Vascular Calcification in Rodent Models—Keeping Track with an Extented Method Assortment
Abstract
Simple Summary
Abstract
1. Introduction
2. Rodent Models for Induction of Vascular Calcification
| Method of Induction | Model | Aspects of Model/ Indication for Animal Burden |
|---|---|---|
| Naturally occurring | DBA2 [8] |
|
| CY+ rat with autosomal dominant PKD [9,10,11] |
| |
| LPK disease rat [12,13] |
| |
| Operation | Kidney reduction (electrocautery, nephrectomy) [14,15] |
|
| Feeding/ Substance application | Adenine [16,17,18,19,20,21,22,23,24,25,26] |
|
| Vitamin D [27,28,29,30,31,32,33,34] |
| |
| Phosphate [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] |
| |
| Streptozotocin [42] |
| |
| Cholesterol Rich Chow [43] |
| |
| PCSK9-AAV [44,45] |
| |
| Genetic modification | Klotho−/− [46,47] |
|
| Fgf-23−/− [48,49] |
| |
| Galnt−/− [50] |
| |
| Tcal/Tcal [51] |
| |
| Abcc6−/− [52,53] |
| |
| Enpp1 (Enpp−/−, Enppttw/ttw, Enpp1asj/asj) [54,55,56,57,58] |
| |
| Lmna [59,60,61,62,63] |
| |
| Fetuin-A−/− [64,65] |
| |
| Opg−/− [66] |
| |
| Mgp−/− [67] |
| |
| Opn−/− [68,69] |
| |
| Madh6−/− [70] |
| |
| ApoE−/− [71,72] |
| |
| Ldlr−/− [73,74] |
| |
| ApoE3 Leiden [75,76] |
|
3. Methods for the Detection of Vascular Calcification in Rodents
3.1. Biochemical Markers for Calcification
3.1.1. Blood Biomarkers of Vascular Calcification
3.1.2. Resident Biomarkers of Vascular Calcification Localized in Tissue
3.2. Functional Markers of Vessel Stiffness
3.2.1. Pulse Wave Velocity
3.2.2. Pulse Pressure
3.2.3. Wire Myography
3.3. Quantification and Imaging of Calcification
3.3.1. Biochemical
3.3.2. Histological Staining for Calcium Deposits
Von Kossa Staining
Alizarin Red S Staining
Fluorescent Staining
Near-Infrared Fluorescence Tracer (NIRF)
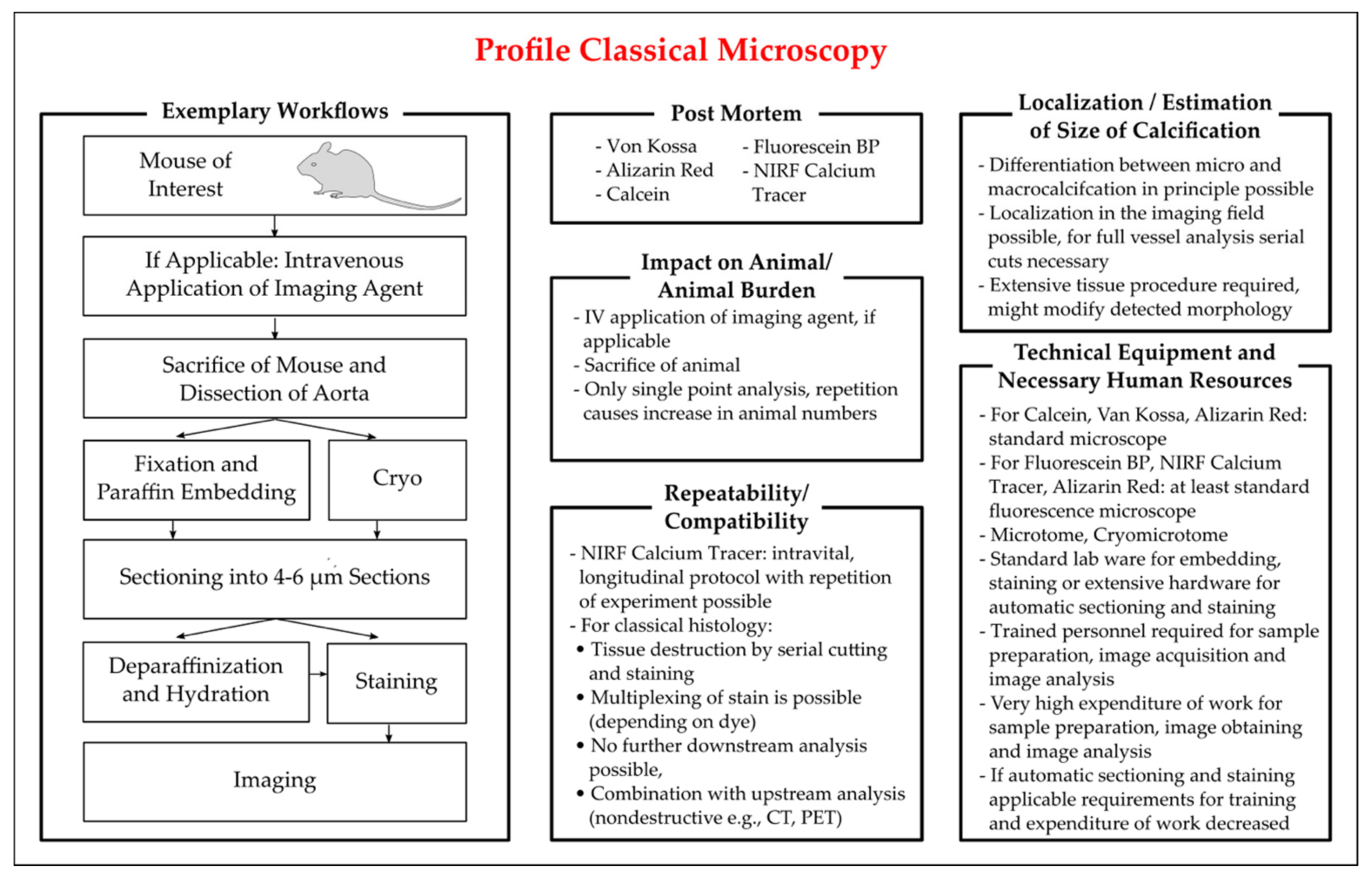
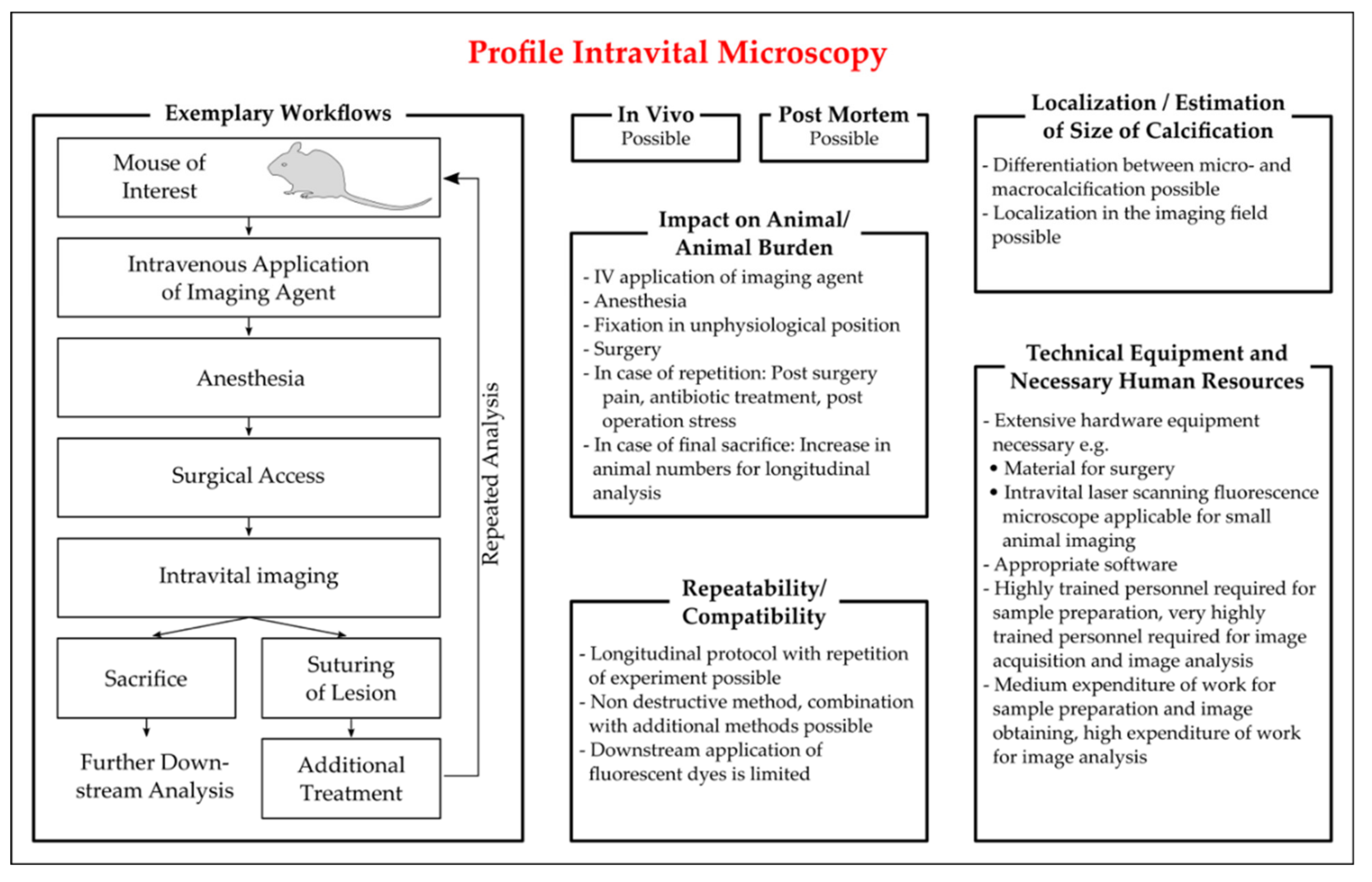
Intravital Microscopy
3.3.3. Magnetic Resonance Imaging
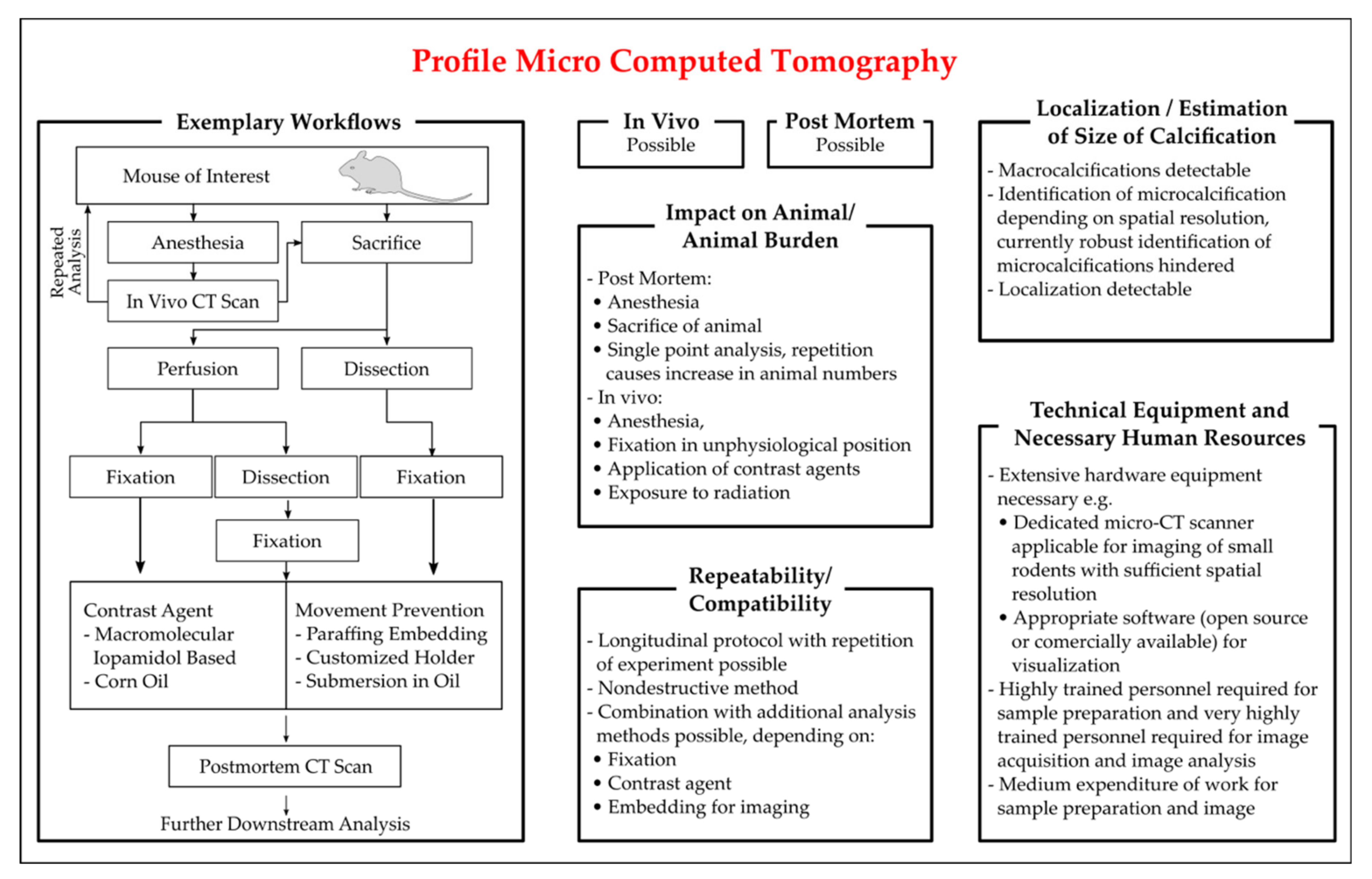
3.3.4. X-ray
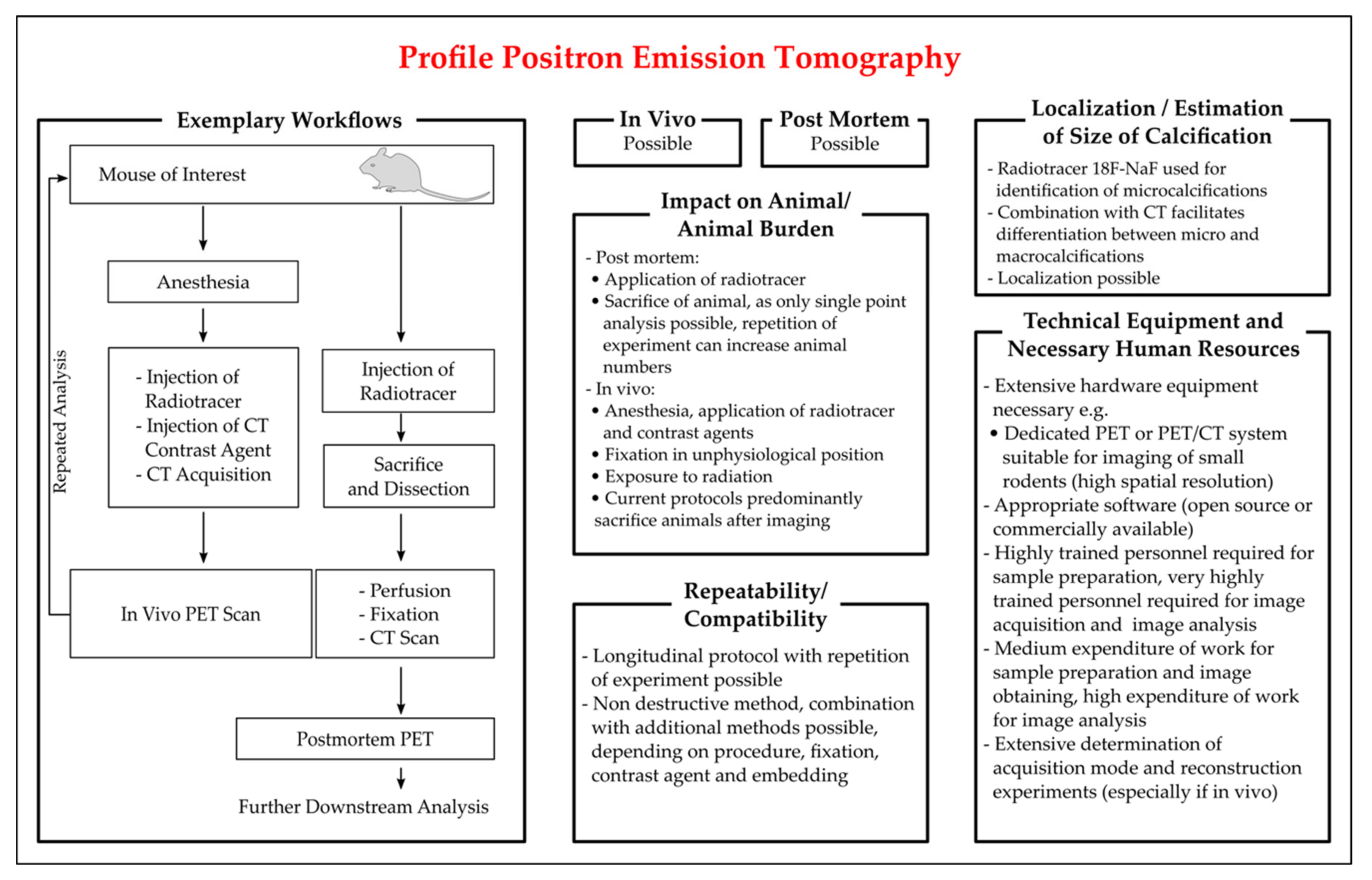
3.3.5. Positron Emission Tomography
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abcc6 | ATP binding cassette subfamily C member 6 |
| ABI | Ankle-brachial index |
| Alp | Alkaline phosphatase |
| α-SMA | Alpha smooth muscle actin |
| ApoE | Apolipoprotein E |
| Bmp-2 | Bone morphogenetic protein 2 |
| Cbfa1 | Core binding factor alpha 1 |
| CKD | Chronic kidney disease |
| CT | Computed tomography |
| Enpp1 | Ectonucleotide pyrophosphatase phosphodiesterase |
| Fgf-23 | Fibroblast growth factor 23 |
| Galnt | GalNAc transferase |
| IVM | Intravital microscopy |
| Ldlr | Low density lipoprotein receptor |
| Lmna | Gene encoding the Lamin A/C protein |
| LPK | Lewis polycystic kidney |
| Madh6 | Mothers against decapentaplegic homolog 6 |
| MAP | Mean arterial pressure |
| Mgp | Matrix Gla protein |
| MRI | Magnetic resonance imaging |
| Msx | Msh homebox |
| NIR | Near infrared |
| NIRF | Near infrared fluorescence |
| Opg | Osteoprotegerin |
| Opn | Osteopontin |
| PCSK9-AAV | Proprotein convertase subtilisin/kexin type 9 adeno-associated virus vector |
| PET | Positron emission tomography |
| PKD | Polycystic kidney disease |
| PP | Pulse pressure |
| PWV | Pulse wave velocity |
| Runx2 | Runt-related transcription factor 2 |
| Sm22 | Smooth muscle-specific 22-kDa protein |
| Tcal | Tumoral calcinosis |
| TPM | Two photon microscopy |
References
- Tolle, M.; Reshetnik, A.; Schuchardt, M.; Hohne, M.; van der Giet, M. Arteriosclerosis and vascular calcification: Causes, clinical assessment and therapy. Eur. J. Clin. Investig. 2015, 45, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Babic, M.; Tolle, M.; van der Giet, M.; Schuchardt, M. Research Models for Studying Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 2204. [Google Scholar] [CrossRef]
- Voelkl, J.; Lang, F.; Eckardt, K.U.; Amann, K.; Kuro, O.M.; Pasch, A.; Pieske, B.; Alesutan, I. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol. Life Sci. 2019, 76, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, Y.; Rutsch, F. Modulators of networks: Molecular targets of arterial calcification identified in man and mice. Curr. Pharm. Des. 2014, 20, 5839–5852. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Bruch, R.L. The Principles of Human Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Ross, R.; Glomset, J.A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science 1973, 180, 1332–1339. [Google Scholar] [CrossRef]
- Schuchardt, M.; Siegel, N.V.; Babic, M.; Reshetnik, A.; Lutzenberg, R.; Zidek, W.; van der Giet, M.; Tolle, M. A Novel Long-Term ex vivo Model for Studying Vascular Calcification Pathogenesis: The Rat Isolated-Perfused Aorta. J. Vasc. Res. 2020, 57, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Rings, R.W.; Wagner, J.E. Incidence of cardiac and other soft tissue mineralized lesions in DNA-2 mice. Lab. Anim. Sci. 1972, 22, 344–352. [Google Scholar]
- Moe, S.M.; Chen, N.X.; Seifert, M.F.; Sinders, R.M.; Duan, D.; Chen, X.; Liang, Y.; Radcliff, J.S.; White, K.E.; Gattone, V.H., 2nd. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009, 75, 176–184. [Google Scholar] [CrossRef]
- Kaspareit-Rittinghausen, J.; Rapp, K.; Deerberg, F.; Wcislo, A.; Messow, C. Hereditary polycystic kidney disease associated with osteorenal syndrome in rats. Vet. Pathol. 1989, 26, 195–201. [Google Scholar] [CrossRef]
