Alleviation of Copper-Induced Stress in Pea (Pisum sativum L.) through Foliar Application of Gibberellic Acid
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Growth Conditions
2.2. Exogenous Application of GA3
2.3. Plant Growth Attributes
2.4. Physiological Variables
2.5. Oxidative Stress Indicators and Antioxidant Response
2.6. Copper Concentration in Roots, Leaves and Stems
2.7. Statistical Analysis
3. Results
3.1. Plant Growth Attributes
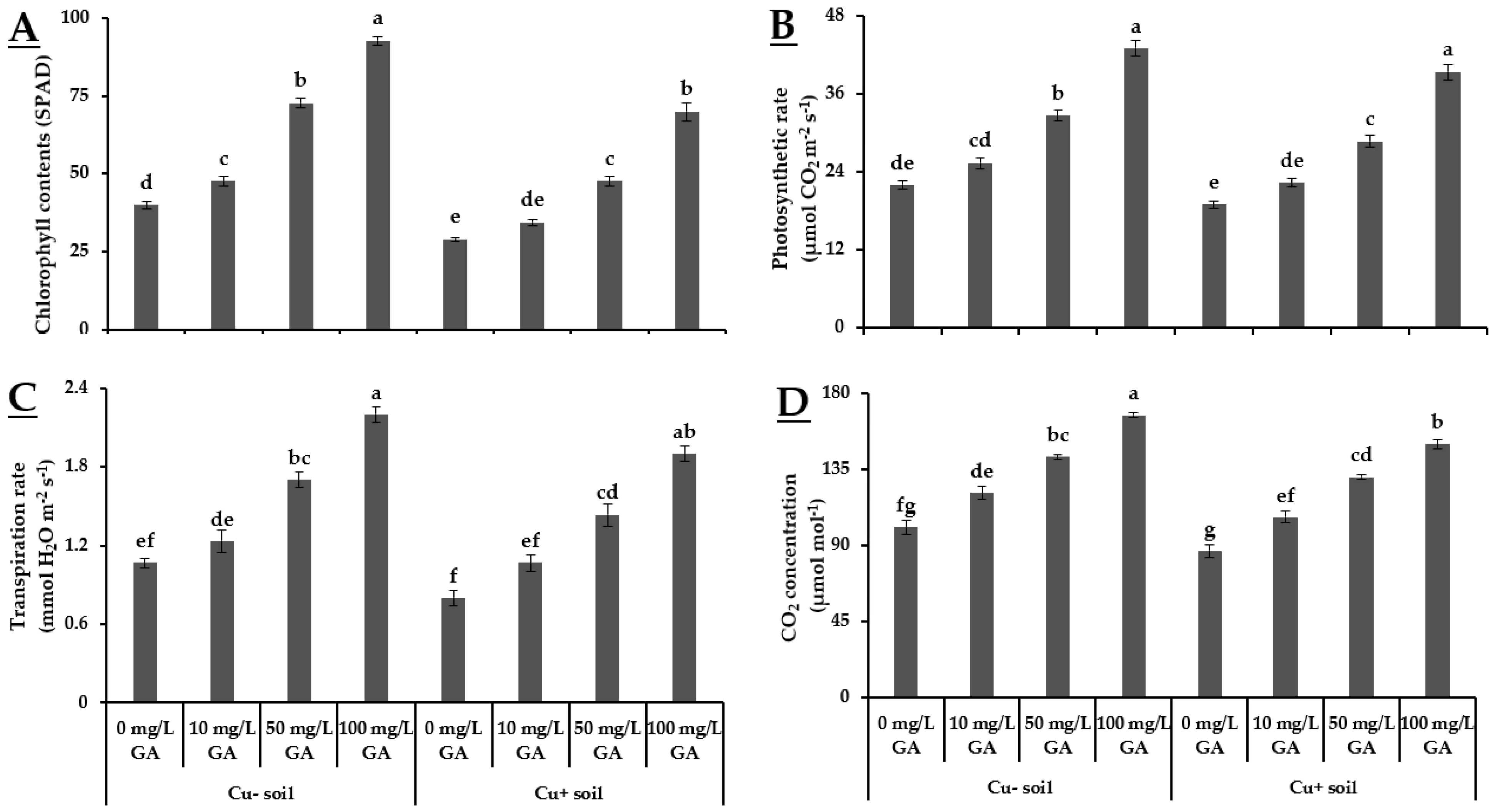
3.2. Physiological Variables
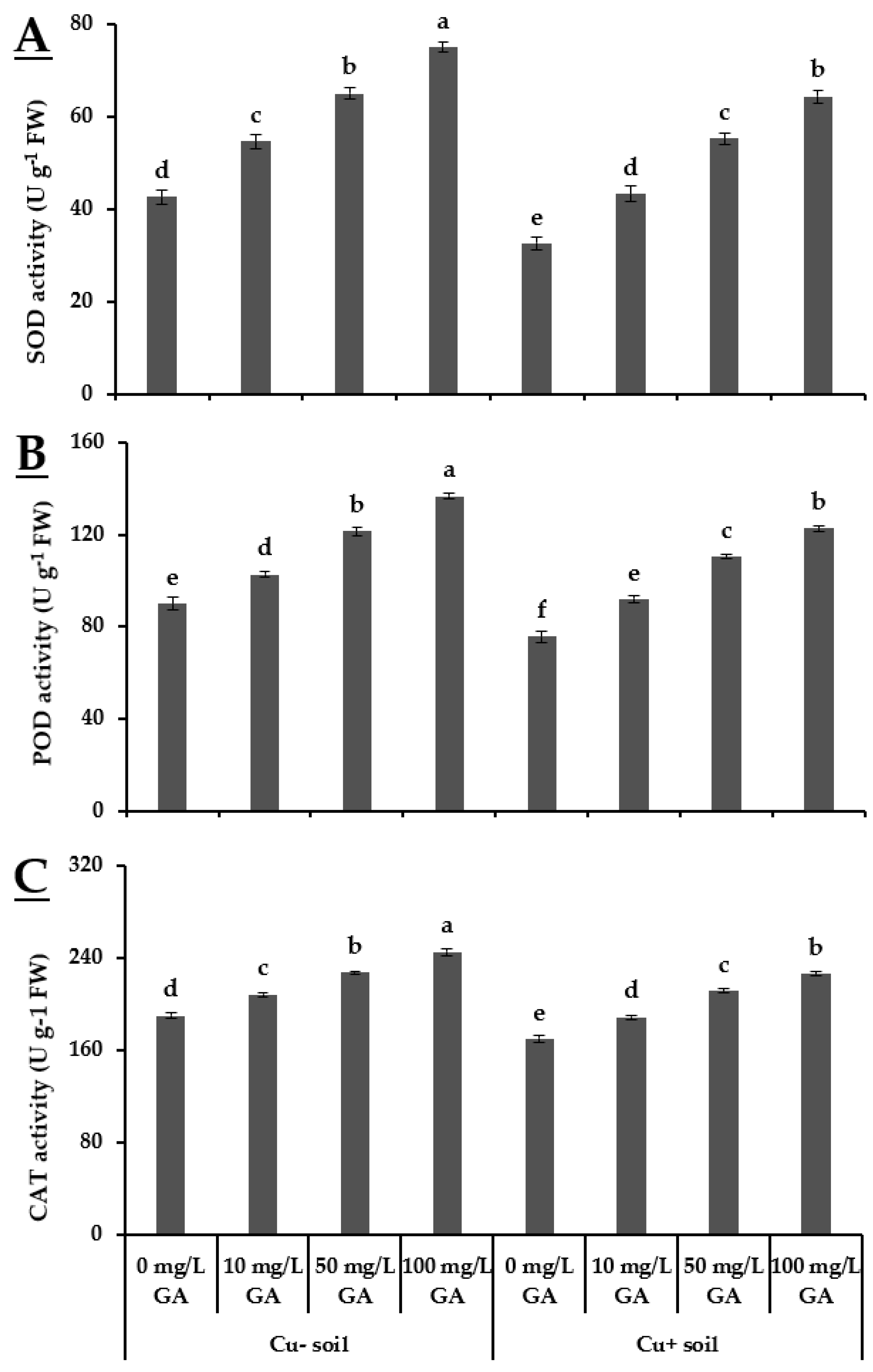
3.3. Oxidative Stress Indicators and Antioxidant Response
3.4. Cu Concentration in Roots, Leaves and Stem
3.5. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. World Food and Agriculture 2018: Statistical Pocketbook; FAO: Rome, Italy, 2018. [Google Scholar]
- Davies, P.J.; Muehlbauer, F.J. The Physiology of Vegetable Crops, 2nd ed.; CABI: Wallingford, UK, 2020. [Google Scholar]
- Slima, D.F.; Ahmed, D.A.E.-A. Trace Metals Accumulated in Pea Plant (Pisum sativum L.) as a Result of Irrigation with Wastewater. J. Soil Sci. Plant Nutr. 2020, 20, 2749–2760. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Adnan, M.; Ali, M.; Rana, M.S.; Kamran, M.; Ali, Q.; Hashem, I.A.; Bhantana, P.; Ali, M.; et al. Foliar application of gibberellic acid endorsed phytoextraction of copper and alleviates oxidative stress in jute (Corchorus capsularis L.) plant grown in highly copper-contaminated soil of China. Environ. Sci. Pollut. Res. 2020, 27, 37121–37133. [Google Scholar] [CrossRef] [PubMed]
- Allevato, E.; Mauro, R.P.; Stazi, S.R.; Marabottini, R.; Leonardi, C.; Ierna, A.; Giuffrida, F. Arsenic accumulation in grafted melon plants: Role of rootstock in modulating root-to-shoot translocation and physiological response. Agronomy 2019, 9, 828. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Fahad, S.; Rehman, A.; Shahzad, B.; Tanveer, M.; Saud, S.; Kamran, M.; Ihtisham, M.; Khan, S.U.; Turan, V.; ur Rahman, M.H. Rice Responses and Tolerance to Metal/Metalloid Toxicity. In Advances in Rice Research for Abiotic Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–312. [Google Scholar]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C.; et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef]
- Saleem, M.; Asghar, H.N.; Khan, M.Y.; Zahir, Z.A. Gibberellic acid in combination with pressmud enhances the growth of sunflower and stabilizes chromium(VI)-contaminated soil. Environ. Sci. Pollut. Res. 2015, 22, 10610–10617. [Google Scholar] [CrossRef]
