Three-Dimensional Transesophageal Echocardiography as an Alternative to Multidetector Computed Tomography in Aortic Annular Diameter Measurements for Transcatheter Aortic Valve Implantation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. TAVI: Procedure, Prosthetic Valves, and Aortic Annular Sizing
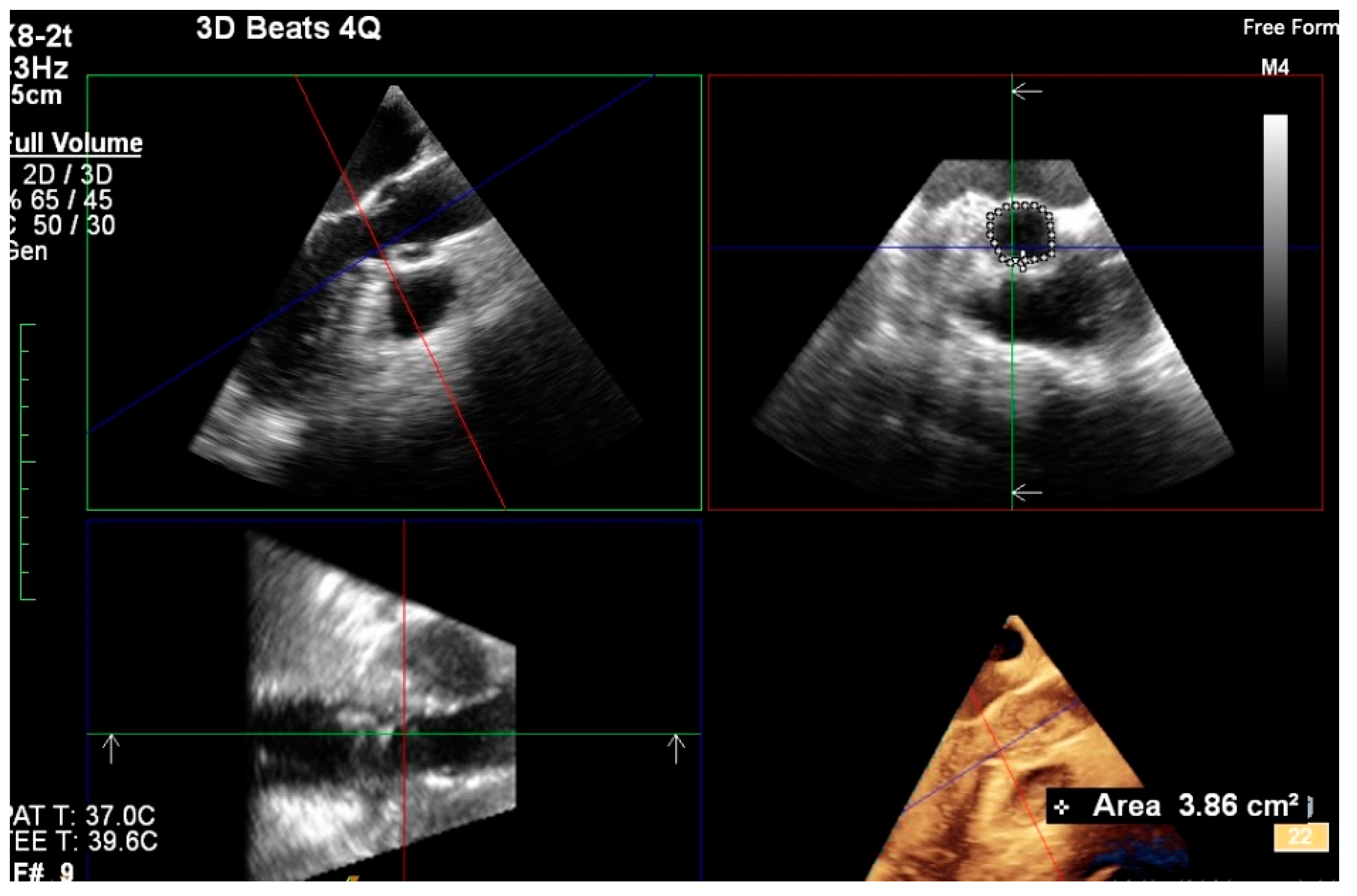
2.3. 3D TEE
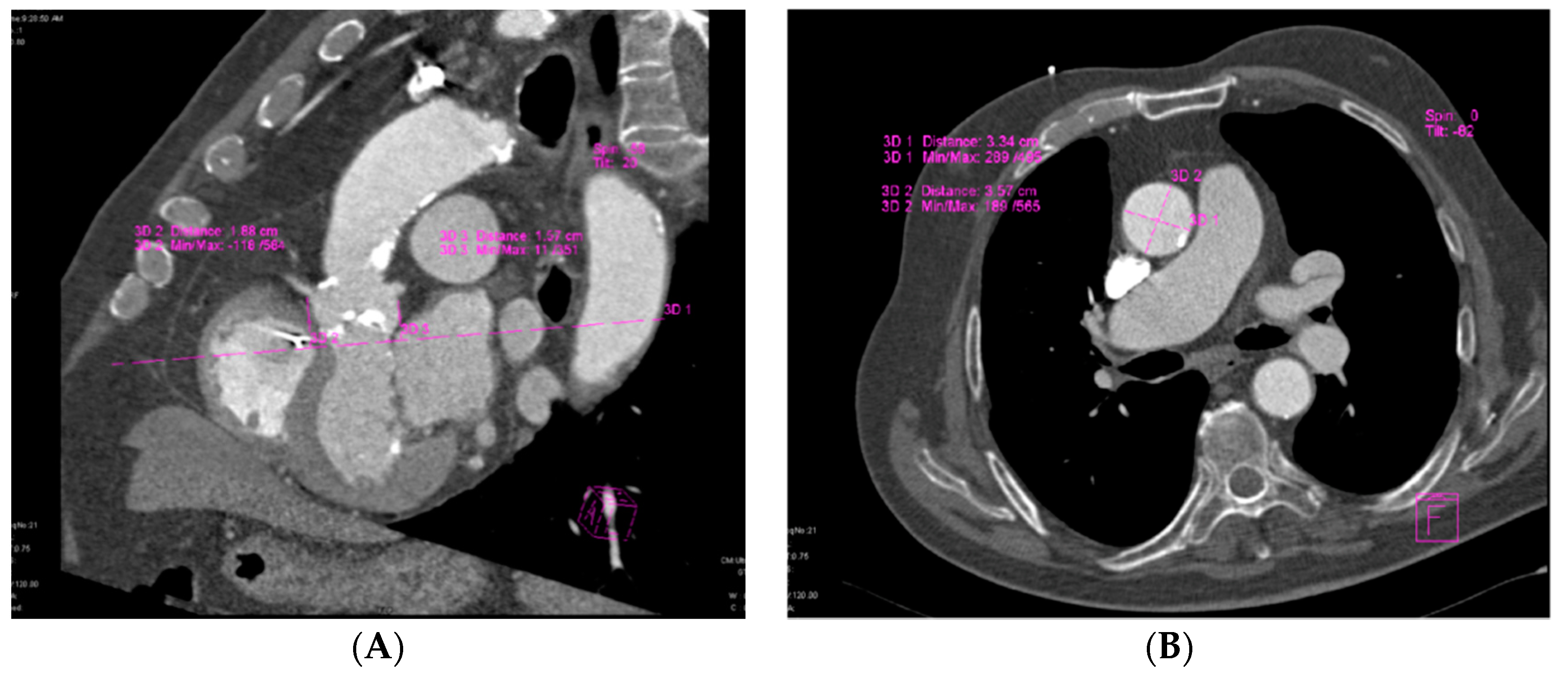
2.4. MDCT
2.5. Statistical Analysis
3. Results
3.1. Population
3.2. Measurements
3.3. Agreement of MDCT and 3D TEE Aortic Annular Diameter Measurements
3.4. Implantation of Prosthetic Valves
4. Discussion
Complications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hutter, A.; Opitz, A.; Bleiziffer, S.; Ruge, H.; Hettich, I.; Mazzitelli, D.; Will, A.; Tassani, P.; Bauernschmitt, R.; Lange, R. Aortic Annulus Evaluation in Transcatheter Aortic Valve Implantation. Catheter. Cardiovasc. Interv. 2010, 76, 1009–1019. [Google Scholar] [CrossRef]
- Chin, D. Echocardiography for transcatheter aortic valve implantation. Eur. J. Echocardiogr. 2009, 10, i21–i29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jilaihawi, H.; Kashif, M.; Fontana, G.; Furugen, A.; Shiota, T.; Friede, G.; Makhija, R.; Doctor, N.; Leon, M.B.; Makkar, R.R. Cross-Sectional Computed Tomographic Assessment Improves Accuracy of Aortic Annular Sizing for Transcatheter Aortic Valve Replacement and Reduces the Incidence of Paravalvular Aortic Regurgitation. J. Am. Coll. Cardiol. 2012, 59, 1275–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, L.Q.; Hameed, I.; Salemi, A.; Rahouma, M.; Khan, F.M.; Wijeysundera, H.C.; Angiolillo, D.J.; Shore-Lesserson, L.; Biondi-Zoccai, G.; Girardi, L.N.; et al. Three-Dimensional Echocardiography for Transcatheter Aortic Valve Replacement Sizing: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e013463. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, G.; Fusini, L.; Muratori, M.; Cefalu, C.; Gripari, P.; Ali, S.G.; Pontone, G.; Andreini, D.; Bartorelli, A.L.; Alamanni, F.; et al. Feasibility and accuracy of three-dimensional transthoracic echocardiography vs. multidetector computed tomography in the evaluation of aortic valve annulus in patient candidates to transcatheter aortic valve implantation. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1316–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, S.A.; Evangelista, A.; Abbara, S.; Arai, A.; Asch, F.M.; Badano, L.P.; Bolen, M.A.; Connolly, H.M.; Cuellar-Calabria, H.; Czerny, M.; et al. Multimodality Imaging of Diseases of the Thoracic Aorta in Adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 119–182. [Google Scholar] [CrossRef]
