Canine Angiostrongylus vasorum-Induced Early Innate Immune Reactions Based on NETs Formation and Canine Vascular Endothelial Cell Activation In Vitro
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Gastropod Maintenance and Isolation of Axenic Angiostrongylus vasorum Third-Stage Larvae (L3)
2.2. Isolation of Canine PMN
2.3. Scanning Electron Microscopy (SEM) Analysis
2.4. NET Visualization by Immunofluorescence
2.5. Assessment of Different NET Phenotypes
2.6. Nuclear Decondensation-Based Quantification Using DANA Software
2.7. Isolation of Primary Canine Aortic Endothelial Cells (CAEC)
2.8. Preparation of Angiostrongylus vasorum L3 Soluble Antigen (AvAg)
2.9. Total RNA Isolation and qRT-PCR
2.10. Protein Isolation and Western Blot Analyses
2.11. Statistical Analysis
3. Results
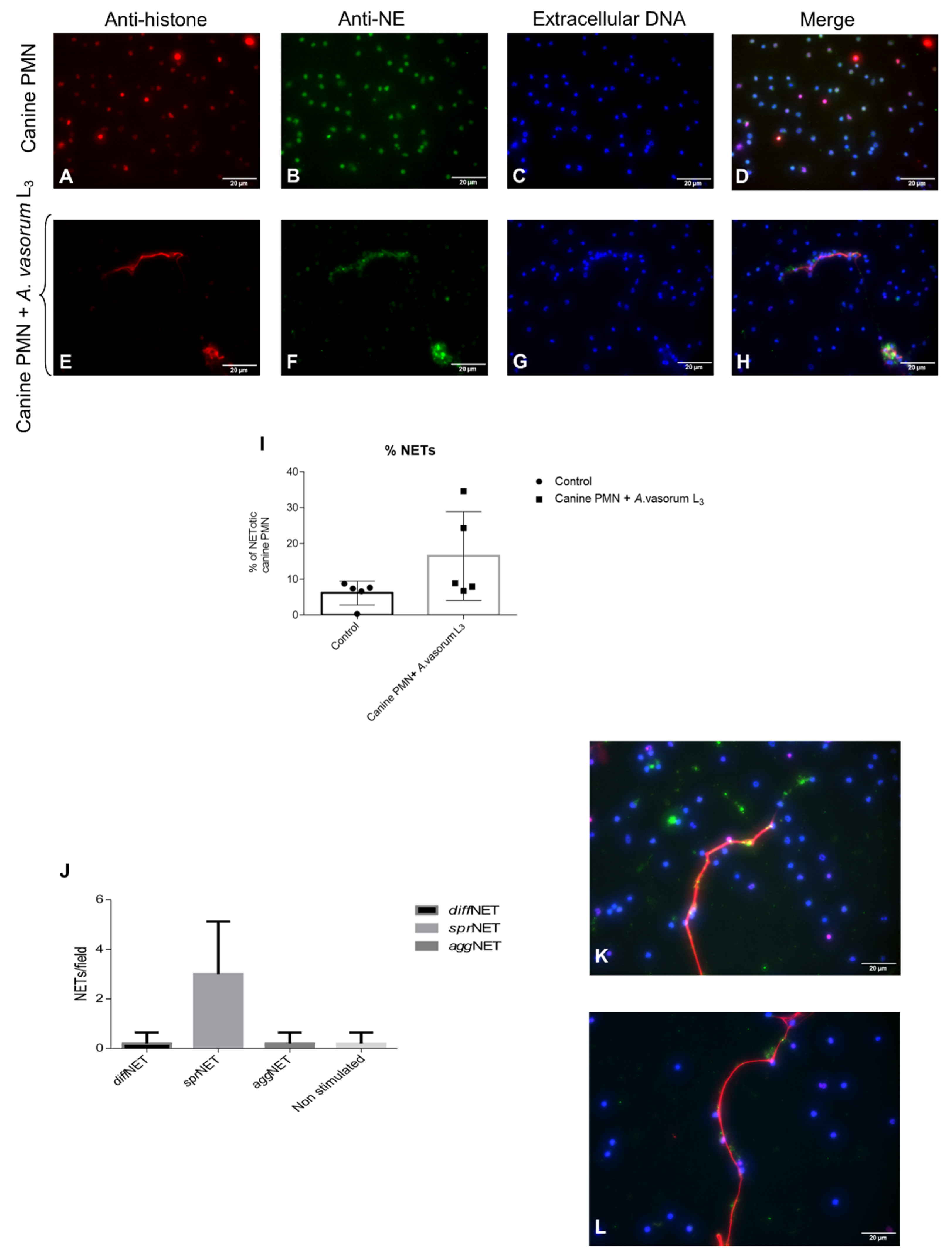
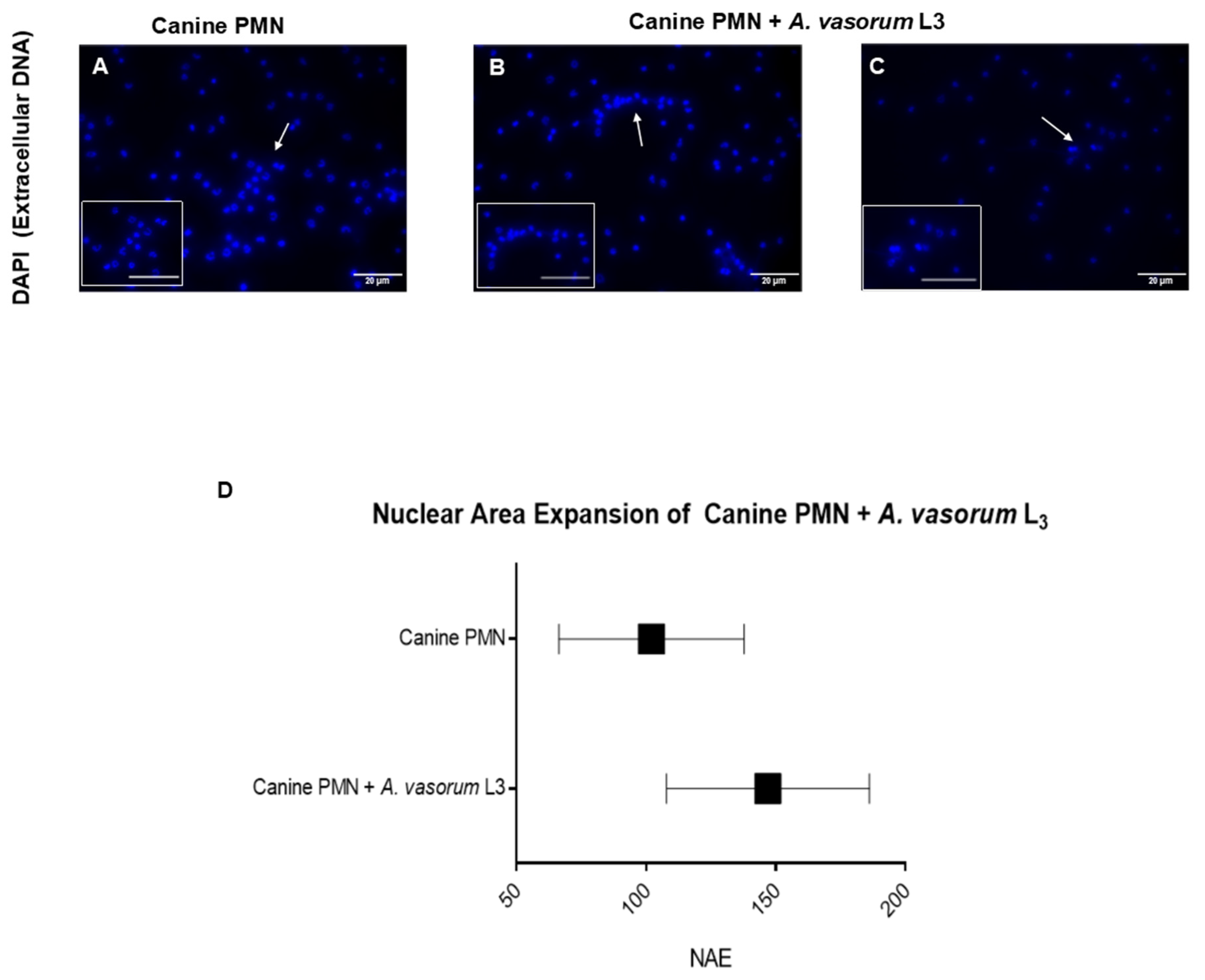
3.1. Angiostrongylus vasorum L3 Trigger NET Formation in Canine PMN and Led to Differential NET Phenotype Formation
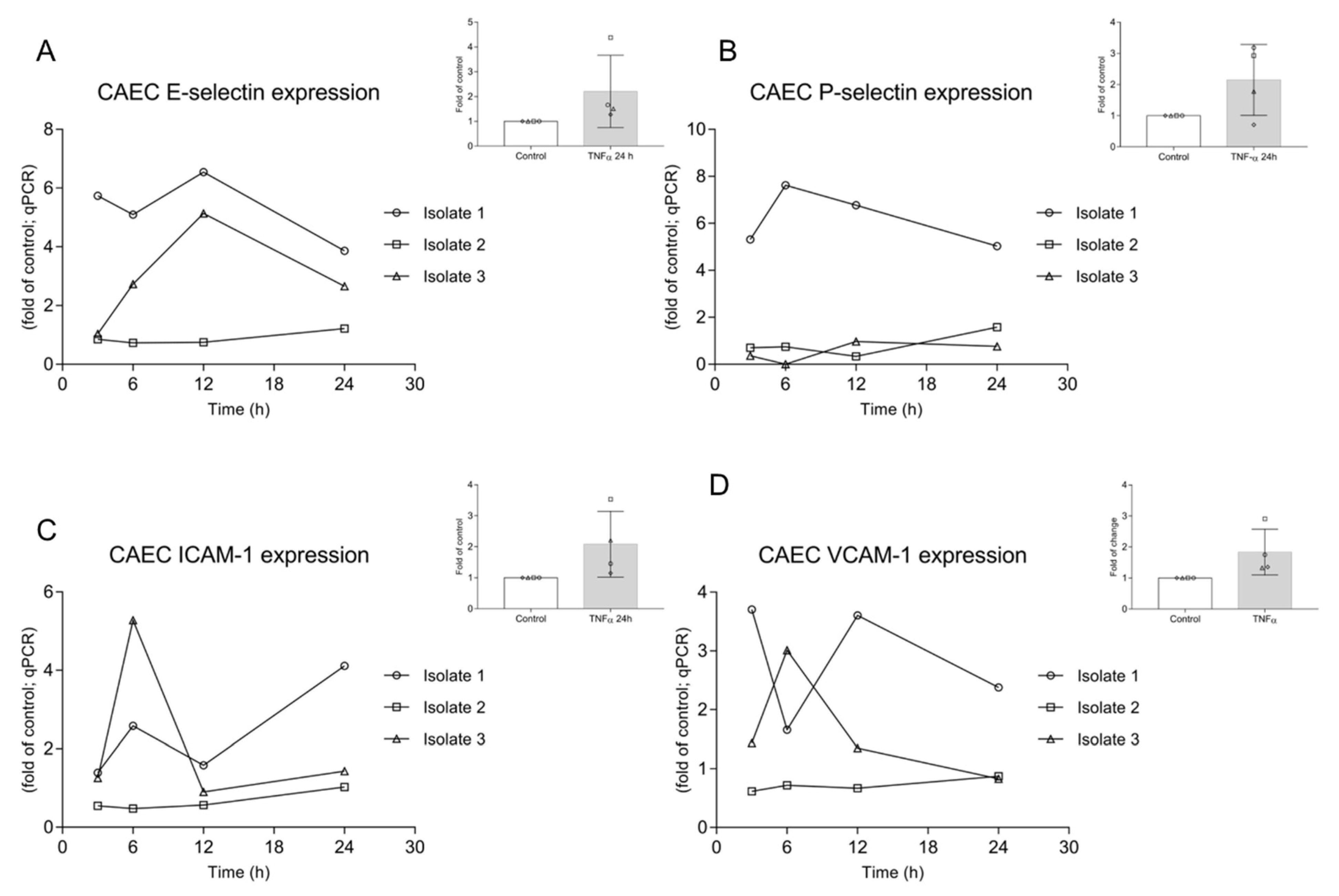
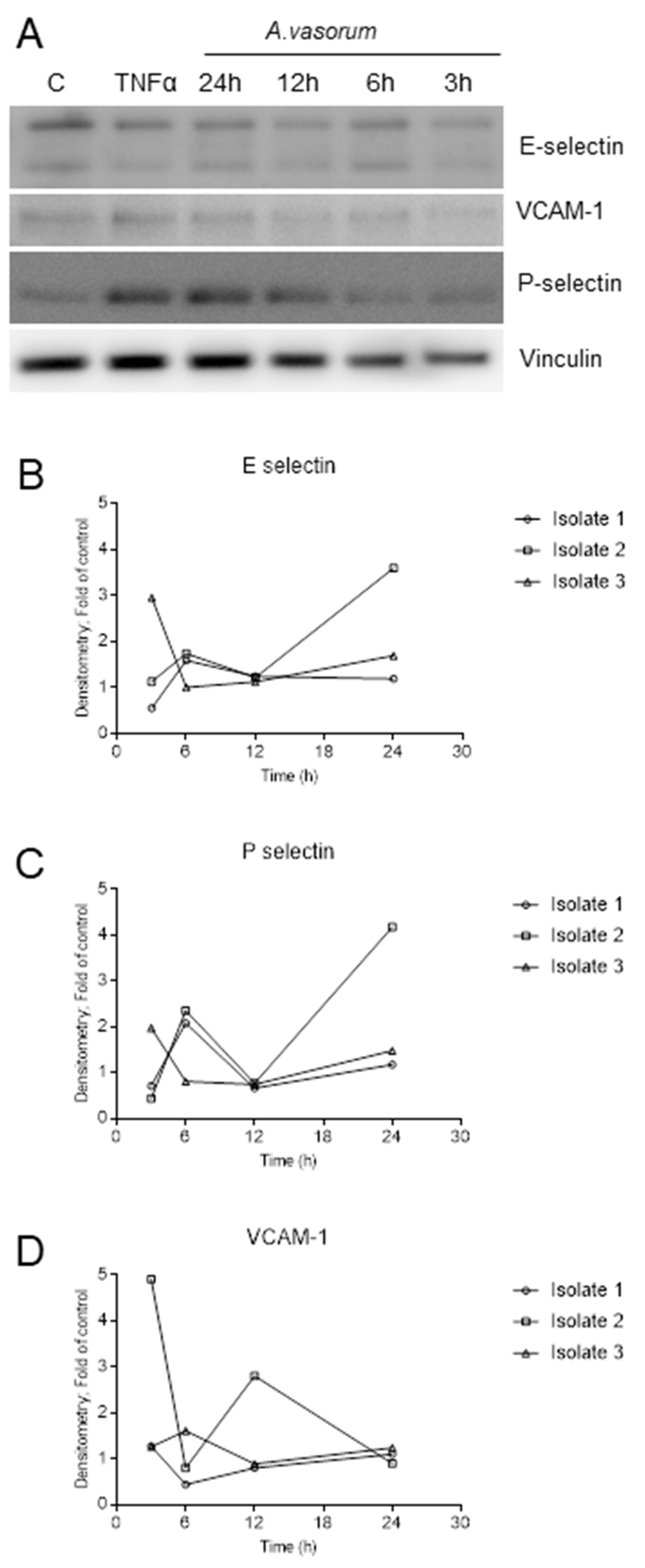
3.2. AvAg Induces Canine Endothelial Cell Activation and Donor-Dependent Adhesion Molecule Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Traversa, D.; Di Cesare, A.; Conboy, G. Canine and feline cardiopulmonary parasitic nematodes in Europe: Emerging and underestimated. Parasites Vectors 2010, 3, 62. [Google Scholar] [CrossRef]
- Schnyder, M.; Bilbrough, G.; Hafner, C.; Schaper, R. Angiostrongylus vasorum, “The French Heartworm”: A Serological Survey in Dogs from France Introduced by a Brief Historical Review. Parasitol. Res. 2017, 116, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Alho, A.M.; Meireles, J.; Schnyder, M.; Cardoso, L.; Belo, S.; Deplazes, P.; de Carvalho, L.M. Dirofilaria immitis and Angiostrongylus vasorum: The current situation of two major canine heartworms in Portugal. Vet. Parasitol. 2018, 252, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla, C.; Kleinertz, S.; Silva, L.M.; Hirzmann, J.; Huber, D.; Kusak, J.; Taubert, A. Protozoan and helminth parasite fauna of free-living Croatian wild wolves (Canis lupus) analyzed by scat collection. Vet. Parasitol. 2017, 233, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Schug, K.; Kramer, F.; Schaper, R.; Hirzmann, J.; Failing, K.; Hermosilla, C.; Taubert, A. Prevalence survey on lungworm (Angiostrongylus vasorum, Crenosoma vulpis, Eucoleus aerophilus) infections of wild red foxes (Vulpes vulpes) in central Germany. Parasites Vectors 2018, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Gavrilović, P.; Dobrosavljević, I.; Vasković, N.; Todorović, I.; Živulj, A.; Kureljušić, B.; Pavlović, I. Cardiopulmonary parasitic nematodes of the red fox (Vulpes vulpes) in Serbia. Acta Vet. Hung. 2019, 67, 60–69. [Google Scholar] [CrossRef]