- Nagao, S.; Morita, M.; Kugita, M.; Yoshihara, D.; Yamaguchi, T.; Kurahashi, H.; Calvet, J.P.; Wallace, D.P. Polycystic kidney disease in Han:SPRD Cy rats is associated with elevated expression and mislocalization of SamCystin. Am. J. Physiol. Ren. Physiol. 2010, 299, F1078–F1086. [Google Scholar] [CrossRef][Green Version]
- Ng, K.; Hildreth, C.M.; Phillips, J.K.; Avolio, A.P. Aortic stiffness is associated with vascular calcification and remodeling in a chronic kidney disease rat model. Am. J. Physiol. Ren. Physiol. 2011, 300, F1431–F1436. [Google Scholar] [CrossRef]
- Phillips, J.K.; Hopwood, D.; Loxley, R.A.; Ghatora, K.; Coombes, J.D.; Tan, Y.S.; Harrison, J.L.; McKitrick, D.J.; Holobotvskyy, V.; Arnolda, L.F.; et al. Temporal relationship between renal cyst development, hypertension and cardiac hypertrophy in a new rat model of autosomal recessive polycystic kidney disease. Kidney Blood Press. Res. 2007, 30, 129–144. [Google Scholar] [CrossRef]
- Chauntin, A.; Ferris, E. Experimental renal insufficiency produced by partial nephrectomy. Arch. Intern. Med. 1932, 49, 767–787. [Google Scholar] [CrossRef]
- Gagnon, R.F.; Duguid, W.P. A reproducible model for chronic renal failure in the mouse. Urol. Res. 1983, 11, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.O.; Ferreira, J.C.; Cavallari, R.T.; Neves, K.R.; dos Reis, L.M.; Dominguez, W.V.; Oliveira, E.C.; Graciolli, F.G.; Passlick-Deetjen, J.; Jorgetti, V.; et al. Mineral bone disorder in chronic kidney disease: Head-to-head comparison of the 5/6 nephrectomy and adenine models. BMC Nephrol. 2014, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Oura, H.; Okada, T. Metabolic effects of dietary purine in rats. J. Nutr. Sci. Vitaminol. 1982, 28, 519–526. [Google Scholar] [CrossRef]
- Matsui, I.; Hamano, T.; Mikami, S.; Fujii, N.; Takabatake, Y.; Nagasawa, Y.; Kawada, N.; Ito, T.; Rakugi, H.; Imai, E.; et al. Fully phosphorylated fetuin-A forms a mineral complex in the serum of rats with adenine-induced renal failure. Kidney Int. 2009, 75, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Shobeiri, N.; Pang, J.; Adams, M.A.; Holden, R.M. Cardiovascular disease in an adenine-induced model of chronic kidney disease: The temporal link between vascular calcification and haemodynamic consequences. J. Hypertens. 2013, 31, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, K.; Kusano, K.; Hirata, M.; Tsunemi, K.; Nagano, N.; Burke, S.K.; Fukushima, N. Sevelamer hydrochloride prevents ectopic calcification and renal osteodystrophy in chronic renal failure rats. Kidney Int. 2003, 64, 441–450. [Google Scholar] [CrossRef]
- Price, P.A.; Roublick, A.M.; Williamson, M.K. Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int. 2006, 70, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Neven, E.; Dauwe, S.; De Broe, M.E.; D’Haese, P.C.; Persy, V. Endochondral bone formation is involved in media calcification in rats and in men. Kidney Int. 2007, 72, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Henley, C.; Davis, J.; Miller, G.; Shatzen, E.; Cattley, R.; Li, X.; Martin, D.; Yao, W.; Lane, N.; Shalhoub, V. The calcimimetic AMG 641 abrogates parathyroid hyperplasia, bone and vascular calcification abnormalities in uremic rats. Eur. J. Pharmacol. 2009, 616, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Neven, E.; Dams, G.; Postnov, A.; Chen, B.; De Clerck, N.; De Broe, M.E.; D’Haese, P.C.; Persy, V. Adequate phosphate binding with lanthanum carbonate attenuates arterial calcification in chronic renal failure rats. Nephrol. Dial. Transplant. 2009, 24, 1790–1799. [Google Scholar] [CrossRef][Green Version]
- Lomashvili, K.A.; Monier-Faugere, M.C.; Wang, X.; Malluche, H.H.; O’Neill, W.C. Effect of bisphosphonates on vascular calcification and bone metabolism in experimental renal failure. Kidney Int. 2009, 75, 617–625. [Google Scholar] [CrossRef]
- Persy, V.; Postnov, A.; Neven, E.; Dams, G.; De Broe, M.; D’Haese, P.; De Clerck, N. High-resolution X-ray microtomography is a sensitive method to detect vascular calcification in living rats with chronic renal failure. Arter. Thromb. Vasc. Biol. 2006, 26, 2110–2116. [Google Scholar] [CrossRef]
- Ejerblad, S.; Eriksson, I.; Johansson, H. Uraemic arterial disease. An experimental study with special reference to the effect of parathyroidectomy. Scand. J. Urol. Nephrol. 1979, 13, 161–169. [Google Scholar] [CrossRef]
- Krog, M.; Ejerblad, S.; Eriksson, I.; Johansson, H. Arterial calcifications in uraemic rats treated with 1-alpha-hydroxycholecalciferol and parathyroidectomy. Scand. J. Urol. Nephrol. 1984, 18, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Katsumata, K.; Endo, K.; Fukushima, N.; Ohkawa, H.; Fukagawa, M. In subtotally nephrectomized rats 22-oxacalcitriol suppresses parathyroid hormone with less risk of cardiovascular calcification or deterioration of residual renal function than 1,25(OH)2 vitamin D3. Nephrol Dial. Transplant. 2003, 18, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Henley, C.; Colloton, M.; Cattley, R.C.; Shatzen, E.; Towler, D.A.; Lacey, D.; Martin, D. 1,25-Dihydroxyvitamin D3 but not cinacalcet HCl (Sensipar/Mimpara) treatment mediates aortic calcification in a rat model of secondary hyperparathyroidism. Nephrol. Dial. Transplant. 2005, 20, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Lopez, I.; Aguilera-Tejero, E.; Mendoza, F.J.; Almaden, Y.; Perez, J.; Martin, D.; Rodriguez, M. Calcimimetic R-568 decreases extraosseous calcifications in uremic rats treated with calcitriol. J. Am. Soc. Nephrol. 2006, 17, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Wu-Wong, J.R.; Noonan, W.; Ma, J.; Dixon, D.; Nakane, M.; Bolin, A.L.; Koch, K.A.; Postl, S.; Morgan, S.J.; Reinhart, G.A. Role of phosphorus and vitamin D analogs in the pathogenesis of vascular calcification. J. Pharmacol. Exp. Ther. 2006, 318, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Cardus, A.; Panizo, S.; Parisi, E.; Fernandez, E.; Valdivielso, J.M. Differential effects of vitamin D analogs on vascular calcification. J. Bone Min. Res. 2007, 22, 860–866. [Google Scholar] [CrossRef]
