The Role of Serum 5-HIAA as a Predictor of Progression and an Alternative to 24-h Urine 5-HIAA in Well-Differentiated Neuroendocrine Neoplasms
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Disease Classification
2.3. 5-HIAA and CT/MRI-Surveillance Protocol
2.4. Comparison Analysis of 5HIAA and CT/MRI
2.5. Laboratory Specifications for Serum and 24-h Urine 5HIAA Measurement
2.6. Statistics
3. Results
3.1. Baseline Characteristics
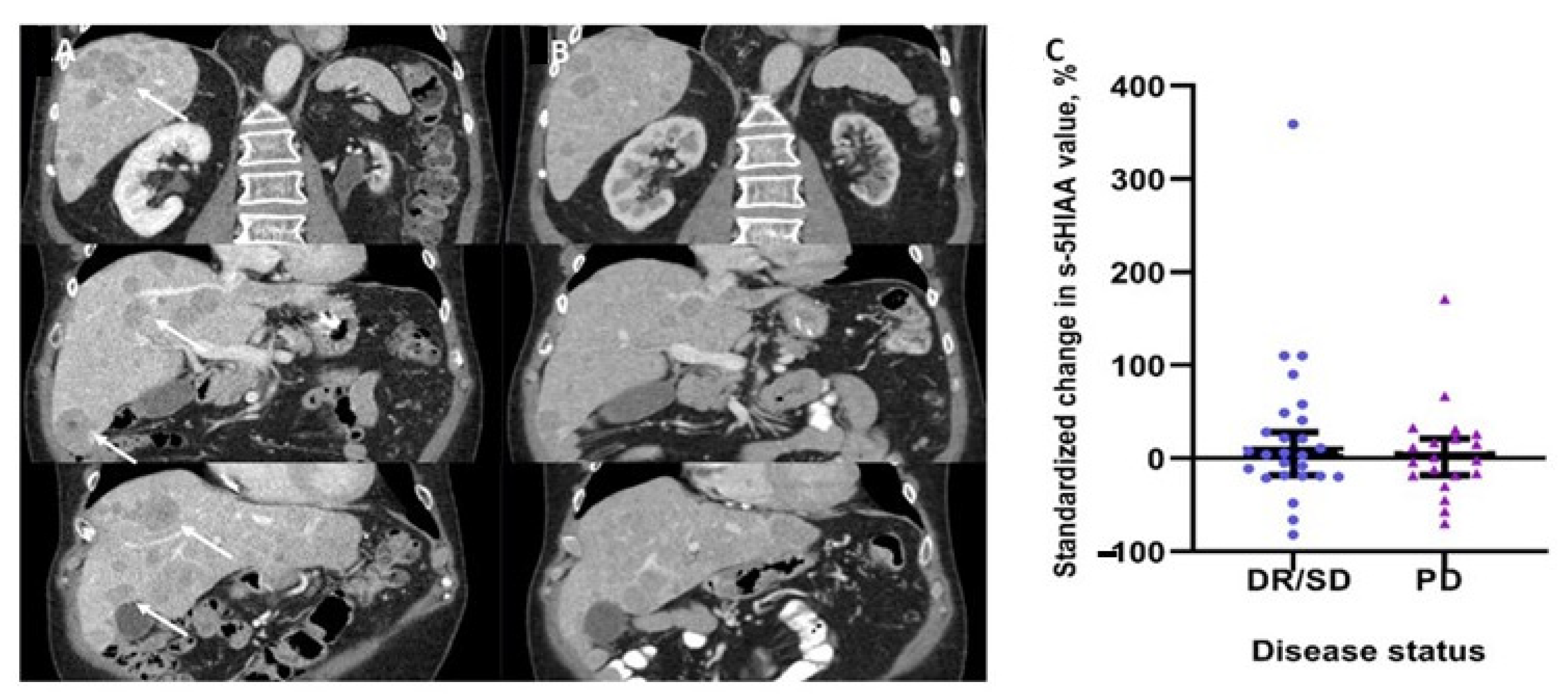
3.2. Changes in Serum 5HIAA Levels and Disease Status
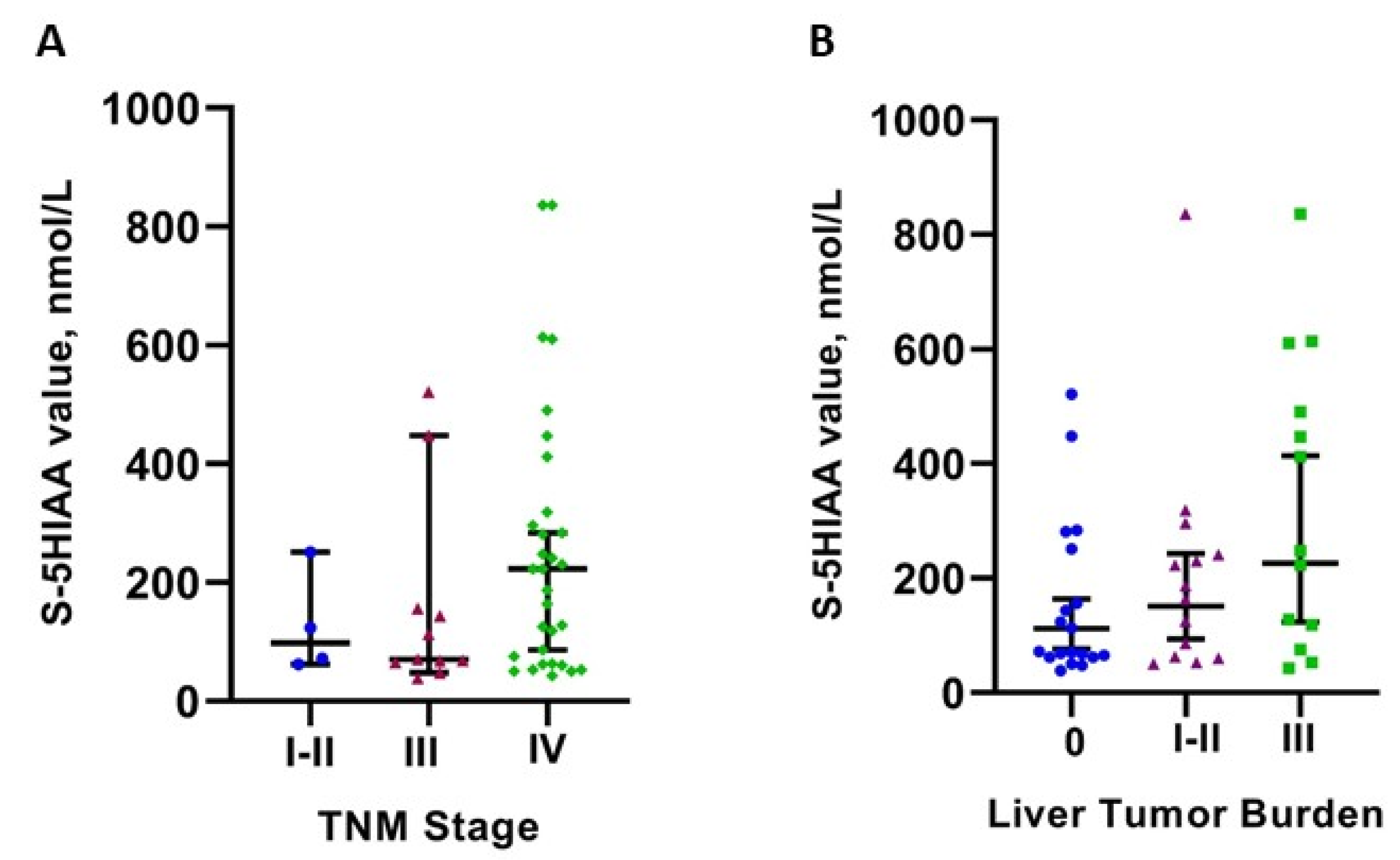
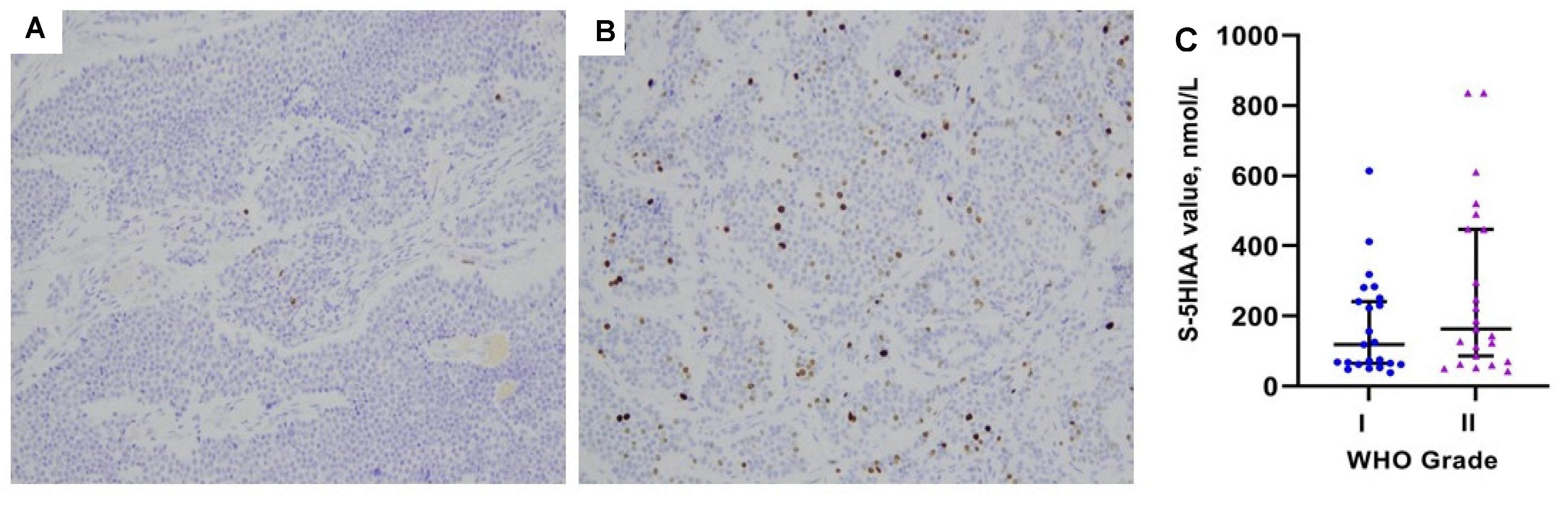
3.3. Serum 5HIAA Levels in Relation to Patient- and Tumor-Related Parameters
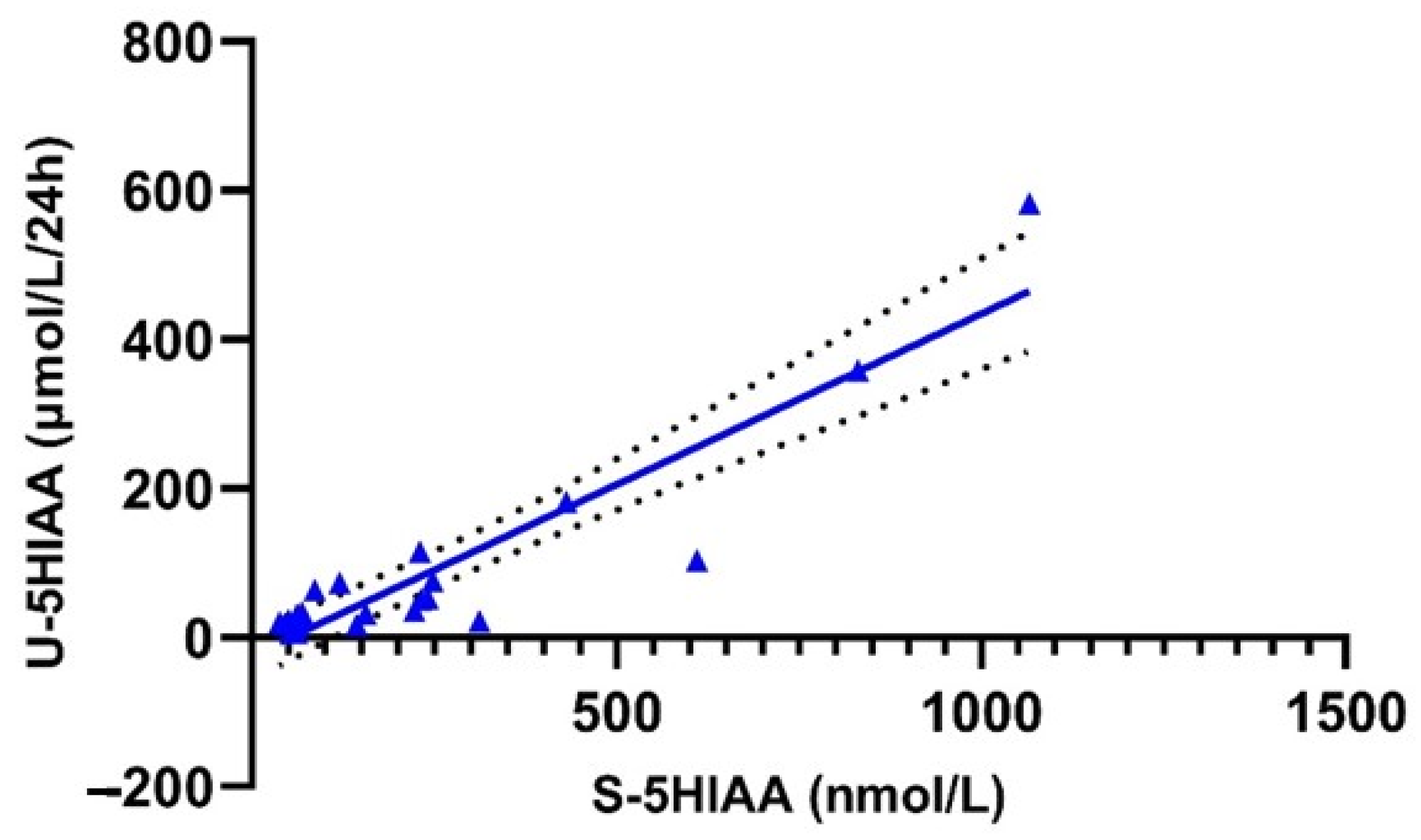
3.4. Correlation and Concordance of Urinary and Serum 5HIAA Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Kaderli, R.M.; Spanjol, M.; Kollar, A.; Butikofer, L.; Gloy, V.; Dumont, R.A.; Seiler, C.A.; Christ, E.R.; Radojewski, P.; Briel, M.; et