Diagnostic and Prognostic Value of miRNAs after Coronary Artery Bypass Grafting: A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. MiRNA Utility to Diagnose Type 5 Myocardial Infarction
3. Myocardial Injury during Coronary Artery Bypass Grafting
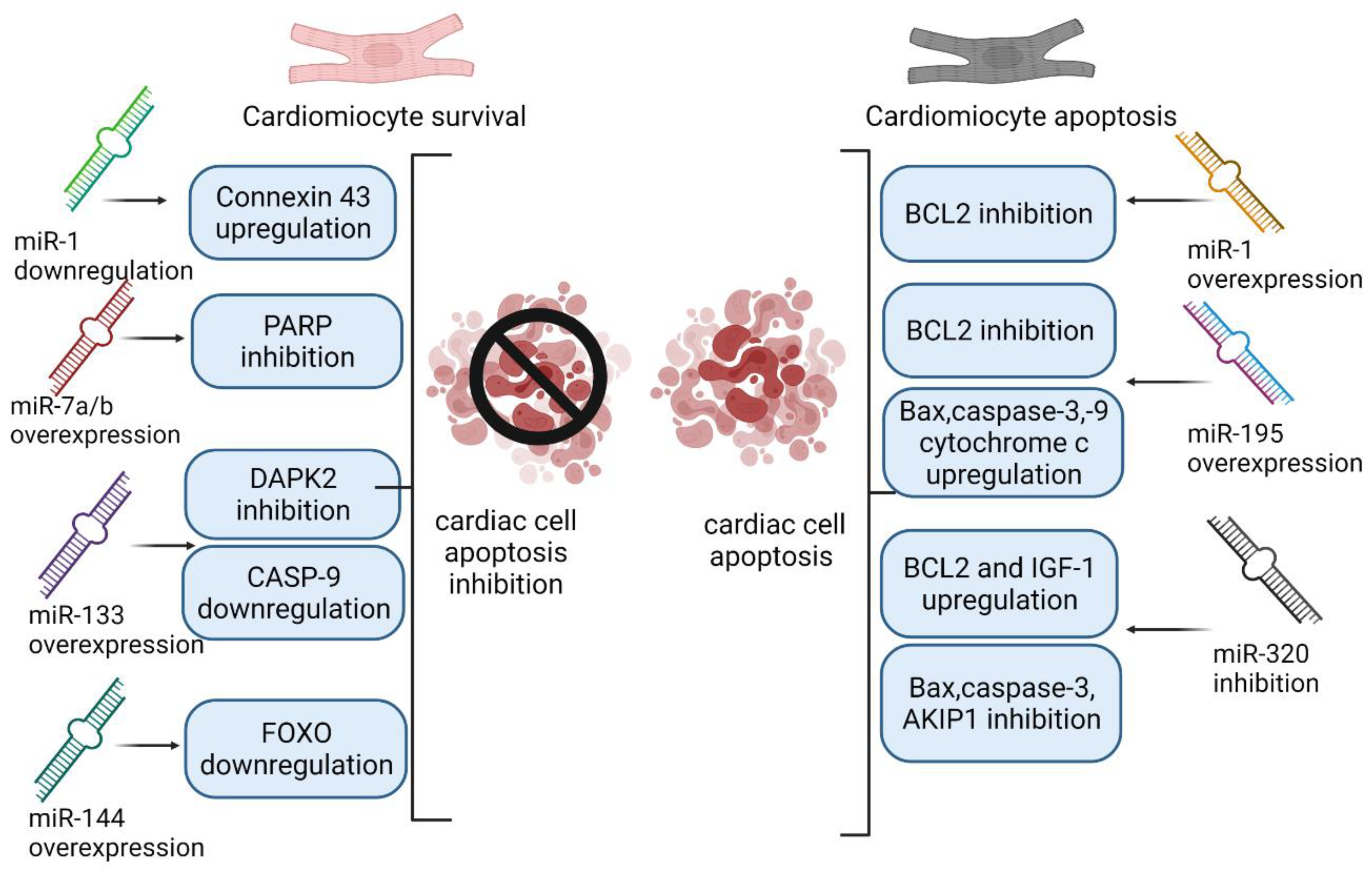
4. MiRNAs as Markers of Ischemia/Reperfusion Injury, Inflammation and Apoptosis
4.1. miR-1
4.2. miR-7a/b
4.3. miR-126a-5p
4.4. miR-133
4.5. miR-144
4.6. miR-195
4.7. miR-320
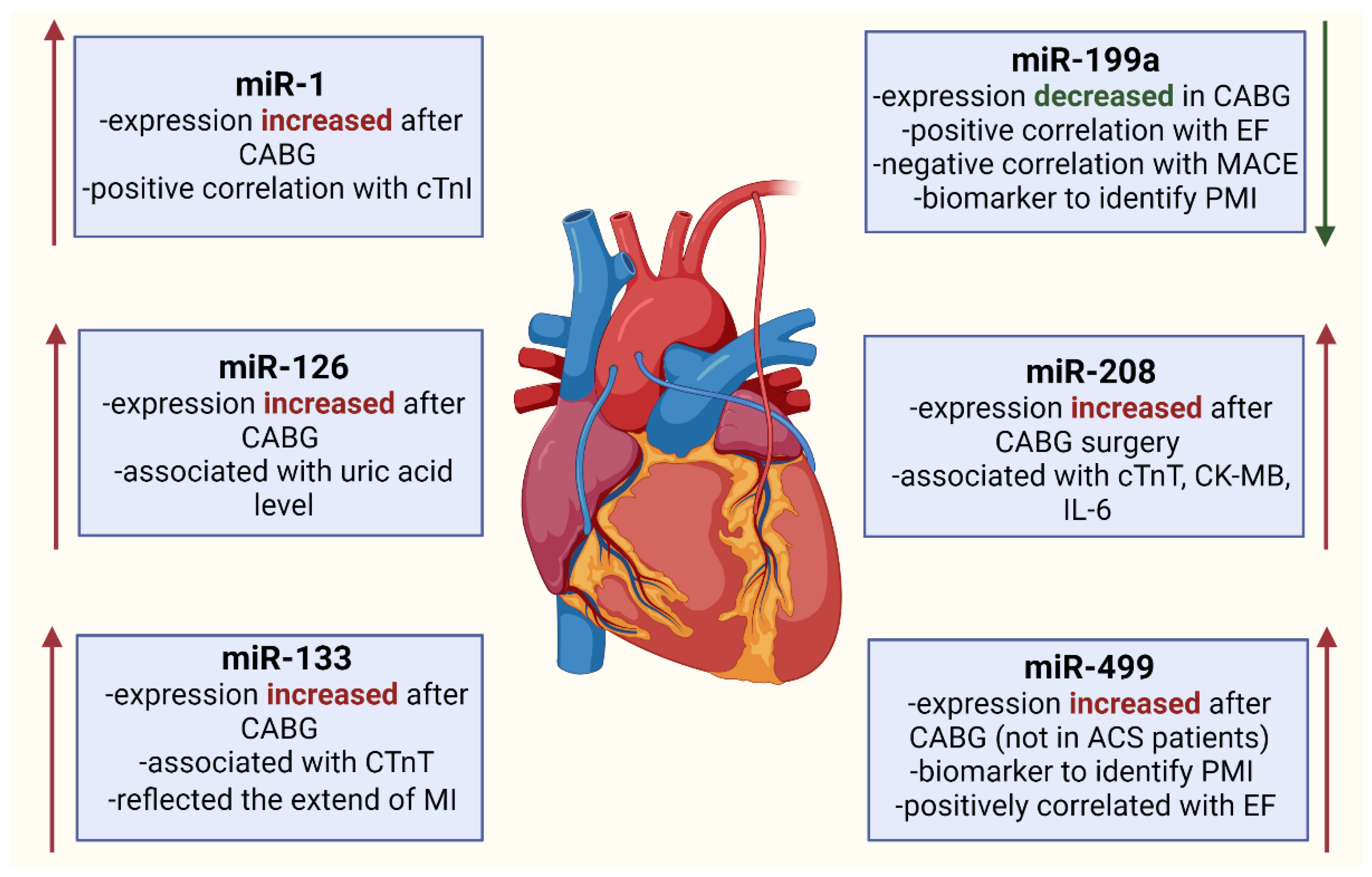
5. miRNA Expression Changes in Patients Undergoing CABG
5.1. miR-1
5.2. miR-126
5.3. miR-133
5.4. miR-199a
5.5. miR-208a
5.6. miR-499
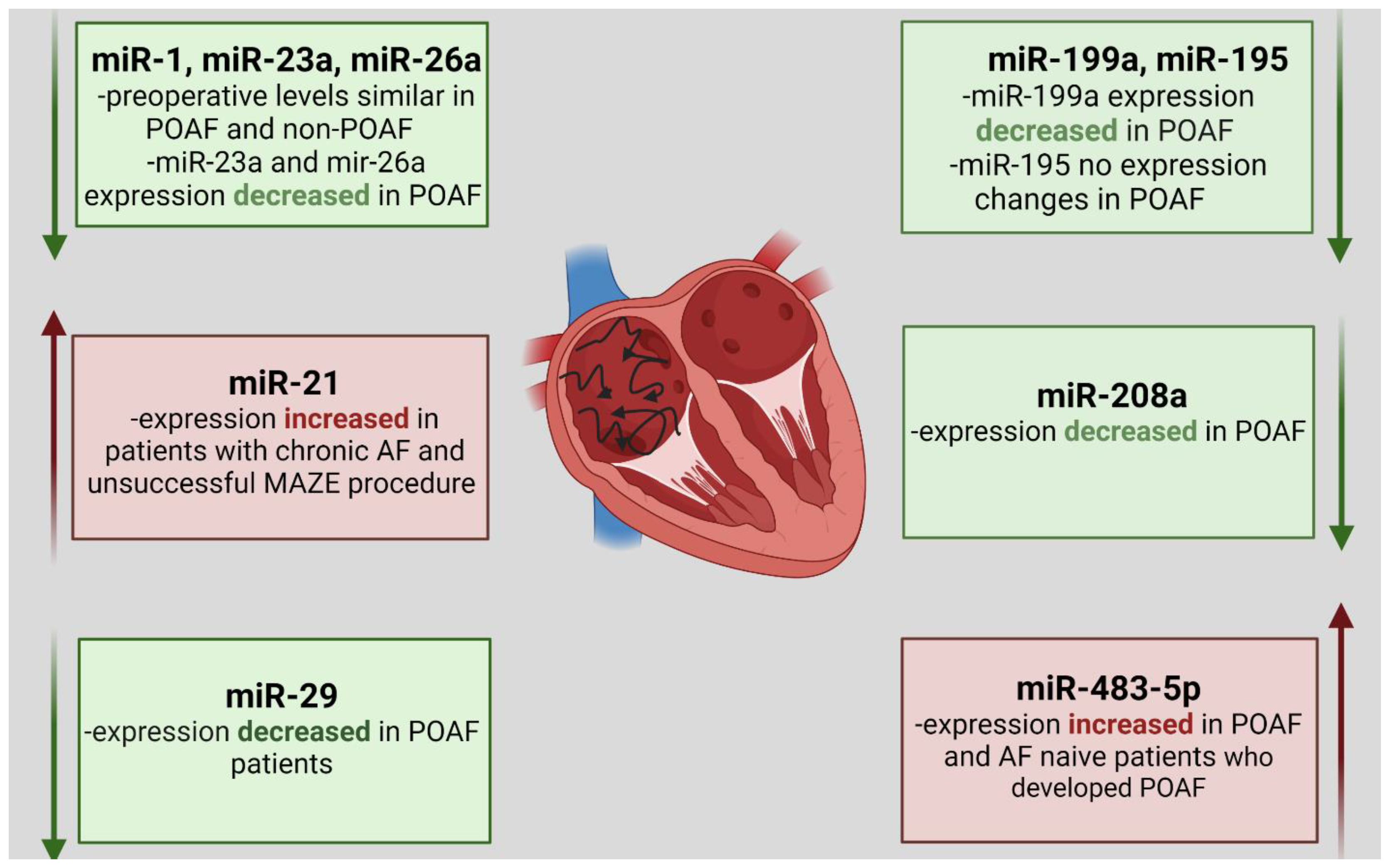
6. MiRNA Expression Changes in Patients with Atrial Fibrillation Undergoing CABG Procedure
7. Perioperative Prognostic Value of miRNAs
8. Economic Aspects of miRNA-Based Diagnostic and Prognostic Strategies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hillis, L.D.; Smith, P.K.; Anderson, J.L.; Bittl, J.A.; Bridges, C.R.; Byrne, J.G.; Cigarroa, J.E.; Disesa, V.J.; Hiratzka, L.F.; Winniford, M.D.; et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation 2011, 124, e652–e735. [Google Scholar] [CrossRef]
- Mohr, F.W.; Rastan, A.J.; Serruys, P.W.; Kappetein, A.P.; Holmes, D.R.; Pomar, J.L.; Westaby, S.; Leadley, K.; Dawkins, K.D.; Mack, M.J. Complex coronary anatomy in coronary artery bypass graft surgery: Impact of complex coronary anatomy in modern bypass surgery? Lessons learned from the SYNTAX trial after two years. J. Thorac. Cardiovasc. Surg. 2011, 141, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Goetz, R.H.; Rohman, M.; Haller, J.D.; Dee, R.; Rosenak, S.S. Internal mammary-coronary artery anastomosis. J. Thorac. Cardiovasc. Surg. 1961, 41, 378–386. [Google Scholar] [CrossRef]