- Lopez, I.; Mendoza, F.J.; Aguilera-Tejero, E.; Perez, J.; Guerrero, F.; Martin, D.; Rodriguez, M. The effect of calcitriol, paricalcitol, and a calcimimetic on extraosseous calcifications in uremic rats. Kidney Int. 2008, 73, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Suzuki, Y.; Matsushita, M.; Fujii, H.; Miyaura, C.; Aizawa, S.; Kogo, H. Prevention of aortic calcification by etidronate in the renal failure rat model. Eur. J. Pharmacol. 2007, 558, 159–166. [Google Scholar] [CrossRef]
- Mendoza, F.J.; Lopez, I.; Montes de Oca, A.; Perez, J.; Rodriguez, M.; Aguilera-Tejero, E. Metabolic acidosis inhibits soft tissue calcification in uremic rats. Kidney Int. 2008, 73, 407–414. [Google Scholar] [CrossRef]
- Haut, L.L.; Alfrey, A.C.; Guggenheim, S.; Buddington, B.; Schrier, N. Renal toxicity of phosphate in rats. Kidney Int. 1980, 17, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Staniforth, M.E.; Liapis, H.; Finch, J.; Burke, S.K.; Dusso, A.S.; Slatopolsky, E. Sevelamer hydrochloride attenuates kidney and cardiovascular calcifications in long-term experimental uremia. Kidney Int. 2003, 64, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, M.; Ogata, H.; Hatamura, I.; Koiwa, F.; Saji, F.; Shiizaki, K.; Negi, S.; Kinugasa, E.; Ooshima, A.; Koshikawa, S.; et al. Up-regulation of Cbfa1 and Pit-1 in calcified artery of uraemic rats with severe hyperphosphataemia and secondary hyperparathyroidism. Nephrol. Dial. Transplant. 2006, 21, 911–916. [Google Scholar] [CrossRef][Green Version]
- Graciolli, F.G.; Neves, K.R.; dos Reis, L.M.; Graciolli, R.G.; Noronha, I.L.; Moyses, R.M.; Jorgetti, V. Phosphorus overload and PTH induce aortic expression of Runx2 in experimental uraemia. Nephrol. Dial. Transplant. 2009, 24, 1416–1421. [Google Scholar] [CrossRef]
- El-Abbadi, M.M.; Pai, A.S.; Leaf, E.M.; Yang, H.Y.; Bartley, B.A.; Quan, K.K.; Ingalls, C.M.; Liao, H.W.; Giachelli, C.M. Phosphate feeding induces arterial medial calcification in uremic mice: Role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int. 2009, 75, 1297–1307. [Google Scholar] [CrossRef]
- Ren, X.; Wei, Q.; Shao, H.; Sun, Z.; Liu, N. A rat model of diabetic artery calcification. J. Endocrinol. Investig. 2012, 35, 497–503. [Google Scholar] [CrossRef]
- Assmann, A.; Zwirnmann, K.; Heidelberg, F.; Schiffer, F.; Horstkotter, K.; Munakata, H.; Gremse, F.; Barth, M.; Lichtenberg, A.; Akhyari, P. The degeneration of biological cardiovascular prostheses under pro-calcific metabolic conditions in a small animal model. Biomaterials 2014, 35, 7416–7428. [Google Scholar] [CrossRef] [PubMed]
- Roche-Molina, M.; Sanz-Rosa, D.; Cruz, F.M.; Garcia-Prieto, J.; Lopez, S.; Abia, R.; Muriana, F.J.; Fuster, V.; Ibanez, B.; Bernal, J.A. Induction of sustained hypercholesterolemia by single adeno-associated virus-mediated gene transfer of mutant hPCSK9. Arter. Thromb. Vasc. Biol. 2015, 35, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Goettsch, C.; Hutcheson, J.D.; Hagita, S.; Rogers, M.A.; Creager, M.D.; Pham, T.; Choi, J.; Mlynarchik, A.K.; Pieper, B.; Kjolby, M.; et al. A single injection of gain-of-function mutant PCSK9 adeno-associated virus vector induces cardiovascular calcification in mice with no genetic modification. Atherosclerosis 2016, 251, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Hum, J.M.; O’Bryan, L.M.; Tatiparthi, A.K.; Cass, T.A.; Clinkenbeard, E.L.; Cramer, M.S.; Bhaskaran, M.; Johnson, R.L.; Wilson, J.M.; Smith, R.C.; et al. Chronic Hyperphosphatemia and Vascular Calcification Are Reduced by Stable Delivery of Soluble Klotho. J. Am. Soc. Nephrol. 2017, 28, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S.; Sitara, D.; Taguchi, T.; St-Arnaud, R.; Lanske, B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 720–722. [Google Scholar] [CrossRef]
- Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004, 113, 561–568. [Google Scholar] [CrossRef]
- Ichikawa, S.; Sorenson, A.H.; Austin, A.M.; Mackenzie, D.S.; Fritz, T.A.; Moh, A.; Hui, S.L.; Econs, M.J. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology 2009, 150, 2543–2550. [Google Scholar] [CrossRef]
- Esapa, C.T.; Head, R.A.; Jeyabalan, J.; Evans, H.; Hough, T.A.; Cheeseman, M.T.; McNally, E.G.; Carr, A.J.; Thomas, G.P.; Brown, M.A.; et al. A mouse with an N-Ethyl-N-nitrosourea (ENU) Induced Trp589Arg Galnt3 mutation represents a model for hyperphosphataemic familial tumoural calcinosis. PLoS ONE 2012, 7, e43205. [Google Scholar] [CrossRef]