al. Therapeutic Options for Neuroendocrine Tumors: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2019, 5, 480–489. [Google Scholar] [CrossRef]
- Van der Zwan, J.M.; Trama, A.; Otter, R.; Larranaga, N.; Tavilla, A.; Marcos-Gragera, R.; Dei Tos, A.P.; Baudin, E.; Poston, G.; Links, T.; et al. Rare neuroendocrine tumours: Results of the surveillance of rare cancers in Europe project. Eur. J. Cancer 2013, 49, 2565–2578. [Google Scholar] [CrossRef]
- Pavel, M.; Oberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- De Herder, W.W. Biochemistry of neuroendocrine tumours. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 33–41. [Google Scholar] [CrossRef]
- Tsoukalas, N.; Chatzellis, E.; Rontogianni, D.; Alexandraki, K.I.; Boutzios, G.; Angelousi, A.; Kaltsas, G. Pancreatic carcinoids (serotonin-producing pancreatic neuroendocrine neoplasms): Report of 5 cases and review of the literature. Medicine 2017, 96, e6201. [Google Scholar] [CrossRef]
- Milanetto, A.C.; Fassan, M.; David, A.; Pasquali, C. Serotonin-Secreting Neuroendocrine Tumours of the Pancreas. J. Clin. Med. 2020, 9, 1363. [Google Scholar] [CrossRef]
- Arnold, R.; Wilke, A.; Rinke, A.; Mayer, C.; Kann, P.H.; Klose, K.J.; Scherag, A.; Hahmann, M.; Muller, H.H.; Barth, P. Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin. Gastroenterol. Hepatol. 2008, 6, 820–827. [Google Scholar] [CrossRef]
- Nolting, S.; Kuttner, A.; Lauseker, M.; Vogeser, M.; Haug, A.; Herrmann, K.A.; Hoffmann, J.N.; Spitzweg, C.; Goke, B.; Auernhammer, C.J. Chromogranin a as serum marker for gastroenteropancreatic neuroendocrine tumors: A single center experience and literature review. Cancers 2012, 4, 141–155. [Google Scholar] [CrossRef]