- Patel, Z.; Gill, A.C.; Fox, M.T.; Hermosilla, C.; Backeljau, T.; Breugelmans, K.; Keevash, E.; McEwan, C.; Aghazadeh, M.; Elson-Riggins, J.G. Molecular identification of novel intermediate host species of Angiostrongylus vasorum in Greater London. Parasitol. Res. 2014, 113, 4363–4369. [Google Scholar] [CrossRef]
- Lange, M.K.; Penagos-Tabares, F.; Muñoz-Caro, T.; Gärtner, U.; Mejer, H.; Schaper, R.; Hermosilla, C.; Taubert, A. Gastropod-derived haemocyte extracellular traps entrap metastrongyloid larval stages of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Troglostrongylus brevior. Parasites Vectors 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Tabares, F.; Lange, M.K.; Vélez, J.; Hirzmann, J.; Gutiérrez-Arboleda, J.; Taubert, A.; Hermosilla, C.; Chaparro Gutiérrez, J.J. The invasive giant African snail Lissachatina fulica as natural intermediate host of Aelurostrongylus abstrusus, Angiostrongylus vasorum, Troglostrongylus brevior, and Crenosoma vulpis in Colombia. PLoS Negl. Trop. Dis. 2019, 13, e0007277. [Google Scholar] [CrossRef]
- Morgan, E.R.; Shaw, S.E.; Brennan, S.F.; De Waal, T.D.; Jones, B.R.; Mulcahy, G. Angiostrongylus vasorum: A real heartbreaker. Trends Parasitol. 2005, 21, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Taubert, A.; Pantchev, N.; Vrhovec, M.G.; Bauer, C.; Hermosilla, C. Lungworm infections (Angiostrongylus vasorum, Crenosoma vulpis, Aelurostrongylus abstrusus) in dogs and cats in Germany and Denmark in 2003–2007. Vet. Parasitol. 2009, 159, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Adamantos, S.; Waters, S.; Boag, A. Coagulation status in dogs with naturally occurring Angiostrongylus vasorum infection. J. Small Anim. Pr. 2015, 56, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Helm, J.; Roberts, L.; Jefferies, R.; Shaw, S.E.; Morgan, E.R. Epidemiological survey of Angiostrongylus vasorum in dogs and slugs around a new endemic focus in Scotland. Vet. Rec. 2015, 177, 46. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, P.; Hermosilla, C.; Taubert, A.; Staubach, C.; Sauter-Louis, C.; Conraths, F.J.; Vrhovec, M.G.; Pantchev, N. GIS-supported epidemiological analysis on canine Angiostrongylus vasorum and Crenosoma vulpis infections in Germany. Parasites Vectors 2017, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Conboy, G.A. Canine angiostrongylosis: The French heartworm: An emerging threat in North America. Vet. Parasitol. 2011, 176, 382–389. [Google Scholar] [CrossRef]