- Kauffenstein, G.; Pizard, A.; Le Corre, Y.; Vessieres, E.; Grimaud, L.; Toutain, B.; Labat, C.; Mauras, Y.; Gorgels, T.G.; Bergen, A.A.; et al. Disseminated arterial calcification and enhanced myogenic response are associated with abcc6 deficiency in a mouse model of pseudoxanthoma elasticum. Arter. Thromb. Vasc. Biol. 2014, 34, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Gorgels, T.G.; Hu, X.; Scheffer, G.L.; van der Wal, A.C.; Toonstra, J.; de Jong, P.T.; van Kuppevelt, T.H.; Levelt, C.N.; de Wolf, A.; Loves, W.J.; et al. Disruption of Abcc6 in the mouse: Novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum. Mol. Genet. 2005, 14, 1763–1773. [Google Scholar] [CrossRef]
- Mackenzie, N.C.; Zhu, D.; Milne, E.M.; van ’t Hof, R.; Martin, A.; Darryl Quarles, L.; Millan, J.L.; Farquharson, C.; MacRae, V.E. Altered bone development and an increase in FGF-23 expression in Enpp1(-/-) mice. PLoS ONE 2012, 7, e32177. [Google Scholar] [CrossRef]
- Watanabe, R.; Fujita, N.; Sato, Y.; Kobayashi, T.; Morita, M.; Oike, T.; Miyamoto, K.; Kuro, O.M.; Michigami, T.; Fukumoto, S.; et al. Enpp1 is an anti-aging factor that regulates Klotho under phosphate overload conditions. Sci. Rep. 2017, 7, 7786. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, H.; Chou, D.W.; Berndt, A.; Sundberg, J.P.; Uitto, J. Mutant Enpp1asj mice as a model for generalized arterial calcification of infancy. Dis. Models Mech. 2013, 6, 1227–1235. [Google Scholar] [CrossRef]
- Albright, R.A.; Stabach, P.; Cao, W.; Kavanagh, D.; Mullen, I.; Braddock, A.A.; Covo, M.S.; Tehan, M.; Yang, G.; Cheng, Z.; et al. ENPP1-Fc prevents mortality and vascular calcifications in rodent model of generalized arterial calcification of infancy. Nat. Commun. 2015, 6, 10006. [Google Scholar] [CrossRef] [PubMed]
- Sali, A.; Favaloro, J.L.; Terkeltaub, R.; Goding, J.W. Germline deletion of the nucleoside triphosphate pyrophosphohydrolase (NTPPPH) plasma cell membrane glycoprotein (PC-1) produces abnormal calcification of periarticular tissues. In Proceedings of the Second International Workshop on Ecto-ATPases and Related Ectonucleotidases, Maastricht, The Netherlands, 1 January 1999; pp. 267–282. [Google Scholar]
- Mounkes, L.C.; Kozlov, S.; Hernandez, L.; Sullivan, T.; Stewart, C.L. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 2003, 423, 298–301. [Google Scholar] [CrossRef]
- Varga, R.; Eriksson, M.; Erdos, M.R.; Olive, M.; Harten, I.; Kolodgie, F.; Capell, B.C.; Cheng, J.; Faddah, D.; Perkins, S.; et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 2006, 103, 3250–3255. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Rivera-Torres, J.; Osorio, F.G.; Acin-Perez, R.; Enriquez, J.A.; Lopez-Otin, C.; Andres, V. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation 2013, 127, 2442–2451. [Google Scholar] [CrossRef] [PubMed]
- Osorio, F.G.; Navarro, C.L.; Cadinanos, J.; Lopez-Mejia, I.C.; Quiros, P.M.; Bartoli, C.; Rivera, J.; Tazi, J.; Guzman, G.; Varela, I.; et al. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci. Transl. Med. 2011, 3, 106ra107. [Google Scholar] [CrossRef]
- Cubria, M.B.; Suarez, S.; Masoudi, A.; Oftadeh, R.; Kamalapathy, P.; DuBose, A.; Erdos, M.R.; Cabral, W.A.; Karim, L.; Collins, F.S.; et al. Evaluation of musculoskeletal phenotype of the G608G progeria mouse model with lonafarnib, pravastatin, and zoledronic acid as treatment groups. Proc. Natl. Acad. Sci. USA 2020, 117, 12029–12040. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Schinke, T.; Trindl, A.; Muller-Esterl, W.; Sablitzky, F.; Kaiser, S.; Blessing, M. Cloning and targeted deletion of the mouse fetuin gene. J. Biol. Chem. 1997, 272, 31496–31503. [Google Scholar] [CrossRef] [PubMed]
- Schafer, C.; Heiss, A.; Schwarz, A.; Westenfeld, R.; Ketteler, M.; Floege, J.; Muller-Esterl, W.; Schinke, T.; Jahnen-Dechent, W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J. Clin. Investig. 2003, 112, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Bucay, N.; Sarosi, I.; Dunstan, C.R.; Morony, S.; Tarpley, J.; Capparelli, C.; Scully, S.; Tan, H.L.; Xu, W.; Lacey, D.L.; et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998, 12, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Speer, M.Y.; McKee, M.D.; Guldberg, R.E.; Liaw, L.; Yang, H.Y.; Tung, E.; Karsenty, G.; Giachelli, C.M. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: Evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J. Exp. Med. 2002, 196, 1047–1055. [Google Scholar] [CrossRef]
- Rittling, S.R.; Matsumoto, H.N.; McKee, M.D.; Nanci, A.; An, X.R.; Novick, K.E.; Kowalski, A.J.; Noda, M.; Denhardt, D.T. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J. Bone Miner. Res. 1998, 13, 1101–1111. [Google Scholar] [CrossRef]
- Galvin, K.M.; Donovan, M.J.; Lynch, C.A.; Meyer, R.I.; Paul, R.J.; Lorenz, J.N.; Fairchild-Huntress, V.; Dixon, K.L.; Dunmore, J.H.; Gimbrone, M.A., Jr.; et al. A role for smad6 in development and homeostasis of the cardiovascular system. Nat. Genet. 2000, 24, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Reddick, R.L.; Piedrahita, J.A.; Maeda, N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992, 258, 468–471. [Google Scholar] [CrossRef]
- Rattazzi, M.; Bennett, B.J.; Bea, F.; Kirk, E.A.; Ricks, J.L.; Speer, M.; Schwartz, S.M.; Giachelli, C.M.; Rosenfeld, M.E. Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: Potential role of chondrocyte-like cells. Arter. Thromb. Vasc. Biol. 2005, 25, 1420–1425. [Google Scholar] [CrossRef]