- Dam, G.; Gronbaek, H.; Sorbye, H.; Thiis Evensen, E.; Paulsson, B.; Sundin, A.; Jensen, C.; Ebbesen, D.; Knigge, U.; Tiensuu Janson, E. Prospective Study of Chromogranin A as a Predictor of Progression in Patients with Pancreatic, Small-Intestinal, and Unknown Primary Neuroendocrine Tumors. Neuroendocrinology 2020, 110, 217–224. [Google Scholar] [CrossRef]
- Modlin, I.M.; Oberg, K.; Taylor, A.; Drozdov, I.; Bodei, L.; Kidd, M. Neuroendocrine tumor biomarkers: Current status and perspectives. Neuroendocrinology 2014, 100, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Gedde-Dahl, M.; Thiis-Evensen, E.; Tjolsen, A.M.; Mordal, K.S.; Vatn, M.; Bergestuen, D.S. Comparison of 24-h and overnight samples of urinary 5-hydroxyindoleacetic acid in patients with intestinal neuroendocrine tumors. Endocr. Connect. 2013, 2, 50–54. [Google Scholar] [CrossRef]
- Adaway, J.E.; Dobson, R.; Walsh, J.; Cuthbertson, D.J.; Monaghan, P.J.; Trainer, P.J.; Valle, J.W.; Keevil, B.G. Serum and plasma 5-hydroxyindoleacetic acid as an alternative to 24-h urine 5-hydroxyindoleacetic acid measurement. Ann. Clin. Biochem. 2016, 53, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, M.; Tohmola, N.; Renkonen, R.; Hamalainen, E.; Schalin-Jantti, C.; Itkonen, O. Comparison of serum serotonin and serum 5-HIAA LC-MS/MS assays in the diagnosis of serotonin producing neuroendocrine neoplasms: A pilot study. Clin. Chim. Acta 2018, 482, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Tohmola, N.; Itkonen, O.; Sane, T.; Markkanen, H.; Joenvaara, S.; Renkonen, R.; Hamalainen, E. Analytical and preanalytical validation of a new mass spectrometric serum 5-hydroxyindoleacetic acid assay as neuroendocrine tumor marker. Clin. Chim. Acta 2014, 428, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Klöppel, G. WHO Classification of Tumours of Endocrine Organs; IARC: Lyon, France, 2017.
- Travis, W.D.; Marx, A.; Nicolson, A.G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2015.