- Ullah, S.; Anwar, S.; Rehman, M.; Khan, S.; Zafar, S.; Liu, L.; Peng, D. Interactive effect of gibberellic acid and NPK fertilizer combinations on ramie yield and bast fibre quality. Sci. Rep. 2017, 7, 10647. [Google Scholar] [CrossRef]
- UZAL, O.; Yasar, F. Effects of GA3 hormone treatments on ion uptake and growth of pepper plants under cadmium stress. Appl. Ecol. Environ. Res. 2017, 15, 1347–1357. [Google Scholar] [CrossRef]
- Ji, P.; Tang, X.; Jiang, Y.; Tong, Y.; Gao, P.; Han, W. Potential of gibberellic acid 3 (GA3) for enhancing the phytoremediation efficiency of Solanum nigrum L. Bull. Environ. Contam. Toxicol. 2015, 95, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Hadi, F.; Bano, A.; Fuller, M.P. The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): The role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere 2010, 80, 457–462. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, Y.; Zhou, Q.; Wang, L.; Lin, D.; Liang, X. The potential of gibberellic acid 3 (GA3) and Tween-80 induced phytoremediation of co-contamination of Cd and Benzo[a]pyrene (B[a]P) using Tagetes patula. J. Environ. Manag. 2013, 114, 202–208. [Google Scholar] [CrossRef]
- Isogai, S.; Touno, K.; Shimomura, K. Gibberellic acid improved shoot multiplication in Cephaelis ipecacuanha. Vitr. Cell. Dev. Biol. Plant 2008, 44, 216–220. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Maehly, A.C. The Assay of Catalases and Peroxidases. In Methods of Biochemical Analysis; Wiley: Hoboken, NJ, USA, 2006; pp. 357–424. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Sfaxi-Bousbih, A.; Chaoui, A.; El Ferjani, E. Unsuitable availability of nutrients in germinating bean embryos exposed to copper excess. Biol. Trace Elem. Res. 2010, 135, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Karmous, I.; Khadija, J.; Chaoui, A.; El Ferjani, E. Proteolytic activities in Phaseolus vulgaris cotyledons under copper stress. Physiol. Mol. Biol. Plants 2012, 18, 337–343. [Google Scholar] [CrossRef]
- Chaoui, A.; El Ferjani, E. β-Estradiol protects embryo growth from heavy-metal toxicity in germinating lentil seeds. J. Plant Growth Regul. 2013, 32, 636–645. [Google Scholar] [CrossRef]