- Ishibashi, S.; Goldstein, J.L.; Brown, M.S.; Herz, J.; Burns, D.K. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J. Clin. Investig. 1994, 93, 1885–1893. [Google Scholar] [CrossRef]
- Awan, Z.; Denis, M.; Bailey, D.; Giaid, A.; Prat, A.; Goltzman, D.; Seidah, N.G.; Genest, J. The LDLR deficient mouse as a model for aortic calcification and quantification by micro-computed tomography. Atherosclerosis 2011, 219, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Van Vlijmen, B.J.; van den Maagdenberg, A.M.; Gijbels, M.J.; van der Boom, H.; HogenEsch, H.; Frants, R.R.; Hofker, M.H.; Havekes, L.M. Diet-induced hyperlipoproteinemia and atherosclerosis in apolipoprotein E3-Leiden transgenic mice. J. Clin. Investig. 1994, 93, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, E.; Daemen, M.; Kockx, M.; Doevendans, P.; Hofker, M.; Havekes, L.; Wellens, H.; de Muinck, E.D. Atherosclerosis in APOE*3-Leiden transgenic mice: From proliferative to atheromatous stage. Circulation 1999, 99, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Cejka, D.; Alesutan, I. An overview of the mechanisms in vascular calcification during chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 289–296. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef]
- Shroff, R.C.; Shanahan, C.M. The vascular biology of calcification. Semin. Dial. 2007, 20, 103–109. [Google Scholar] [CrossRef]
- Song, J.-S.; Wang, R.-S.; Leopold, J.A.; Loscalzo, J. Network determinants of cardiovascular calcification and repositioned drug treatments. FASEB J. 2020, 34, 11087–11100. [Google Scholar] [CrossRef]
- Koppert, S.; Buscher, A.; Babler, A.; Ghallab, A.; Buhl, E.M.; Latz, E.; Hengstler, J.G.; Smith, E.R.; Jahnen-Dechent, W. Cellular Clearance and Biological Activity of Calciprotein Particles Depend on Their Maturation State and Crystallinity. Front. Immunol. 2018, 9, 1991. [Google Scholar] [CrossRef]
- Back, M.; Aranyi, T.; Cancela, M.L.; Carracedo, M.; Conceicao, N.; Leftheriotis, G.; Macrae, V.; Martin, L.; Nitschke, Y.; Pasch, A.; et al. Endogenous Calcification Inhibitors in the Prevention of Vascular Calcification: A Consensus Statement From the COST Action EuroSoftCalcNet. Front. Cardiovasc. Med. 2018, 5, 196. [Google Scholar] [CrossRef]
- Qunibi, W.Y. Consequences of hyperphosphatemia in patients with end-stage renal disease (ESRD). Kidney Int. Suppl. 2004, 90, S8–S12. [Google Scholar] [CrossRef]
- Azpiazu, D.; Gonzalo, S.; Villa-Bellosta, R. Tissue Non-Specific Alkaline Phosphatase and Vascular Calcification: A Potential Therapeutic Target. Curr. Cardiol. Rev. 2019, 15, 91–95. [Google Scholar] [CrossRef]
- Pasch, A.; Farese, S.; Graber, S.; Wald, J.; Richtering, W.; Floege, J.; Jahnen-Dechent, W. Nanoparticle-based test measures overall propensity for calcification in serum. J. Am. Soc. Nephrol. 2012, 23, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Ford, M.L.; Tomlinson, L.A.; Bodenham, E.; McMahon, L.P.; Farese, S.; Rajkumar, C.; Holt, S.G.; Pasch, A. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J. Am. Soc. Nephrol. 2014, 25, 339–348. [Google Scholar] [CrossRef]
- Chen, N.X.; Duan, D.; O’Neill, K.D.; Moe, S.M. High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol. Dial. Transplant. 2006, 21, 3435–3442. [Google Scholar] [CrossRef] [PubMed]
- Steitz, S.A.; Speer, M.Y.; Curinga, G.; Yang, H.Y.; Haynes, P.; Aebersold, R.; Schinke, T.; Karsenty, G.; Giachelli, C.M. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001, 89, 1147–1154. [Google Scholar] [CrossRef]
- Mori, K.; Shioi, A.; Jono, S.; Nishizawa, Y.; Morii, H. Dexamethasone enhances In vitro vascular calcification by promoting osteoblastic differentiation of vascular smooth muscle cells. Arter. Thromb. Vasc. Biol. 1999, 19, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.L.; Shao, J.S.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J. Biol. Chem. 2003, 278, 45969–45977. [Google Scholar] [CrossRef]
- Todd, G.M.; Gao, Z.; Hyvonen, M.; Brazil, D.P.; Ten Dijke, P. Secreted BMP antagonists and their role in cancer and bone metastases. Bone 2020, 137, 115455. [Google Scholar] [CrossRef] [PubMed]
- Shin, V.; Zebboudj, A.F.; Bostrom, K. Endothelial cells modulate osteogenesis in calcifying vascular cells. J. Vasc. Res. 2004, 41, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A.; Mathew, S.; Saab, G. Bone morphogenetic proteins in vascular calcification. Circ. Res. 2005, 97, 105–114. [Google Scholar] [CrossRef]
- Sorescu, G.P.; Song, H.; Tressel, S.L.; Hwang, J.; Dikalov, S.; Smith, D.A.; Boyd, N.L.; Platt, M.O.; Lassegue, B.; Griendling, K.K.; et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ. Res. 2004, 95, 773–779. [Google Scholar] [CrossRef]
- Moe, S.M.; Duan, D.; Doehle, B.P.; O’Neill, K.D.; Chen, N.X. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int. 2003, 63, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Cobb, A.M.; Yusoff, S.; Hayward, R.; Ahmad, S.; Sun, M.; Verhulst, A.; D’Haese, P.C.; Shanahan, C.M. Runx2 (Runt-Related Transcription Factor 2) Links the DNA Damage Response to Osteogenic Reprogramming and Apoptosis of Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2021, 41, 1339–1357. [Google Scholar] [CrossRef] [PubMed]
- Byon, C.H.; Javed, A.; Dai, Q.; Kappes, J.C.; Clemens, T.L.; Darley-Usmar, V.M.; McDonald, J.M.; Chen, Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008, 283, 15319–15327. [Google Scholar] [CrossRef]