- Vaquerizo, B.; Spaziano, M.; Alali, J.; Mylote, D.; Theriault-Lauzier, P.; Alfagih, R.; Martucci, G.; Buithieu, J.; Piazza, N. Three-dimensional echocardiography vs. computed tomography for transcatheter aortic valve replacement sizing. Eur. Heart J. Cardiovasc. Imaging 2015, 17, 15–23. [Google Scholar] [CrossRef]
- Prihadi, E.A.; van Rosendael, P.J.; Vollema, E.M.; Bax, J.J.; Delgado, V.; Marsan, N.A. Feasibility, Accuracy, and Reproducibility of Aortic Annular and Root Sizing for Transcatheter Aortic Valve Replacement Using Novel Automated Three-Dimensional Echocardiographic Software: Comparison with Multi–Detector Row Computed Tomography. J. Am. Soc. Echocardiogr. 2018, 31, 505–514. [Google Scholar] [CrossRef]
- Maeno, Y.; Yoon, S.H.; Abramowitz, Y.; Watanabe, Y.; Jilaihawi, H.; Lin, M.S.; Chan, J.; Sharma, R.; Kawashima, H.; Israr, S.; et al. Effect of ascending aortic dimension on acute procedural success following self-expanding transcatheter aortic valve replacement. Int. J. Cardiol. 2017, 244, 100–105. [Google Scholar] [CrossRef]
- Alkhouli, M.; Sengupta, P.; Badhwar, V. Toward Precision in Balloon-Expandable TAVR. JACC: Cardiovascular Interventions. JACC Cardiovasc. Interv. 2017, 10, 821–823. [Google Scholar] [CrossRef]
- Dvir, D.; Webb, J.G.; Piazza, N.; Blanke, P.; Barbanti, M.; Bleiziffer, S.; Wood, D.A.; Mylotte, D.; Wilson, A.B.; Tan, J. Multicenter evaluation of transcatheter aortic valve replacement using either SAPIEN XT or CoreValve: Degree of device oversizing by computed-tomography and clinical outcomes. Catheter. Cardiovasc. Interv. 2015, 86, 508–515. [Google Scholar] [CrossRef]
- Biasco, L.; Ferrari, E.; Pedrazzini, G.; Faletra, F.; Moccetti, T.; Petracca, F.; Moccetti, M. Access Sites for TAVI: Patient Selection Criteria, Technical Aspects, and Outcomes. Front. Cardiovasc. Med. 2018, 5, 88. [Google Scholar] [CrossRef]
- Achenbach, S.; Delgado, V.; Hausleiter, J.; Schoenhagen, P.; Min, J.K.; Leipsic, J.A. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J. Cardiovasc. Comput. Tomogr. 2012, 6, 366–380. [Google Scholar] [CrossRef]
- Hoinoiu, B.; Jiga, L.P.; Nistor, A.; Dornean, V.; Barac, S.; Miclaus, G.; Ionac, M.; Hoinoiu, T. Chronic Hindlimb Ischemia Assessment; Quantitative Evaluation Using Laser Doppler in a Rodent Model of Surgically Induced Peripheral Arterial Occlusion. Diagnostics 2019, 9, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwan, M.; Achenbach, S. Role of Cardiac CT before Transcatheter Aortic Valve Implantation (TAVI). Curr. Cardiol. Rep. 2016, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Dankerl, P.; Hammon, M.; Seuss, H.; Tröbs, M.; Schuhbaeck, A.; Hell, M.M.; Cavallaro, A.; Achenbach, S.; Uder, M.; Marwan, M. Computer-aided evaluation of low-dose and low-contrast agent third-generation dual-source CT angiography prior to transcatheter aortic valve implantation (TAVI). Int. J. Comput. Assist. Radiol. Surg. 2016, 12, 795–802. [Google Scholar] [CrossRef]
- Bittner, D.O.; Arnold, M.; Klinghammer, L.; Schuhbaeck, A.; Hell, M.M.; Muschiol, G.; Gauss, S.; Lell, M.; Uder, M.; Hoffmann, U.; et al. Contrast volume reduction using third generation dual source computed tomography for the evaluation of patients prior to transcatheter aortic valve implantation. Eur. Radiol. 2016, 26, 4497–4504. [Google Scholar] [CrossRef] [PubMed]
- Guez, D.; Boroumand, G.; Ruggiero, N.J.; Mehrotra, P.; Halpern, E.J. Automated and Manual Measurements of the Aortic Annulus with ECG-Gated Cardiac CT Angiography Prior to Transcatheter Aortic Valve Replacement: Comparison with 3D-Transesophageal Echocardiography. Acad. Radiol. 2017, 24, 587–593. [Google Scholar] [CrossRef]
- Hammerstingl, C.; Schueler, R.; Weber, M.; Ghanem, A.; Werner, N.; Vasa Nicotera, M.; Thomas, D.; Mellert, F.; Schiller, W.; Schild, H.H.; et al. Three-dimensional imaging of the aortic valve geometry for prosthesis sizing prior to transcatheter aortic valve replacement. Int. J. Cardiol. 2014, 174, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Shibayama, K.; Noguchi, M.; Makihara, Y.; Okumura, H.; Obunai, K.; Isobe, M.; Hirao, K.; Watanabe, H. Superiority of novel automated assessment of aortic annulus by intraoperative three-dimensional transesophageal echocardiography in patients with severe aortic stenosis: Comparison with conventional cross-sectional assessment. J. Cardiol. 2018, 72, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Ng, A.C.T.; Delgado, V.; van der Kley, F.; Shanks, M.; van de Veire, N.R.L.; Bertini, M.; Nucifora, G.; van Bommel, R.J.; Tops, L.F.; de Weger, A.; et al. Comparison of Aortic Root Dimensions and Geometries Before and After Transcatheter Aortic Valve Implantation by 2- and 3-Dimensional Transesophageal Echocardiography and Multislice Computed Tomography. Circ. Cardiovasc. Imaging 2009, 3, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Chien-Chia Wu, V.; Kaku, K.; Takeuchi, M.; Otani, K.; Yoshitani, H.; Tamura, M.; Abe, H.; Lin, F.C.; Otsuji, Y. Aortic Root Geometry in Patients with Aortic Stenosis Assessed by Real-Time Three-Dimensional Transesophageal Echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 32–41. [Google Scholar] [CrossRef]
- Machida, T.; Izumo, M.; Suzuki, K.; Yoneyama, K.; Kamijima, R.; Mizukoshi, K.; Takai, M.; Kobayashi, Y.; Harada, T.; Miyake, F.; et al. Value of anatomical aortic valve area using real-time three-dimensional transoesophageal echocardiography in patients with aortic stenosis: A comparison between tricuspid and bicuspid aortic valves. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Longhitano, Y.; Artico, M.; Cammarota, G.; Barbanera, A.; Racca, F.; Audo, A.; Ravera, E.; Migneco, A.; Piccioni, A.; et al. Bedside Cardiac Pocus in Emergency Setting: A Practice Review. Rev. Recent. Clin. Trials 2020, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Podlesnikar, T.; Delgado, V. Transcatheter Aortic Valve Replacement: Advantages and Limitations of Different Cardiac Imaging Techniques. Rev. Esp. Cardiol. (Engl. Ed.) 2016, 69, 310–321. [Google Scholar] [CrossRef]
- Ebuchi, K.; Yoshitani, K.; Kanemaru, E.; Fujii, T.; Tsukinaga, A.; Shimahara, Y.; Ohnishi, Y. Measurement of the Aortic Annulus Area and Diameter by Three-Dimensional Transesophageal Echocardiography in Transcatheter Aortic Valve Replacement. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Husser, O.; Holzamer, A.; Resch, M.; Endemann, D.H.; Nunez, J.; Bodi, V.; Schmid, C.; Riegger, G.A.J.; Gössmann, H.; Hamer, O.; et al. Prosthesis sizing for transcatheter aortic valve implantation—Comparison of three dimensional transesophageal echocardiography with multislice computed tomography. J. Cardiol. 2013, 168, 3431–3438. [Google Scholar] [CrossRef]
- Willson, A.B.; Webb, J.G.; Freeman, M.; Wood, D.A.; Gurvitch, R.; Thompson, C.R.; Moss, R.R.; Toggweiler, S.; Binder, R.K.; Munt, B.; et al. Computed tomography–based sizing recommendations for transcatheter aortic valve replacement with balloon-expandable valves: Comparison with transesophageal echocardiography and rationale for implementation in a prospective trial. J. Cardiovasc. Comput. Tomogr. 2012, 6, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Takeuchi, M.; Kaku, K.; Sugeng, L.; Yoshitani, H.; Haruki, N.; Ota, T.; Mor-Avi, V.; Lang, R.M.; Otsuji, Y. Assessment of the Aortic Root Using Real-Time 3D Transesophageal Echocardiography. Circ. J. 2002, 74, 2649–2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamborini, G.; Fusini, L.; Gripari, P.; Muratori, M.; Cefalù, C.; Maffessanti, F.; Alamanni, F.; Bartorelli, A.; Pontone, G.; Andreini, D.; et al. Feasibility and Accuracy of 3DTEE Versus CT for the Evaluation of Aortic Valve Annulus to Left Main Ostium Distance Before Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Imaging 