- MedSuite. Cardiac Surgery Market Analysis, Size, Trends | United States | 2020–2026 | COVID19 | MedSuite. 2020. Available online: https://idataresearch.com/product/cardiac-surgery-market-united-states/ (accessed on 29 August 2021).
- Hokkanen, M.; Huhtala, H.; Laurikka, J.; Järvinen, O. The effect of postoperative complications on health-related quality of life and survival 12 years after coronary artery bypass grafting—A prospective cohort study. J. Cardiothorac. Surg. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Baig, K.; Harling, L.; Papanikitas, J.; Attaran, S.; Ashrafian, H.; Casula, R.; Athanasiou, T. Does coronary artery bypass grafting improve quality of life in elderly patients? Interact. Cardiovasc. Thorac. Surg. 2013, 17, 542–553. [Google Scholar] [CrossRef][Green Version]
- Rexius, H.; Brandrup-Wognsen, G.; Ekroth, R.; Odén, A. Does coronary artery bypass surgery improve survival? Scand. Cardiovasc. J. 2012, 46, 269–277. [Google Scholar] [CrossRef]
- Jan, A.; Hayat, M.K.; Khan, M.A.A.; Ullah, R. Trends in per-operative parameters and postoperative complications associated with coronary artery bypass graft surgery (CABG); A four-year retrospective study. Pak. J. Med Sci. 2021, 37, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Adelborg, K.; Horváth-Puhó, E.; Schmidt, M.; Munch, T.; Pedersen, L.; Nielsen, P.H.; Bøtker, H.E.; Sørensen, H.T. Thirty-Year Mortality After Coronary Artery Bypass Graft Surgery. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e002708. [Google Scholar] [CrossRef]
- Montrief, T.; Koyfman, A.; Long, B. Coronary artery bypass graft surgery complications: A review for emergency clinicians. Am. J. Emerg. Med. 2018, 36, 2289–2297. [Google Scholar] [CrossRef]