- Huang, M.; Zheng, L.; Xu, H.; Tang, D.; Lin, L.; Zhang, J.; Li, C.; Wang, W.; Yuan, Q.; Tao, L.; et al. Oxidative stress contributes to vascular calcification in patients with chronic kidney disease. J. Mol. Cell Cardiol. 2020, 138, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.S.; Cheng, S.L.; Pingsterhaus, J.M.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J. Clin. Investig. 2005, 115, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Cary, N.R.; Salisbury, J.R.; Proudfoot, D.; Weissberg, P.L.; Edmonds, M.E. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: Evidence for smooth muscle cell-mediated vascular calcification. Circulation 1999, 100, 2168–2176. [Google Scholar] [CrossRef]
- Willems, B.A.; Furmanik, M.; Caron, M.M.J.; Chatrou, M.L.L.; Kusters, D.H.M.; Welting, T.J.M.; Stock, M.; Rafael, M.S.; Viegas, C.S.B.; Simes, D.C.; et al. Ucma/GRP inhibits phosphate-induced vascular smooth muscle cell calcification via SMAD-dependent BMP signalling. Sci. Rep. 2018, 8, 4961. [Google Scholar] [CrossRef]
- Moss, D.W.; Eaton, R.H.; Smith, J.K.; Whitby, L.G. Association of inorganic-pyrophosphatase activity with human alkaline-phosphatase preparations. Biochem. J. 1967, 102, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.Y.; Yao, L.; Sheng, Z.T.; Wan, P.Z.; Qiu, X.B.; Wang, J.; Xu, T.H. Specific knockdown of WNT8b expression protects against phosphate-induced calcification in vascular smooth muscle cells by inhibiting the Wnt-beta-catenin signaling pathway. J. Cell Physiol. 2019, 234, 3469–3477. [Google Scholar] [CrossRef]
- Adragao, T.; Pires, A.; Branco, P.; Castro, R.; Oliveira, A.; Nogueira, C.; Bordalo, J.; Curto, J.D.; Prata, M.M. Ankle--brachial index, vascular calcifications and mortality in dialysis patients. Nephrol. Dial. Transplant. 2012, 27, 318–325. [Google Scholar] [CrossRef]
- Cheng, S.L.; Behrmann, A.; Shao, J.S.; Ramachandran, B.; Krchma, K.; Bello Arredondo, Y.; Kovacs, A.; Mead, M.; Maxson, R.; Towler, D.A. Targeted reduction of vascular Msx1 and Msx2 mitigates arteriosclerotic calcification and aortic stiffness in LDLR-deficient mice fed diabetogenic diets. Diabetes 2014, 63, 4326–4337. [Google Scholar] [CrossRef] [PubMed]
- Fujikura, K.; Luo, J.; Gamarnik, V.; Pernot, M.; Fukumoto, R.; Tilson, M.D., 3rd; Konofagou, E.E. A novel noninvasive technique for pulse-wave imaging and characterization of clinically-significant vascular mechanical properties in vivo. Ultrason. Imaging 2007, 29, 137–154. [Google Scholar] [CrossRef]
- Di Lascio, N.; Kusmic, C.; Stea, F.; Faita, F. Ultrasound-based Pulse Wave Velocity Evaluation in Mice. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Franklin, S.S.; Gustin, W.t.; Wong, N.D.; Larson, M.G.; Weber, M.A.; Kannel, W.B.; Levy, D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997, 96, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, K.S.; Barreira, T.V. Association between pulse pressure and aortic calcification: Findings from the National Health and Nutrition Examination Survey 2013-2014. J. Clin. Hypertens. (Greenwich) 2020, 22, 879–885. [Google Scholar] [CrossRef]
- Del Campo, L.; Ferrer, M. Wire Myography to Study Vascular Tone and Vascular Structure of Isolated Mouse Arteries. In Methods in Mouse Atherosclerosis; Andrés, V., Dorado, B., Eds.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Spiers, A.; Padmanabhan, N. A guide to wire myography. Methods Mol. Med. 2005, 108, 91–104. [Google Scholar] [CrossRef]
- Kirsch, A.H.; Kirsch, A.; Artinger, K.; Schabhuttl, C.; Goessler, W.; Klymiuk, I.; Gully, C.; Fritz, G.A.; Frank, S.; Wimmer, R.; et al. Heterogeneous susceptibility for uraemic media calcification and concomitant inflammation within the arterial tree. Nephrol. Dial. Transplant. 2015, 30, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Nguy, L.; Nilsson, H.; Lundgren, J.; Johansson, M.E.; Teerlink, T.; Scheffer, P.G.; Guron, G. Vascular function in rats with adenine-induced chronic renal failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1426–R1435. [Google Scholar] [CrossRef]
- Stern, J.; Lewis, W.H. The colorimetric estimation of calcium in serum with ocresolphthalein complexone. Clin. Chim. Acta 1957, 2, 576–580. [Google Scholar] [CrossRef]
- Puchtler, H.; Meloan, S.N.; Terry, M.S. On the history and mechanism of alizarin and alizarin red S stains for calcium. J. Histochem. Cytochem. 1969, 17, 110–124. [Google Scholar] [CrossRef]
- Puchtler, H.; Meloan, S.N. Demonstration of phosphates in calcium deposits: A modification of von Kossa’s reaction. Histochemistry 1978, 56, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.; Hrapchak, B. Theory and Practice of Histotechnology, 2nd ed.; Battelle Press: Columbus, OH, USA, 1980; pp. 226–227. [Google Scholar]
- Lievremont, M.; Potus, J.; Guillou, B. Use of alizarin red S for histochemical staining of Ca2+ in the mouse; some parameters of the chemical reaction in vitro. Acta Anat. 1982, 114, 268–280. [Google Scholar] [CrossRef]
- Bonewald, L.F.; Harris, S.E.; Rosser, J.; Dallas, M.R.; Dallas, S.L.; Camacho, N.P.; Boyan, B.; Boskey, A. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif. Tissue Int. 2003, 72, 537–547. [Google Scholar] [CrossRef]