- Lange, M.K.; Penagos-Tabares, F.; Hirzmann, J.; Failing, K.; Schaper, R.; Van Bourgonie, Y.R.; Backeljau, T.; Hermosilla, C.; Taubert, A. Prevalence of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Crenosoma vulpis larvae in native slug populations in Germany. Vet. Parasitol. 2018, 254, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Tabares, F.; Lange, M.K.; Seipp, A.; Gärtner, U.; Mejer, H.; Taubert, A.; Hermosilla, C. Novel approach to study gastropod-mediated innate immune reactions against metastrongyloid parasites. Parasitol. Res. 2018, 117, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.S.; Boag, A.K.; Guitian, J.; Boswood, A. Angiostrongylus vasorum infection in 23 dogs (1999–2002). J. Small Anim. Pr. 2004, 45, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Willesen, J.L. Canine pulmonary angiostrongylosis: An update. Vet. J. 2009, 179, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, M.; Fahrion, A.; Riond, B.; Ossent, P.; Webster, P.; Kranjc, A.; Glaus, T.; Deplazes, P. Clinical, laboratory and pathological findings in dogs experimentally infected with Angiostrongylus vasorum. Parasitol. Res. 2010, 107, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Koike, Y.; Shimura, T.; Okigami, M.; Ide, S.; Toiyama, Y.; Okugawa, Y.; Inoue, Y.; Araki, T.; Uchida, K.; et al. In Vivo Characterization of Neutrophil Extracellular Traps in Various Organs of a Murine Sepsis Model. PLoS ONE 2014, 9, e111888. [Google Scholar] [CrossRef]
- Grob, D.; Conejeros, I.; Velásquez, Z.D.; Preußer, C.; Gärtner, U.; Alarcón, P.; Burgos, R.A.; Hermosilla, C.; Taubert, A. Trypanosoma brucei brucei Induces Polymorphonuclear Neutrophil Activation and Neutrophil Extracellular Traps Release. Front. Immunol. 2020, 11, 559561. [Google Scholar] [CrossRef] [PubMed]
- Imlau, M.; Conejeros, I.; Muñoz-Caro, T.; Zhou, E.; Gärtner, U.; Ternes, K.; Taubert, A.; Hermosilla, C. Dolphin-derived NETosis results in rapid Toxoplasma gondii tachyzoite ensnarement and different phenotypes of NETs. Dev. Comp. Immunol. 2020, 103, 103527. [Google Scholar] [CrossRef] [PubMed]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhöfer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Conejeros, I.; Zhou, E.; Pikhovych, A.; Gärtner, U.; Hermosilla, C.; Kulke, D.; Taubert, A. Dirofilaria immitis Microfilariae and Third-Stage Larvae Induce Canine NETosis Resulting in Different Types of Neutrophil Extracellular Traps. Front. Immunol. 2018, 9, 968. [Google Scholar] [CrossRef]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2010, 7, 75–77. [Google Scholar] [CrossRef]
- Munoz-Caro, T.; Mena Huertas, S.J.; Conejeros, I.; Alarcon, P.; Hidalgo, M.A.; Burgos, R.A.; Hermosilla, C.; Taubert, A. Eimeria bovis-triggered neutrophil extracellular trap formation is CD11b-, ERK 1/2-, p38 MAP kinase- and SOCE-dependent. Vet. Res. 2015, 46, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.L.; Ng, G.; Tablin, F. Lipopolysaccharide-induced neutrophil extracellular trap formation in canine neutrophils is dependent on histone H3 citrullination by peptidylarginine deiminase. Vet. Immunol. Immunopathol. 2017, 193-194, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, X.; Wang, J.; Wang, Y.; Yang, Z.; Fu, Y. The formation of canine neutrophil extracellular traps induced by sodium arsenic in polymorphonuclear neutrophils. Chemosphere 2018, 196, 297–302. [Google Scholar] [CrossRef]