- Bourassa, M.G.; Fisher, L.D.; Campeau, L.; Gillespie, M.J.; McConney, M.; Lespérance, J. Long-term fate of bypass grafts: The Coronary Artery Surgery Study (CASS) and Montreal Heart Institute experiences. Circulation 1985, 72, 71–78. [Google Scholar]
- SabikIII, J.F. Understanding Saphenous Vein Graft Patency. Circulation 2011, 124, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Royse, A.; Pamment, W.; Pawanis, Z.; Clarke-Errey, S.; Eccleston, D.; Ajani, A.; Wilson, W.; Canty, D.; Royse, C. Patency of conduits in patients who received internal mammary artery, radial artery and saphenous vein grafts. BMC Cardiovasc. Disord. 2020, 20, 148. [Google Scholar] [CrossRef]
- Otsuka, F.; Yahagi, K.; Sakakura, K.; Virmani, R. Why is the mammary artery so special and what protects it from atherosclerosis? Ann. Cardiothorac. Surg. 2013, 2, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.P. Current status of arterial grafts for coronary artery bypass grafting. Ann. Cardiothorac. Surg. 2013, 2, 427–430. [Google Scholar]
- Preeshagul, I.; Gharbaran, R.; Jeong, K.H.; Abdel-Razek, A.; Lee, L.Y.; Elman, E.; Suh, K.S. Potential biomarkers for predicting outcomes in CABG cardiothoracic surgeries. J. Cardiothorac. Surg. 2013, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. microRNAs: Tiny Regulators with Great Potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef]
- Ruvkun, G. Glimpses of a Tiny RNA World. Science 2001, 294, 797–799. [Google Scholar] [CrossRef]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Ranjbar, A.; Saleh, S.H.S.; Bagherian, M.; Sharifzadeh, S.O.; Hushmandi, K.; et al. Regulation of Nuclear Factor-KappaB (NF-κB) signaling pathway by non-coding RNAs in cancer: Inhibiting or promoting carcinogenesis? Cancer Lett. 2021, 509, 63–80. [Google Scholar] [CrossRef]