2012, 5, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Sjoerdsma, M.; Fixsen, L.S.; Schoots, T.; van de Voose, F.N.; Lopata, R.G.P. A demonstration of high field-of-view stability in hands-free echocardiography. Cardiovasc. Ultrasound 2020, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Queirós, S.; Morais, P.; Dubois, C.; Voigt, J.U.; Fehske, W.; Kuhn, A.; Achenbach, T.; Fonseca, J.C.; Vilaca, L.J.; D’hooge, J. Validation of a Novel Software Tool for Automatic Aortic Annular Sizing in Three-Dimensional Transesophageal Echocardiographic Images. J. Am. Soc. Echocardiogr. 2018, 31, 515–525. [Google Scholar] [CrossRef]
- Chen, X.; Owens, C.A.; Huang, E.C.; Maggard, B.D.; Latif, R.K.; Clifford, S.P.; Li, J.; Huang, J. Artificial Intelligence in Echocardiography for Anesthesiologists. J. Cardiothorac. Vasc. Anesth. 2021, 35, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, L.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dima, C.N.; Gaspar, M.; Mornos, C.; Mornos, A.; Deutsch, P.; Cioloca, H.; Cerbu, S.; Dinu, M.; Hoinoiu, B.; Luca, C.T.; et al. Three-Dimensional Transesophageal Echocardiography as an Alternative to Multidetector Computed Tomography in Aortic Annular Diameter Measurements for Transcatheter Aortic Valve Implantation. Biology 2021, 10, 132. https://doi.org/10.3390/biology10020132
Dima CN, Gaspar M, Mornos C, Mornos A, Deutsch P, Cioloca H, Cerbu S, Dinu M, Hoinoiu B, Luca CT, et al. Three-Dimensional Transesophageal Echocardiography as an Alternative to Multidetector Computed Tomography in Aortic Annular Diameter Measurements for Transcatheter Aortic Valve Implantation. Biology. 2021; 10(2):132. https://doi.org/10.3390/biology10020132
Chicago/Turabian StyleDima, Ciprian Nicusor, Marian Gaspar, Cristian Mornos, Aniko Mornos, Petru Deutsch, Horia Cioloca, Simona Cerbu, Mihai Dinu, Bogdan Hoinoiu, Constantin Tudor Luca, and et al. 2021. "Three-Dimensional Transesophageal Echocardiography as an Alternative to Multidetector Computed Tomography in Aortic Annular Diameter Measurements for Transcatheter Aortic Valve Implantation" Biology 10, no. 2: 132. https://doi.org/10.3390/biology10020132
APA StyleDima, C. N., Gaspar, M., Mornos, C., Mornos, A., Deutsch, P., Cioloca, H., Cerbu, S., Dinu, M., Hoinoiu, B., Luca, C. T., & Petrescu, L. (2021). Three-Dimensional Transesophageal Echocardiography as an Alternative to Multidetector Computed Tomography in Aortic Annular Diameter Measurements for Transcatheter Aortic Valve Implantation. Biology, 10(2), 132. https://doi.org/10.3390/biology10020132