- Jeffery, U.; Kimura, K.; Gray, R.; Lueth, P.; Bellaire, B.; LeVine, D. Dogs cast NETs too: Canine neutrophil extracellular traps in health and immune-mediated hemolytic anemia. Vet. Immunol. Immunopathol. 2015, 168, 262–268. [Google Scholar] [CrossRef]
- Wei, Z.; Hermosilla, C.; Taubert, A.; He, X.; Wang, X.; Gong, P.; Li, J.; Yang, Z.; Zhang, X. Canine Neutrophil Extracellular Traps Release Induced by the Apicomplexan Parasite Neospora caninum in vitro. Front. Immunol. 2016, 7, 436. [Google Scholar] [CrossRef]
- Bonne-Année, S.; Kerepesi, L.A.; Hess, J.A.; Wesolowski, J.; Paumet, F.; Lok, J.B.; Nolan, T.J.; Abraham, D. Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microbes Infect. 2014, 16, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Rubio, R.M.; Silva, L.M.; Magdowski, G.; Gartner, U.; McNeilly, T.N.; Taubert, A.; Hermosilla, C. Leucocyte-derived extracellular trap formation significantly contributes to Haemonchus contortus larval entrapment. Parasites Vectors 2015, 8, 607. [Google Scholar] [CrossRef]
- McCoy, C.J.; Reaves, B.J.; Giguère, S.; Coates, R.; Rada, B.; Wolstenholme, A.J. Human Leukocytes Kill Brugia malayi Microfilariae Independently of DNA-Based Extracellular Trap Release. PLoS Negl. Trop. Dis. 2017, 11, e0005279. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.; Sun, D.; Tuo, W.; Xiao, Z. Bovine neutrophils form extracellular traps in response to the gastrointestinal parasite Ostertagia ostertagi. Sci. Rep. 2018, 8, 17598. [Google Scholar] [CrossRef]
- Maksimov, P.; Hermosilla, C.; Kleinertz, S.; Hirzmann, J.; Taubert, A. Besnoitia besnoiti infections activate primary bovine endothelial cells and promote PMN adhesion and NET formation under physiological flow condition. Parasitol. Res. 2016, 115, 1991–2001. [Google Scholar] [CrossRef]
- Conejeros, I.; Velásquez, Z.D.; Grob, D.; Zhou, E.; Salecker, H.; Hermosilla, C.; Taubert, A. Histone H2A and Bovine Neutrophil Extracellular Traps Induce Damage of Besnoitia besnoiti-Infected Host Endothelial Cells but Fail to Affect Total Parasite Proliferation. Biology 2019, 8, 78. [Google Scholar] [CrossRef]
- Zhou, E.; Conejeros, I.; Gärtner, U.; Mazurek, S.; Hermosilla, C.; Taubert, A. Metabolic requirements of Besnoitia besnoiti tachyzoite-triggered NETosis. Parasitol. Res. 2020, 119, 545–557. [Google Scholar] [CrossRef]