- Kuhn, D.E.; Martin, M.M.; Feldman, D.S.; Terry, A.; Nuovo, G.J.; Elton, T.S. Experimental validation of miRNA targets. Methods 2008, 44, 47–54. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.-H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Hogg, D.R.; Harries, L. Human genetic variation and its effect on miRNA biogenesis, activity and function. Biochem. Soc. Trans. 2014, 42, 1184–1189. [Google Scholar] [CrossRef]
- Selbach, M.; Schwanhäusser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008, 455, 58–63. [Google Scholar] [CrossRef]
- Peter, M.E. Targeting of mRNAs by multiple miRNAs: The next step. Oncogene 2010, 29, 2161–2164. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Mohan, M. MicroRNAs: History, biogenesis, and their evolving role in animal development and disease. Vet. Pathol. 2013, 51, 759–774. [Google Scholar] [CrossRef]
- Colpaert, R.M.; Calore, M. MicroRNAs in Cardiac Diseases. Cells 2019, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-S.; Jin, J.-P.; Wang, J.-Q.; Zhang, Z.-G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Ono, K. MicroRNAs and Cardiovascular Diseases. FEBS J. 2013, 477–493. [Google Scholar] [CrossRef]
- Pang, J.K.S.; Phua, Q.H.; Soh, B.-S. Applications of miRNAs in cardiac development, disease progression and regeneration. Stem Cell Res. Ther. 2019, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Gozdowska, R.; Makowska, A.; Gasecka, A.; Chabior, A.; Marchel, M. Circulating microRNA in Heart Failure-Practical Guidebook to Clinical Application. Cardiol. Rev. 2020, 30, 16–23. [Google Scholar] [CrossRef]
- Stenvang, J.; Petri, A.; Lindow, M.; Obad, S.; Kauppinen, S. Inhibition of microRNA function by antimiR oligonucleotides. Silence 2012, 3, 1–17. [Google Scholar] [CrossRef]