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Gress, D.; Greene, F.; Washington, M.; Asare, E.; Brierley, J. Principles of Cancer Staging. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Niederle, B.; Pape, U.F.; Costa, F.; Gross, D.; Kelestimur, F.; Knigge, U.; Oberg, K.; Pavel, M.; Perren, A.; Toumpanakis, C.; et al. ENETS Consensus Guidelines Update for Neuroendocrine Neoplasms of the Jejunum and Ileum. Neuroendocrinology 2016, 103, 125–138. [Google Scholar] [CrossRef]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Tirosh, A.; Nilubol, N.; Patel, D.; Kebebew, E. Prognostic Utility of 24-Hour Urinary 5-HIAA Doubling Time in Patients With Neuroendocrine Tumors. Endocr. Pract. 2018, 24, 710–717. [Google Scholar] [CrossRef]
- Laskaratos, F.M.; Diamantopoulos, L.; Walker, M.; Walton, H.; Khalifa, M.; El-Khouly, F.; Koffas, A.; Demetriou, G.; Caplin, M.; Toumpanakis, C.; et al. Prognostic Factors for Survival among Patients with Small Bowel Neuroendocrine Tumours Associated with Mesenteric Desmoplasia. Neuroendocrinology 2018, 106, 366–380. [Google Scholar] [CrossRef]
| Characteristics | N = 48 | % |
|---|---|---|
| Gender | ||
| Male | 23 | 47.9 |
| Female | 25 | 52.1 |
| Age, years (median, range) | 71 (45–89) | - |
| Time since Initial Diagnosis, Months (Median, Range) | 36 (0–288) | - |
| Inheritance | ||
| Sporadic | 42 | 87.5 |
| Familial | 6 | 12.5 |
| Primary Tumor Site | ||
| Small Intestine | 37 | 77.1 |
| Pancreas | 8 | 16.7 |
| Lung | 1 | 2.1 |
| UPO | 2 | 4.2 |
| Secretory Status | ||
| Functioning | 20 | 41.7 |
| Non-Functioning | 28 | 58.3 |
| Grading | ||
| G1 (Ki67 < 3%) | 23 | 47.9 |
| G2 (Ki67 3–20%) | 19 | 39.6 |
| G3 (Ki67 > 20%) | 0 | 0 |
| Unknown | 6 | 12.5 |
| Liver Metastases | ||
| Yes | 29 | 60.4 |
| No | 19 | 39.6 |
| Liver Tumor Burden | ||
| Type 0 (No Liver Metastases) | 11 | 22.9 |
| Type 1 (<5 Metastases Confined in 1 lobe) | 12 | 25 |
| Type 2 (Bilobar and/or 5–10 Metastases) | 2 | 4.2 |
| Type 3 (>10 Metastases or Diffuse Metastatic Disease) | 15 | 31.3 |
| Extrahepatic Metastases (Distant) | ||
| Yes | 6 | 12.5 |
| No | 42 | 87.5 |
| Peritoneal Carcinomatosis | 5 | |
| Yes | 8 | 16.7 |
| No | 40 | 83.3 |
| Staging | ||
| Stage I | 3 | 2.1 |
| Stage II | 11 | 6.3 |
| Stage III | 33 | 22.9 |
| Stage IV | 68.8 | |
| Prior Surgery | ||
| Yes | 43 | 89.6 |
| No | 5 | 10.4 |
| Concomitant SSA Treatment | ||
| Yes | 28 | 58.3 |
| No | 20 | 41.7 |
| Other Systemic Treatments (IF-a, Chemotherapy. PRRT, MTT) | ||
| Yes | 11 | 22.9 |
| No | 37 | 77.1 |
| Charlson Comorbidity Index | ||
| 0 | 15 | 31.3 |
| 1 | 7 | 14.6 |
| 2 | 4 | 8.3 |
| 3 | 5 | 10.4 |
| ≥4 | 10 | 20.8 |
| Serum 5HIAA levels, nmol/L | ||
| ≤123 | 20 | 41.7 |
| 123–250 | 12 | 25 |
| 250–500 | 9 | 18.8 |
| >500 | 7 | 14.6 |
| Urinary 5HIAA levels, μmol/L/24 h | ||
| ≤50 | 12 | 25 |
| 50–200 | 9 | 18.8 |
| 200–500 | 1 | 2.1 |
| >500 | 2 | 4.1 |
| not available concurrently to serum sampling | 24 | 50 |
| Characteristics | s-5HIAA Value (Median with Range; nmol/L) | p-Value * |
|---|---|---|
| Gender | 0.747 | |
| Male | 144 (43–836) | |
| Female | 156 (39–3199) | |
| Age, Years | 0.709 | |
| Group1: 45–71 years | 124 (39–3199) | |
| Group 2: 71–89 years | 164 (43–836) | |
| Inheritance | 0.909 | |
| Sporadic | 160 (39–3199) | |
| Familial | 99.5 (50–836) | |
| Primary Tumor Site | 0.261 | |
| Small Intestine | 164 (39–3199) | |
| Other (Pancreas, Lung, UPO) | 124 (43–490) | |
| Secretory Status | 0.711 | |
| Functioning | 164 (60–3199) | |
| Non-Functioning | 128 (39–836) | |
| WHO Grading | 0.174 | |
| G1 (Ki67 < 3%) | 119 (39–614) | |
| G2 (Ki67 3–20%) | 164 (43–3199) | |
| Liver Metastases | 0.045 | |
| Yes | 223 (43–3199) | |
| No | 72 (39–521) | |
| Liver Tumor Burden Classification | 0.041 | |
| No Liver Metastases | 72 (39–521) | |
| Type 1 and 2 | 175.5 (50–836) | |
| Type 3 (>10 Metastases or Diffuse Metastatic disease). | 412 (43–3199) | |
| Extrahepatic Metastases (Distant) | 0.599 | |
| Yes | 232 (48–2076) | |
| No | 134.5 (39–3199) | |
| Peritoneal Carcinomatosis | 0.394 | |
| Yes | 255.5 (50–836) | |
| No | 126.5 (39–3199) | |
| TNM Staging | 0.398 | |
| stage I-II | 98 (62–251) | |
| stage III | 113 (48–521) | |
| stage IV | 223 (43–3199) | |
| Prior Surgery | 0.193 | |
| Yes | 144 39–3199) | |
| No | 272 (128–614) | |
| Concomitant SSA Treatment | 0.332 | |
| Yes | 118 (39–2076) | |
| No | 175 (48–3199) | |
| Other Systemic Treatments (IF-a, Chemotherapy. PRRT, MTT) | 0.031 | |
| Yes | 296 (50–3199) | |
| No | 124 (39–2076) | |
| Charlson Comorbidity Index | 0.888 | |
| 0–2 | 160 (39–836) | |
| ≥3 | 187 (8–3199) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wedin, M.; Mehta, S.; Angerås-Kraftling, J.; Wallin, G.; Daskalakis, K. The Role of Serum 5-HIAA as a Predictor of Progression and an Alternative to 24-h Urine 5-HIAA in Well-Differentiated Neuroendocrine Neoplasms. Biology 2021, 10, 76. https://doi.org/10.3390/biology10020076
Wedin M, Mehta S, Angerås-Kraftling J, Wallin G, Daskalakis K. The Role of Serum 5-HIAA as a Predictor of Progression and an Alternative to 24-h Urine 5-HIAA in Well-Differentiated Neuroendocrine Neoplasms. Biology. 2021; 10(2):76. https://doi.org/10.3390/biology10020076
Chicago/Turabian StyleWedin, Maria, Sagar Mehta, Jenny Angerås-Kraftling, Göran Wallin, and Kosmas Daskalakis. 2021. "The Role of Serum 5-HIAA as a Predictor of Progression and an Alternative to 24-h Urine 5-HIAA in Well-Differentiated Neuroendocrine Neoplasms" Biology 10, no. 2: 76. https://doi.org/10.3390/biology10020076
APA StyleWedin, M., Mehta, S., Angerås-Kraftling, J., Wallin, G., & Daskalakis, K. (2021). The Role of Serum 5-HIAA as a Predictor of Progression and an Alternative to 24-h Urine 5-HIAA in Well-Differentiated Neuroendocrine Neoplasms. Biology, 10(2), 76. https://doi.org/10.3390/biology10020076