- Glaus, T.; Schnyder, M.; Dennler, M.; Tschuor, F.; Wenger, M.; Sieber-Ruckstuhl, N. Natural infection with Angiostrongylus vasorum: Characterisation of 3 dogs with pulmonary hypertension. Schweiz. Arch. Tierheilkd. 2010, 152, 331–338. [Google Scholar] [CrossRef]
- Chiu, P.S.; Lai, S.C. Matrix metalloproteinase-9 leads to blood–brain barrier leakage in mice with eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis. Acta Trop. 2014, 140, 141–150. [Google Scholar] [CrossRef]
- Mupanomunda, M.; Williams, J.F.; MacKenzie, C.D.; Kaiser, L. Dirofilaria immitis: Heartworm infection alters pulmonary artery endothelial cell behavior. J. Appl. Physiol. 1997, 82, 389–398. [Google Scholar] [CrossRef]
- Kramer, L.; Grandi, G.; Passeri, B.; Gianelli, P.; Genchi, M.; Dzimianski, M.T.; Supakorndej, P.; Mansour, A.M.; Supakorndej, N.; McCall, S.D.; et al. Evaluation of lung pathology in Dirofilaria immitis-experimentally infected dogs treated with doxycycline or a combination of doxycycline and ivermectin before administration of melarsomine dihydrochloride. Vet. Parasitol. 2011, 176, 357–360. [Google Scholar] [CrossRef]
- Lip, G.Y.; Blann, A. von Willebrand factor: A marker of endothelial dysfunction in vascular disorders? Cardiovasc. Res. 1997, 34, 255–265. [Google Scholar] [CrossRef]
- Brott, D.A.; Katein, A.; Thomas, H.; Lawton, M.; Montgomery, R.R.; Richardson, R.J.; Louden, C.S. Evaluation of von Willebrand Factor and von Willebrand Factor Propeptide in Models of Vascular Endothelial Cell Activation, Perturbation, and/or Injury. Toxicol. Pathol. 2014, 42, 672–683. [Google Scholar] [CrossRef]
- Levine, D.N.; Cianciolo, R.E.; Linder, K.E.; Bizikova, P.; Birkenheuer, A.J.; Brooks, M.B.; Salous, A.K.; Nordone, S.K.; Bellinger, D.A.; Marr, H.; et al. Endothelial alterations in a canine model of immune thrombocytopenia. Platelets 2019, 30, 88–97. [Google Scholar] [CrossRef]
- Denis, C.V. Molecular and Cellular Biology of von Willebrand Factor. Int. J. Hematol. 2002, 75, 3–8. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Di Cesare, A.; Traversa, D. Canine angiostrongylosis: Recent advances in diagnosis, prevention, and treatment. Vet. Med. 2014, 5, 181–192. [Google Scholar] [CrossRef]
- Rinaldi, L.; Cortese, L.; Meomartino, L.; Pagano, T.B.; Pepe, P.; Cringoli, G.; Papparella, S. Angiostrongylus vasorum: Epidemiological, clinical and histopathological insights. BMC Vet. Res. 2014, 10, 236. [Google Scholar] [CrossRef]
- Barçante, J.M.; Barçante, T.A.; Dias, S.R.; Vieira, L.Q.; Lima, W.S.; Negrão-Corrêa, D. A method to obtain axenic Angiostrongylus vasorum first-stage larvae from dog feces. Parasitol. Res. 2003, 89, 89–93. [Google Scholar] [CrossRef]