- Wang, Z. The Guideline of the Design and Validation of MiRNA. MicroRNA Cancer 2011, 676, 211–223. [Google Scholar] [CrossRef]
- Sonkoly, E.; Pivarcsi, A. microRNAs in Inflammation. Int. Rev. Immunol. 2009, 28, 535–561. [Google Scholar] [CrossRef] [PubMed]
- Shirjang, S.; Mansoori, B.; Asghari, S.; Duijf, P.; Mohammadi, A.; Gjerstorff, M.; Baradaran, B. MicroRNAs in cancer cell death pathways: Apoptosis and necroptosis. Free Radic. Biol. Med. 2019, 139, 1–15. [Google Scholar] [CrossRef]
- Jovanovic, M.; Hengartner, M. miRNAs and apoptosis: RNAs to die for. Oncogene 2006, 25, 6176–6187. [Google Scholar] [CrossRef]
- Ye, Y.; Perez-Polo, J.R.; Qian, J.; Birnbaum, Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol. Genom. 2011, 43, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, L.; Schmid, C.; Debl, K.; Lunz, D.; Flörchinger, B.; Keyser, A. Impact of coronary angiography early after CABG for suspected postoperative myocardial ischemia. J. Cardiothorac. Surg. 2019, 14, 54. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction. Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Thielmann, M.; Sharma, V.; Al-Attar, N.; Bulluck, H.; Bisleri, G.; Bunge, J.J.; Czerny, M.; Ferdinandy, P.; Frey, U.H.; Heusch, G.; et al. ESC Joint Working Groups on Cardiovascular Surgery and the Cellular Biology of the Heart Position Paper: Peri-operative myocardial injury and infarction in patients undergoing coronary artery bypass graft surgery. Eur. Heart J. 2017, 38, 2392–2411. [Google Scholar] [CrossRef]
- Alam, S.R.; Stirrat, C.; Spath, N.; Zamvar, V.; Pessotto, R.; Dweck, M.R.; Moore, C.; Semple, S.; El-Medany, A.; Manoharan, D.; et al. Myocardial inflammation, injury and infarction during on-pump coronary artery bypass graft surgery. J. Cardiothorac. Surg. 2017, 12, 115. [Google Scholar] [CrossRef]