- Sim, A.M.; Rashdan, N.A.; Cui, L.; Moss, A.J.; Nudelman, F.; Dweck, M.R.; MacRae, V.E.; Hulme, A.N. A novel fluorescein-bisphosphonate based diagnostic tool for the detection of hydroxyapatite in both cell and tissue models. Sci. Rep. 2018, 8, 17360. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Goettsch, C.; Bertazzo, S.; Maldonado, N.; Ruiz, J.L.; Goh, W.; Yabusaki, K.; Faits, T.; Bouten, C.; Franck, G.; et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat. Mater. 2016, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, D.; Skepper, J.N.; Hegyi, L.; Bennett, M.R.; Shanahan, C.M.; Weissberg, P.L. Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circ. Res. 2000, 87, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Tung, C.H. Osteocalcin biomimic recognizes bone hydroxyapatite. ChemBioChem 2011, 12, 1669–1673. [Google Scholar] [CrossRef]
- Zorlu, Y.; Brown, C.; Keil, C.; Ayhan, M.M.; Haase, H.; Thompson, R.B.; Lengyel, I.; Yucesan, G. Fluorescent Arylphosphonic Acids: Synergic Interactions between Bone and the Fluorescent Core. Chemistry 2020, 26, 11129–11134. [Google Scholar] [CrossRef]
- Celeng, C.; de Keizer, B.; Merkely, B.; de Jong, P.; Leiner, T.; Takx, R.A.P. PET Molecular Targets and Near-Infrared Fluorescence Imaging of Atherosclerosis. Curr. Cardiol. Rep. 2018, 20, 11. [Google Scholar] [CrossRef]
- Zaheer, A.; Lenkinski, R.E.; Mahmood, A.; Jones, A.G.; Cantley, L.C.; Frangioni, J.V. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat. Biotechnol. 2001, 19, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.D.; Hohl, T.; Hutcheson, J.D.; Moss, A.J.; Schlotter, F.; Blaser, M.C.; Park, M.A.; Lee, L.H.; Singh, S.A.; Alcaide-Corral, C.J.; et al. (18)F-Fluoride Signal Amplification Identifies Microcalcifications Associated With Atherosclerotic Plaque Instability in Positron Emission Tomography/Computed Tomography Images. Circ. Cardiovasc. Imaging 2019, 12, e007835. [Google Scholar] [CrossRef]
- Aikawa, E.; Nahrendorf, M.; Figueiredo, J.L.; Swirski, F.K.; Shtatland, T.; Kohler, R.H.; Jaffer, F.A.; Aikawa, M.; Weissleder, R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007, 116, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Derwall, M.; Malhotra, R.; Lai, C.S.; Beppu, Y.; Aikawa, E.; Seehra, J.S.; Zapol, W.M.; Bloch, K.D.; Yu, P.B. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Pittet, M.J.; Weissleder, R. Intravital imaging. Cell 2011, 147, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, E.; Aikawa, M.; Libby, P.; Figueiredo, J.L.; Rusanescu, G.; Iwamoto, Y.; Fukuda, D.; Kohler, R.H.; Shi, G.P.; Jaffer, F.A.; et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation 2009, 119, 1785–1794. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Aikawa, M.; Zheng, C.; Aaron, J.; Lax, L.; Libby, P.; de Lima Filho, J.L.; Gruener, S.; Fingerle, J.; Haap, W.; et al. Selective cathepsin S inhibition attenuates atherosclerosis in apolipoprotein E-deficient mice with chronic renal disease. Am. J. Pathol. 2015, 185, 1156–1166. [Google Scholar] [CrossRef]
- Fayad, Z.A.; Fallon, J.T.; Shinnar, M.; Wehrli, S.; Dansky, H.M.; Poon, M.; Badimon, J.J.; Charlton, S.A.; Fisher, E.A.; Breslow, J.L.; et al. Noninvasive In vivo high-resolution magnetic resonance imaging of atherosclerotic lesions in genetically engineered mice. Circulation 1998, 98, 1541–1547. [Google Scholar] [CrossRef]
- Santos, A.; Fernandez-Friera, L.; Villalba, M.; Lopez-Melgar, B.; Espana, S.; Mateo, J.; Mota, R.A.; Jimenez-Borreguero, J.; Ruiz-Cabello, J. Cardiovascular imaging: What have we learned from animal models? Front. Pharm. 2015, 6, 227. [Google Scholar] [CrossRef]
- Wang, Y.; Osborne, M.T.; Tung, B.; Li, M.; Li, Y. Imaging Cardiovascular Calcification. J. Am. Heart Assoc. 2018, 7, e008564. [Google Scholar] [CrossRef]
- Huesa, C.; Millan, J.L.; van ‘tHof, R.J.; MacRae, V.E. A new method for the quantification of aortic calcification by three-dimensional micro-computed tomography. Int. J. Mol. Med. 2013, 32, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Shamsuzzaman, S.; Onal, M.; St John, H.C.; Jeffery, J.J.; Pike, J.W. Absence of the Vitamin D Receptor Inhibits Atherosclerotic Plaque Calcification in Female Hypercholesterolemic Mice. J. Cell Biochem. 2017, 118, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Borland, S.J.; Behnsen, J.; Ashton, N.; Francis, S.E.; Brennan, K.; Sherratt, M.J.; Withers, P.J.; Canfield, A.E. X-ray Micro-Computed Tomography: An Emerging Technology to Analyze Vascular Calcification in Animal Models. Int. J. Mol. Sci. 2020, 21, 4538. [Google Scholar] [CrossRef] [PubMed]
- Irkle, A.; Vesey, A.T.; Lewis, D.Y.; Skepper, J.N.; Bird, J.L.; Dweck, M.R.; Joshi, F.R.; Gallagher, F.A.; Warburton, E.A.; Bennett, M.R.; et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat. Commun. 2015, 6, 7495. [Google Scholar] [CrossRef] [PubMed]
- Rucher, G.; Cameliere, L.; Fendri, J.; Abbas, A.; Dupont, K.; Kamel, S.; Delcroix, N.; Dupont, A.; Berger, L.; Manrique, A. Performance Evaluation of a Dedicated Preclinical PET/CT System for the Assessment of Mineralization Process in a Mouse Model of Atherosclerosis. Mol. Imaging Biol. 2018, 20, 984–992. [Google Scholar] [CrossRef]
- MacAskill, M.G.; McDougald, W.; Alcaide-Corral, C.; Newby, D.E.; Tavares, A.A.S.; Hadoke, P.W.F.; Wu, J. Characterisation of an atherosclerotic micro-calcification model using ApoE(−/−) mice and PET/CT. Int. J. Cardiol. Heart Vasc. 2020, 31, 100672. [Google Scholar] [CrossRef]