- Bourque, A.C.; Conboy, G.; Miller, L.M.; Whitney, H. Pathological Findings in Dogs Naturally Infected with Angiostrongylus Vasorum in Newfoundland and Labrador, Canada. J. Vet. Diagn. Investig. 2008, 20, 11–20. [Google Scholar] [CrossRef]
- Neumann, A.; Brogden, G.; Von Köckritz-Blickwede, M. Extracellular Traps: An Ancient Weapon of Multiple Kingdoms. Biology 2020, 9, 34. [Google Scholar] [CrossRef]
- Zhou, E.; Conejeros, I.; Velásquez, Z.D.; Muñoz-Caro, T.; Gärtner, U.; Hermosilla, C.; Taubert, A. Simultaneous and Positively Correlated NET Formation and Autophagy in Besnoitia besnoiti Tachyzoite-Exposed Bovine Polymorphonuclear Neutrophils. Front. Immunol. 2019, 10, 1131. [Google Scholar] [CrossRef]
- Hahn, J.; Schauer, C.; Czegley, C.; Kling, L.; Petru, L.; Schmid, B.; Weidner, D.; Reinwald, C.; Biermann, M.H.C.; Blunder, S.; et al. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J. 2019, 33, 1401–1414. [Google Scholar] [CrossRef]
- Knopf, J.; Leppkes, M.; Schett, G.; Herrmann, M.; Muñoz, L.E. Aggregated NETs Sequester and Detoxify Extracellular Histones. Front. Immunol. 2019, 10, 2176. [Google Scholar] [CrossRef]
- Mahajan, A.; Grüneboom, A.; Petru, L.; Podolska, M.J.; Kling, L.; Maueröder, C.; Dahms, F.; Christiansen, S.; Günter, L.; Krenn, V.; et al. Frontline Science: Aggregated neutrophil extracellular traps prevent inflammation on the neutrophil-rich ocular surface. J. Leukoc. Biol. 2019, 105, 1087–1098. [Google Scholar] [CrossRef]
- Peixoto, R.; Silva, L.M.R.; López-Osório, S.; Zhou, E.; Gärtner, U.; Conejeros, I.; Taubert, A.; Hermosilla, C. Fasciola hepatica induces weak NETosis and low production of intra- and extracellular ROS in exposed bovine polymorphonuclear neutrophils. Dev. Comp. Immunol. 2021, 114, 103787. [Google Scholar] [CrossRef]
- Van Breda, S.V.; Vokalova, L.; Neugebauer, C.; Rossi, S.W.; Hahn, S.; Hasler, P. Computational Methodologies for the in vitro and in situ Quantification of Neutrophil Extracellular Traps. Front. Immunol. 2019, 10, 1562. [Google Scholar] [CrossRef] [PubMed]
- Rebernick, R.; Fahmy, L.; Glover, C.; Bawadekar, M.; Shim, D.; Holmes, C.L.; Rademacher, N.; Potluri, H.; Bartels, C.M.; Shelef, M.A. DNA Area and NETosis Analysis (DANA): A High-Throughput Method to Quantify Neutrophil Extracellular Traps in Fluorescent Microscope Images. Biol. Proced. Online 2018, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.J.; Wang, L.; Meng, X.L.; Zhang, S.H.; Sheng, Z.A.; Wei, Z.K.; Luo, X.N.; Huang, W.Y.; Zhu, X.Q.; Zhang, X.C.; et al. Newly excysted juveniles of Fasciola gigantica trigger the release of water buffalo neutrophil extracellular traps in vitro. Exp. Parasitol. 2020, 211, 107828. [Google Scholar] [CrossRef] [PubMed]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef]