- Bonnefoy, E.; Filley, S.; Kirkorian, G.; Guidollet, J.; Roriz, R.; Robin, J.; Touboul, P. Troponin I, troponin T, or Creatine Kinase-MB to Detect Perioperative Myocardial Damage After Coronary Artery Bypass Surgery. Chest 1998, 114, 482–486. [Google Scholar] [CrossRef]
- Agewall, S.; Giannitsis, E.; Jernberg, T.; Katus, H. Troponin elevation in coronary vs. non-coronary disease. Eur. Heart J. 2010, 32, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Chuang, A.; Nguyen, M.T.; Kung, W.-M.; Lehman, S.; Chew, D. High-sensitivity troponin in chronic kidney disease: Considerations in myocardial infarction and beyond. Rev. Cardiovasc. Med. 2020, 21, 191–203. [Google Scholar] [CrossRef]
- Long, B.; Long, D.A.; Tannenbaum, L.; Koyfman, A. An emergency medicine approach to troponin elevation due to causes other than occlusion myocardial infarction. Am. J. Emerg. Med. 2019, 38, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Mascia, G.; Pescetelli, F.; Baldari, A.; Gatto, P.; Seitun, S.; Sartori, P.; Pieroni, M.; Calò, L.; Della Bona, R.; Porto, I. Interpretation of elevated high-sensitivity cardiac troponin I in elite soccer players previously infected by severe acute respiratory syndrome coronavirus 2. Int. J. Cardiol. 2020, 326, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Du, J.; Cao, X.; Wang, Y.; Huang, Y.; Hu, S.; Zheng, Z. Plasma Levels of MicroRNA-499 Provide an Early Indication of Perioperative Myocardial Infarction in Coronary Artery Bypass Graft Patients. PLoS ONE 2014, 9, e104618. [Google Scholar] [CrossRef]
- Jeremias, A. The utility of troponin measurement to detect myocardial infarction: Review of the current findings. Vasc. