H.; Nishiyama, T. Effects of pulsed electrical stimulation on growth factor gene expression and proliferation in human dermal fibroblasts. Mol. Cell. Biochem. 2021, 476, 361–368. [Google Scholar] [CrossRef]
- Wang, Y.; Rouabhia, M.; Lavertu, D.; Zhang, Z. Pulsed electrical stimulation modulates fibroblasts’ behaviour through the Smad signalling pathway. J. Tissue Eng. Regen. Med. 2017, 11, 1110–1121. [Google Scholar] [CrossRef]
- Moravvej, H.; Memariani, H.; Memariani, M.; Kabir-Salmani, M.; Shoae-Hassani, A.; Abdollahimajd, F. Evaluation of Fibroblast Viability Seeded on Acellular Human Amniotic Membrane. BioMed Res. Int. 2021, 2021, 5597758. [Google Scholar] [CrossRef]
- Gkotsoulias, E. Split Thickness Skin Graft of the Foot and Ankle Bolstered with Negative Pressure Wound Therapy in a Diabetic Population: The Results of a Retrospective Review and Review of the Literature. Foot Ankle Spec. 2019, 13, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Mostow, E.N.; Haraway, G.D.; Dalsing, M.; Hodde, J.P.; King, D. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: A randomized clinical trial. J. Vasc. Surg. 2005, 41, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Marston, W.A.; Hanft, J.; Norwood, P.; Pollak, R. The Efficacy and Safety of Dermagraft in Improving the Healing of Chronic Diabetic Foot Ulcers: Results of a prospective randomized trial. Diabetes Care 2003, 26, 1701–1705. [Google Scholar] [CrossRef]
- Sinkin, J.C.; Reilly, M.; Cralley, A.; Kim, P.J.; Steinberg, J.S.; Cooper, P.; Evans, K.; Attinger, C.E. Multidisciplinary Approach to Soft-Tissue Reconstruction of the Diabetic Charcot Foot. Plast. Reconstr. Surg. 2015, 135, 611–616. [Google Scholar] [CrossRef]
- Tzeng, Y.-S.; Deng, S.-C.; Wang, C.-H.; Tsai, J.-C.; Chen, T.-M.; Burnouf, T. Treatment of Nonhealing Diabetic Lower Extremity Ulcers with Skin Graft and Autologous Platelet Gel: A Case Series. BioMed Res. Int. 2013, 2013, 837620. [Google Scholar] [CrossRef]
- Zhang, H.; Wallace, G.; Higgins, M.J. Effect of monophasic pulsed stimulation on live single cell de-adhesion on conducting polymers with adsorbed fibronectin as revealed by single cell force spectroscopy. Biointerphases 2019, 14, 021003. [Google Scholar] [CrossRef]
- Rahbar Layegh, E.; Fadaei Fathabadi, F.; Lotfinia, M.; Zare, F.; Mohammadi Tofigh, A.; Abrishami, S.; Piryaei, A. Photobiomodulation therapy improves the growth factor and cytokine secretory profile in human type 2 diabetic fibroblasts. J. Photochem. Photobiol. B 2020, 210, 111962. [Google Scholar] [CrossRef]
- Marchese, C.; Messina, A.; Faggioni, A.; Torrisi, M.R.; Frati, L.; Ron, D.; Rubin, J.; Aaronson, S.A. Human keratinocyte growth factor activity on proliferation and differentiation of human keratinocytes: Differentiation response distinguishes KGF from EGF family. J. Cell. Physiol. 1990, 144, 326–332. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Cardinali, G.; Aspite, N.; Picardo, M.; Marchese, C.; Torrisi, M.R.; Mancini, P. Cortactin involvement in the keratinocyte growth factor and fibroblast growth factor 10 promotion of migration and cortical actin assembly in human keratinocytes. Exp. Cell Res. 2007, 313, 1758–1777. [Google Scholar] [CrossRef]
- Finch, P.W.; Rubin, J.S.; Miki, T.; Ron, D.; Aaronson, S.A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science 1989, 245, 752–755. [Google Scholar] [CrossRef]
- Marchese, C.; Chedid, M.; Dirsch, O.R.; Csaky, K.G.; Santanelli, F.; Latini, C.; LaRochelle, W.J.; Torrisi, M.R.; Aaronson, S.A. Modulation of keratinocyte growth factor and its receptor in reepithelializing human skin. J. Exp. Med. 1995, 182, 1369–1376. [Google Scholar] [CrossRef]
- Ko, K.I.; Sculean, A.; Graves, D.T. Diabetic wound healing in soft and hard oral tissues. Transl. Res. 2021. [Google Scholar] [CrossRef]
- Meng, W.; Veluchamy, A.; Hébert, H.; Campbell, A.; Colhoun, H.; Palmer, C. A genome-wide association study suggests that MAPK14 is associated with diabetic foot ulcers. Br. J. Dermatol. 2017, 177, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Zhao, M.; Forrester, J.V.; McCaig, C.D. Electric Fields and MAP Kinase Signaling Can Regulate Early Wound Healing in Lens Epithelium. Investig. Opthalmol. Vis. Sci. 2003, 44, 244–249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, C.; Jacobson, K.; Schaller, M.D. MAP kinases and cell migration. J. Cell Sci. 2004, 117, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-R.; Nam, B.; Han, A.-R.; Kim, J.-B.; Jin, C.H. Isoegomaketone from Perilla frutescens (L.) Britt Stimulates MAPK/ERK Pathway in Human Keratinocyte to Promote Skin Wound Healing. Evid. Based Complement. Altern. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kowtharapu, B.S.; Murín, R.; Jünemann, A.G.M.; Stachs, O. Role of Corneal Stromal Cells on Epithelial Cell Function during Wound Healing. Int. J. Mol. Sci. 2018, 19, 464. [Google Scholar] [CrossRef]
| 6 h | 24 h | ||||||
|---|---|---|---|---|---|---|---|
| Control | 20 mV/mm | 40 mV/mm | Control | 20 mV/mm | 40 mV/mm | ||
| Cytokine levels (pg/mL) measured immediately after stopping ES | |||||||
| GM-CSF | Con. | 10.8 ± 1.14 | 16.32 ± 0.59 | 30.4 ± 2.84 | 162.0 ± 16.69 | 118.7 ± 6.47 | 131.1 ± 7.01 |
| PV | <0.01 | <0.001 | <0.001 | <0.01 | |||
| IL-1β | Con. | 3.7 ± 0.27 | 4.3 ± 0.21 | 4.6 ± 0.46 | 5.0 ± 0.25 | 5.0 ± 0.35 | 4.4 ± 0.12 |
| PV | Ns | <0.01 | Ns | <0.05 | |||
| IL-6 | Con. | 892.4 ± 109.9 | 1187.4 ± 125.4 | 1245.8 ± 171.9 | 1285.0 ± 54.6 | 1459.9 ± 299.6 | 2189.2 ± 911.5 |
| PV | <0.05 | <0.01 | Ns | Ns | |||
| IL-8 | Con. | 2105 ± 175 | 3894 ± 263 | 5791 ± 853 | 8667 ± 2270 | 9167 ± 376 | 8253 ± 1069 |
| PV | <0.05 | <0.01 | Ns | Ns | |||
| IL-10 | Con. | OOR | OOR | OOR | 0.21 ± 0.14 | 0.38 ± 0.06 | 0.11 ± 0.04 |
| PV | - | - | Ns | Ns | |||
| Cytokine levels (pg/mL) measured 48 h post-exposure to ES | |||||||
| GM-CSF | Con. | 197.2 ± 17.5 | 225.6 ± 14.28 | 309.4 ± 10.62 | 220.4 ± 27.4 | 182.6 ± 3.76 | 240.0 ± 13.6 |
| PV | <0.05 | <0.001 | <0.05 | Ns | |||
| IL-1β | Con. | 4.1 ± 0.3 | 4.4 ± 0.2 | 4.7 ± 0.5 | 4.0 ± 0.3 | 4.6 ± 0.3 | 4.5 ± 0.2 |
| PV | Ns | Ns | <0.01 | <0.05 | |||
| IL-6 | Con. | 2187.6 ± 633 | 2083.4 ± 690 | 1414.2 ± 249 | 1447.7 ± 434 | 1976.3 ± 293 | 1555.7 ± 110 |
| PV | Ns | Ns | <0.05 | Ns | |||
| IL-8 | Con. | 17168.7 ± 6959.7 | 8712.4 ± 1213 | 8808.7 ± 1080 | 8580.8 ± 537 | 9391.1 ± 74 | 9252.1 ± 1373 |
| PV | <0.05 | <0.05 | Ns | Ns | |||
| IL-10 | Con. | 0.8 ± 0.17 | 0.6 ± 0.08 | 0.3 ± 0.07 | 1.0 ± 0.27 | 1.4 ± 0.16 | 0.9 ± 0.17 |
| PV | <0.05 | <0.001 | <0.05 | Ns | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedin-Do, A.; Zhang, Z.; Douville, Y.; Méthot, M.; Rouabhia, M. Effect of Electrical Stimulation on Diabetic Human Skin Fibroblast Growth and the Secretion of Cytokines and Growth Factors Involved in Wound Healing. Biology 2021, 10, 641. https://doi.org/10.3390/biology10070641
Abedin-Do A, Zhang Z, Douville Y, Méthot M, Rouabhia M. Effect of Electrical Stimulation on Diabetic Human Skin Fibroblast Growth and the Secretion of Cytokines and Growth Factors Involved in Wound Healing. Biology. 2021; 10(7):641. https://doi.org/10.3390/biology10070641
Chicago/Turabian StyleAbedin-Do, Atieh, Ze Zhang, Yvan Douville, Mireille Méthot, and Mahmoud Rouabhia. 2021. "Effect of Electrical Stimulation on Diabetic Human Skin Fibroblast Growth and the Secretion of Cytokines and Growth Factors Involved in Wound Healing" Biology 10, no. 7: 641. https://doi.org/10.3390/biology10070641
APA StyleAbedin-Do, A., Zhang, Z., Douville, Y., Méthot, M., & Rouabhia, M. (2021). Effect of Electrical Stimulation on Diabetic Human Skin Fibroblast Growth and the Secretion of Cytokines and Growth Factors Involved in Wound Healing. Biology, 10(7), 641. https://doi.org/10.3390/biology10070641