- Ben Massoud, M.; Karmous, I.; El Ferjani, E.; Chaoui, A. Alleviation of copper toxicity in germinating pea seeds by IAA, GA 3, Ca and citric acid. J. Plant Interact. 2018, 13, 21–29. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka, E.; Godlewska-Żyłkiewicz, B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 2012, 52, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Song, H. Calcium protects Trifolium repens L. seedlings against cadmium stress. Plant Cell Rep. 2009, 28, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Tassi, E.; Pouget, J.; Petruzzelli, G.; Barbafieri, M. The effects of exogenous plant growth regulators in the phytoextraction of heavy metals. Chemosphere 2008, 71, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Pazmiño, D.M.; Testillano, P.S.; Risueño, M.C.; del Río, L.A.; Sandalio, L.M. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef]
- Shafigh, M.; Ghasemi-Fasaei, R.; Ronaghi, A. Influence of plant growth regulators and humic acid on the phytoremediation of lead by maize in a Pb-polluted calcareous soil. Arch. Agron. Soil Sci. 2016, 62, 1733–1740. [Google Scholar] [CrossRef]
- Wen, F.; Zhang, Z.; Bai, T.; Xu, Q.; Pan, Y. Proteomics reveals the effects of gibberellic acid (GA3) on salt-stressed rice (Oryza sativa L.) shoots. Plant Sci. 2010, 178, 170–175. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Dikilitas, M.; Higgs, D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008, 62, 1–9. [Google Scholar] [CrossRef]
- Alonso-Ramírez, A.; Rodríguez, D.; Reyes, D.; Jiménez, J.A.; Nicolás, G.; López-Climent, M.; Gómez-Cadenas, A.; Nicolás, C. Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol. 2009, 150, 1335–1344. [Google Scholar] [CrossRef]
- Lüttge, U. Plant Physiology. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 549–557. [Google Scholar]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Parveen, A.; Saleem, M.H.; Kamran, M.; Haider, M.Z.; Chen, J.-T.; Malik, Z.; Rana, M.S.; Hassan, A.; Hur, G.; Javed, M.T.; et al. Effect of citric acid on growth, ecophysiology, chloroplast ultrastructure, and phytoremediation potential of jute (Corchorus capsularis L.) seedlings exposed to copper stress. Biomolecules 2020, 10, 592. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Din, M.; Ullah, A.; El Sabagh, A.; Hossain, A.; Llanes, A.; Liu, L. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 2020, 27, 5211–5221. [Google Scholar] [CrossRef]
- Habiba, U.; Ali, S.; Farid, M.; Shakoor, M.B.; Rizwan, M.; Ibrahim, M.; Abbasi, G.H.; Hayat, T.; Ali, B. EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 1534–1544. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Lee, S.; Wen, R. Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS ONE 2018, 13, e0203612. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2019, 21, 148. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Panda, P.; Mazumdar, P.; Kumar, D.; Sharma, G.D.; Sahoo, L.; Sanjib, P. Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol. Biochem. 2012, 53, 33–39. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Farid, M.; Shakoor, M.B.; Gill, R.A.; Najeeb, U.; Iqbal, N.; Ahmad, R. Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol. Environ. Saf. 2015, 120, 310–317. [Google Scholar] [CrossRef]
- Kanwal, U.; Ali, S.; Shakoor, M.B.; Farid, M.; Hussain, S.; Yasmeen, T.; Adrees, M.; Bharwana, S.A.; Abbas, F. EDTA ameliorates phytoextraction of lead and plant growth by reducing morphological and biochemical injuries in Brassica napus L. under lead stress. Environ. Sci. Pollut. Res. 2014, 21, 9899–9910. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shafi, M.; Li, S.; Wang, Y.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Liu, D. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci. Rep. 2015, 5, 13554. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Plant Height (cm) | Plant Relative Fresh Weight (g) | Plant Dry Weight (g) | Leaves per Plant | |
|---|---|---|---|---|---|
| Cu− soil | 0 mg/L GA3 | 24.3 ± 0.33 e | 20.6 ± 0.38 ef | 9.0 ± 0.23 ef | 16.7 ± 0.38 e |
| 10 mg/L GA3 | 27.3 ± 0.57 de | 22.1 ± 0.25 e | 11.3 ± 0.65 de | 18.3 ± 0.12 de | |
| 50 mg/L GA3 | 32.1 ± 1.15 bc | 28.1 ± 0.52 c | 14.4 ± 0.16 bc | 24.5 ± 0.54 bc | |
| 100 mg/L GA3 | 40.1 ± 1.13 a | 34 ± 1.2 a | 17.2 ± 0.12 a | 30.1 ± 0.82 a | |
| Cu+ soil | 0 mg/L GA3 | 19.3 ± 0.88 f | 18.1 ± 0.57 f | 8.0 ± 0.54 f | 13.7 ± 0.23 e |
| 10 mg/L GA3 | 23.3 ± 0.3 e | 20.3 ± 0.12 ef | 9.7 ± 0.43 def | 15.3 ± 0.13 e | |
| 50 mg/L GA3 | 28.7 ± 0.33 cd | 25.1 ± 0.92 d | 12.3 ± 0.27 cd | 21.7 ± 0.43 cd | |
| 100 mg/L GA3 | 34.2 ± 0.58 b | 31 ± 1.12 b | 15.2 ± 0.74 ab | 28.3 ± 0.73 ab | |
| HSD (Interaction) | 3.773 | 2.614 | 2.669 | 4.76 | |
| Treatments | Root Cu (mg·kg−1) | Leaves Cu (mg·kg−1) | Stem Cu (mg·kg−1) | |
|---|---|---|---|---|
| Cu− soil | 0 mg/L GA3 | 16.7 ± 0.81 g | 31.7 ± 0.88 h | 22.1 ± 0.52 g |
| 10 mg/L GA3 | 20.7 ± 0.78 g | 36.0 ± 1.15 g | 27.2 ± 0.57 fg | |
| 50 mg/L GA3 | 26.3 ± 0.88 f | 43.7 ± 1.76 f | 32.7 ± 0.72 f | |
| 100 mg/L GA3 | 34.7 ± 1.25 e | 58.1 ± 1.52 e | 39.7 ± 0.89 e | |
| Cu+ soil | 0 mg/L GA3 | 61.3 ± 0.69 d | 145.2 ± 2.31 d | 82.7 ± 1.45 d |
| 10 mg/L GA3 | 66.7 ± 0.89 c | 161.9 ± 1.15 c | 95.3 ± 0.88 c | |
| 50 mg/L GA3 | 74.3 ± 0.82 b | 177.3 ± 1.73 b | 119.9 ± 2.3 b | |
| 100 mg/L GA3 | 90.7 ± 1.2 a | 193.3 ± 1.85 a | 145.3 ± 2.33 a | |
| HSD (Interaction) | 4.371 | 3.527 | 6.332 | |
| Variables | Principle Component Factors | |
|---|---|---|
| F1 | F2 | |
| Plant height | 0.992 | −0.056 |
| Plant relative fresh weight | 0.989 | 0.082 |
| Plant dry weight | 0.997 | 0.025 |
| Number of leaves | 0.986 | 0.091 |
| Chlorophyll content | 0.974 | −0.148 |
| Photosynthetic rate | 0.982 | 0.105 |
| Transpiration rate | 0.995 | 0.033 |
| CO2 index | 0.997 | 0.026 |
| Malondialdehyde content | −0.983 | 0.056 |
| H2O2 content | −0.979 | −0.087 |
| Electrolyte leakage | −0.997 | −0.059 |
| Superoxide dismutase | 0.989 | −0.094 |
| Peroxidase | 0.996 | −0.022 |
| Catalase | 0.990 | −0.098 |
| Root Cu content | 0.027 | 0.998 |
| Leaves Cu content | −0.102 | 0.992 |
| Stem Cu content | 0.042 | 0.995 |
| Eigenvalue | 13.711 | 3.059 |
| Explained variability (%) | 80.652 | 17.997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, T.; Ali, M.M.; Shabbir, R.; Anwar, R.; Afzal, I.; Mauro, R.P. Alleviation of Copper-Induced Stress in Pea (Pisum sativum L.) through Foliar Application of Gibberellic Acid. Biology 2021, 10, 120. https://doi.org/10.3390/biology10020120
Javed T, Ali MM, Shabbir R, Anwar R, Afzal I, Mauro RP. Alleviation of Copper-Induced Stress in Pea (Pisum sativum L.) through Foliar Application of Gibberellic Acid. Biology. 2021; 10(2):120. https://doi.org/10.3390/biology10020120
Chicago/Turabian StyleJaved, Talha, Muhammad Moaaz Ali, Rubab Shabbir, Raheel Anwar, Irfan Afzal, and Rosario Paolo Mauro. 2021. "Alleviation of Copper-Induced Stress in Pea (Pisum sativum L.) through Foliar Application of Gibberellic Acid" Biology 10, no. 2: 120. https://doi.org/10.3390/biology10020120
APA StyleJaved, T., Ali, M. M., Shabbir, R., Anwar, R., Afzal, I., & Mauro, R. P. (2021). Alleviation of Copper-Induced Stress in Pea (Pisum sativum L.) through Foliar Application of Gibberellic Acid. Biology, 10(2), 120. https://doi.org/10.3390/biology10020120