- Silva, L.M.; Muñoz-Caro, T.; Burgos, R.A.; Hidalgo, M.A.; Taubert, A.; Hermosilla, C. Far beyond Phagocytosis: Phagocyte-Derived Extracellular Traps Act Efficiently against Protozoan Parasites in vitro and In Vivo. Mediat. Inflamm. 2016, 2016, 5898074. [Google Scholar] [CrossRef]
- Deckert-Schlüter, M.; Schlüter, D.; Hof, H.; Wiestler, O.D.; Lassmann, H. Differential Expression of ICAM-1, VCAM-1 and Their Ligands LFA-1, Mac-1, CD43, VLA-4, and MHC Class II Antigens in MurineToxoplasmaEncephalitis: A Light Microscopic and Ultrastructural Immunohistochemical Study. J. Neuropathol. Exp. Neurol. 1994, 53, 457–468. [Google Scholar] [CrossRef]
- Hermosilla, C.; Ruiz, A.; Taubert, A. Eimeria bovis: An update on parasite–host cell interactions. Int. J. Med. Microbiol. 2012, 302, 210–215. [Google Scholar] [CrossRef]
- Raza, A.; Ghanchi, N.K.; Sarwar Zubairi, A.; Raheem, A.; Nizami, S.; Beg, M.A. Tumor Necrosis Factor -α, Interleukin-10, Intercellular and Vascular Adhesion Molecules Are Possible Biomarkers of Disease Severity in Complicated Plasmodium vivax Isolates from Pakistan. PLoS ONE 2013, 8, e81363. [Google Scholar] [CrossRef]
- DiStasi, M.R.; Ley, K. Opening the flood-gates: How neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009, 30, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Löf, A.; Müller, J.P.; Brehm, M.A. A biophysical view on von Willebrand factor activation. J. Cell. Physiol. 2018, 233, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, Z.; Long, Q.; Huang, J.; Hong, T.; Liu, W.; Lin, J. Insights into Immunothrombosis: The Interplay among Neutrophil Extracellular Trap, von Willebrand Factor, and ADAMTS13. Front. Immunol. 2020, 11, 610696. [Google Scholar] [CrossRef]
- Whitley, N.T.; Corzo-Menendez, N.; Carmichael, N.G.; McGarry, J.W. Cerebral and conjunctival haemorrhages associated with von Willebrand factor deficiency and canine angiostrongylosis. J. Small Anim. Pr. 2005, 46, 75–78. [Google Scholar] [CrossRef]
- Pillai, V.G.; Bao, J.; Zander, C.B.; McDaniel, J.K.; Chetty, P.S.; Seeholzer, S.H.; Bdeir, K.; Cines, D.B. Human neutrophil peptides inhibit cleavage of von Willebrand factor by ADAMTS13: A potential link of inflammation to TTP. Blood 2016, 128, 110–119. [Google Scholar] [CrossRef]
- Grässle, S.; Huck, V.; Pappelbaum, K.I.; Gorzelanny, C.; Aponte-Santamaría, C.; Baldauf, C.; Gräter, F.; Schneppenheim, R.; Obser, T.; Schneider, S.W. von Willebrand Factor Directly Interacts With DNA From Neutrophil Extracellular Traps. Arter. Thromb. Vasc. Biol. 2014, 34, 1382–1389. [Google Scholar] [CrossRef]
- Thålin, C.; Hisada, Y.; Lundström, S.; Mackman, N.; Wallén, H. Neutrophil Extracellular Traps: Villains and Targets in Arterial, Venous, and Cancer-Associated Thrombosis. Arter. Thromb. Vasc. Biol. 2019, 39, 1724–1738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Halvorsen, K.; Zhang, C.Z.; Wong, W.P.; Springer, T.A. Mechanoenzymatic Cleavage of the Ultralarge Vascular Protein von Willebrand Factor. Science 2009, 324, 1330–1334. [Google Scholar] [CrossRef]

| Primers | ||
|---|---|---|
| Target | Forward/Reverse | Tm |
| Canis lupus E-selectin | 5′-TGGCTTCAGAGGTCTCAGGT-3′ 5′-TCAAAGCACTGCACTCAACC-3′ | 60 °C |
| Canis lupus P-selectin | 5′-CAAAAAGCCTCTCACCGAAG-3′ 5′-ATGCATTCTCCTTGCTTGCT-3′ | 60 °C |
| Canis lupus ICAM-1 | 5′-CAGGGTTGCCAGGTACAGTT-3′ 5′-AGTATGGGCTCAGTGGGTTG-3′ | 60 °C |
| Canis lupus VCAM-1 | 5′-TCCATCGTGGAGGAAGGTAG-3′ 5′-CAGCCTGGTTAATCCCTTCA-3′ | 60 °C |
| Canis lupus RPL32 | 5′-CCTCAGACCTCTGGTGAAGC-3′ 5′-TCAAGCTCCTTGACGTTGTG-3′ | 60 °C |
| Canis lupus RPS19 | 5′-TGTCAAGGCTACCTCGGAGT-3′ 5′-GCCTTCAGCCTCCTTCTTCT-3′ | 60 °C |
| Canis lupus HPRT | 5′-AAGCTTGCTGGTGAAAAGGA-3′ 5′-CAATGGGACTCCAGATGCTT-3′ | 60 °C |
| Probes | ||
| Canis lupus E-selectin | 5′-TTTGTCAGCTGTGACAAGGG-3′ | 60 °C |
| Canis lupus P-selectin | 5′-GCTATACAGCCTCCTGCCAG-3′ | 60 °C |
| Canis lupus ICAM-1 | 5′-CATTGGCTAAGCTGCTTTCC-3′ | 60 °C |
| Canis lupus VCAM-1 | 5′-GAGCAGGCGGCTAAGTAATG-3′ | 60 °C |
| Canis lupus RPL32 | 5′-GGCACCAGTCAGACCGATAT-3′ | 60 °C |
| Canis lupus RPS19 | 5′-CAGTCACCCAGCAGATTGTG-3′ | 60 °C |
| Canis lupus HPRT | 5′-CCCCTCGAAGTGTTGGCTAT-3′ | 60 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grob, D.; Conejeros, I.; López-Osorio, S.; Velásquez, Z.D.; Segeritz, L.; Gärtner, U.; Schaper, R.; Hermosilla, C.; Taubert, A. Canine Angiostrongylus vasorum-Induced Early Innate Immune Reactions Based on NETs Formation and Canine Vascular Endothelial Cell Activation In Vitro. Biology 2021, 10, 427. https://doi.org/10.3390/biology10050427
Grob D, Conejeros I, López-Osorio S, Velásquez ZD, Segeritz L, Gärtner U, Schaper R, Hermosilla C, Taubert A. Canine Angiostrongylus vasorum-Induced Early Innate Immune Reactions Based on NETs Formation and Canine Vascular Endothelial Cell Activation In Vitro. Biology. 2021; 10(5):427. https://doi.org/10.3390/biology10050427
Chicago/Turabian StyleGrob, Daniela, Iván Conejeros, Sara López-Osorio, Zahady D. Velásquez, Lisa Segeritz, Ulrich Gärtner, Roland Schaper, Carlos Hermosilla, and Anja Taubert. 2021. "Canine Angiostrongylus vasorum-Induced Early Innate Immune Reactions Based on NETs Formation and Canine Vascular Endothelial Cell Activation In Vitro" Biology 10, no. 5: 427. https://doi.org/10.3390/biology10050427
APA StyleGrob, D., Conejeros, I., López-Osorio, S., Velásquez, Z. D., Segeritz, L., Gärtner, U., Schaper, R., Hermosilla, C., & Taubert, A. (2021). Canine Angiostrongylus vasorum-Induced Early Innate Immune Reactions Based on NETs Formation and Canine Vascular Endothelial Cell Activation In Vitro. Biology, 10(5), 427. https://doi.org/10.3390/biology10050427