- Florea, A.; Sigl, J.P.; Morgenroth, A.; Vogg, A.; Sahnoun, S.; Winz, O.H.; Bucerius, J.; Schurgers, L.J.; Mottaghy, F.M. Sodium [18F]Fluoride PET Can Efficiently Monitor In Vivo Atherosclerotic Plaque Calcification Progression and Treatment. Cells 2021, 10, 275. [Google Scholar] [CrossRef]
- Hsu, J.J.; Fong, F.; Patel, R.; Qiao, R.; Lo, K.; Soundia, A.; Chang, C.C.; Le, V.; Tseng, C.H.; Demer, L.L.; et al. Changes in microarchitecture of atherosclerotic calcification assessed by (18)F-NaF PET and CT after a progressive exercise regimen in hyperlipidemic mice. J. Nucl. Cardiol. 2020. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, P.; Hu, B.; Chen, W.; Cheng, D.; Shi, H. Dynamic monitoring of active calcification in atherosclerosis by (18)F-NaF PET imaging. Int. J. Cardiovasc. Imaging 2021, 37, 731–739. [Google Scholar] [CrossRef] [PubMed]
| Items | Multi Point Analysis Feasible | Single Point Analysis | ||
|---|---|---|---|---|
| Destructive | Non-Destructive | |||
| Biochemical markers of pathological calcification | Circulating blood markers | Detection of resident markers in tissue | - | |
| Markers of vessel stiffness | Pulse wave velocity | Wire myography | - | |
| Pulse pressure | - | |||
| Direct detection of calcification | Biochemical | - | o-cresolpthalein | - |
| Alizarin red | ||||
| Microscopy | Intravital microscopy | Histological staining of dissected tissue | Intravital microscopy | |
| Magnetic Resonance | Magnetic resonance imaging | - | Magnetic resonance imaging | |
| X-ray | (Micro-) Computed tomography | - | (Micro-) Computed tomography | |
| Positron emission | Positron emission tomography | - | Positron emission tomography | |
| Biomarker | Mechanism | Consequences in Genetically Altered Mice/Correlation with Endpoint | Methods of Biomarker Analysis |
|---|---|---|---|
| Calcium [78] | Causal relationship between elevated calcium and calcification unclear | In humans, correlation of high serum calcium with coronary atherosclerosis, cardiovascular events and increased mortality. | Standard serum chemistry |
| Phosphate [78,83] | Pathophysiologic mechanisms between elevated phosphate and calcification are incompletely understood, several pathways likely contribute to increased mortality in ESRD patients | In humans, increased serum phosphate correlate with increased coronary calcification, morbidity and mortality. | Standard serum chemistry |
| Alkaline Phosphatase [84] | Hydrolyzation of extracellular pyrophosphate and formation of hydroxyapatite | Alkaline phosphatase is increased in models with medial calcification. | Standard serum chemistry/Functional assay, Antibody-based technique |
| Calcium Propensity [85,86] | Physiologically, formation of calciprotein particles (CPP) is tightly regulated in serum supersaturated in calcium and phosphate by inhibitors such as Fetuin-A | In human studies, correlation with all-cause mortality in predialysis patients. | In vitro test that monitors the maturation time of calciprotein particles via nephelometry |
| Matrix Gla Protein [67] |
| Mgp−/− mice exhibit extensive mineralization of the aorta located predominantly in the media. | Antibody-based technique |
| Fetuin-A [68,69,81] |
| Mice deficient of Fetuin-A develop soft tissue calcifications, with extent depending on genetic background and chow. | Antibody-based technique |
| Osteopontin [68] |
| Knockout of Opn alone does not induce calcification in mice. Double Knockout of Opn and Mgp in mice exacerbates vascular calcification in comparison to sole knockout of Mgp. | Antibody-based technique |
| Fgf-23 [49] | Regulates phosphate homeostasis and metabolism of Vitamin D, binding of Fgf-23 to receptor requires Klotho as necessary co-factor | Fgf-23−/− exhibit early onset vascular calcification. Elevated Fgf-23 levels associated with higher calcification scores and arterial stiffness. | Antibody-based technique |
| Klotho [46] |
| Klotho−/− mice exhibit severe vascular calcification. | Antibody-based technique |
| Pyrophosphate (PPi) [52,54,55,56] |
| Enpp−/−, Enppttw/ttw and Enppasj exhibit vascular calcification of the aorta. Abcc6−/− mice exhibit increased arterial calcium content. | Enzymatic assay |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrmann, J.; Gummi, M.R.; Xia, M.; van der Giet, M.; Tölle, M.; Schuchardt, M. Vascular Calcification in Rodent Models—Keeping Track with an Extented Method Assortment. Biology 2021, 10, 459. https://doi.org/10.3390/biology10060459
Herrmann J, Gummi MR, Xia M, van der Giet M, Tölle M, Schuchardt M. Vascular Calcification in Rodent Models—Keeping Track with an Extented Method Assortment. Biology. 2021; 10(6):459. https://doi.org/10.3390/biology10060459
Chicago/Turabian StyleHerrmann, Jaqueline, Manasa Reddy Gummi, Mengdi Xia, Markus van der Giet, Markus Tölle, and Mirjam Schuchardt. 2021. "Vascular Calcification in Rodent Models—Keeping Track with an Extented Method Assortment" Biology 10, no. 6: 459. https://doi.org/10.3390/biology10060459
APA StyleHerrmann, J., Gummi, M. R., Xia, M., van der Giet, M., Tölle, M., & Schuchardt, M. (2021). Vascular Calcification in Rodent Models—Keeping Track with an Extented Method Assortment. Biology, 10(6), 459. https://doi.org/10.3390/biology10060459






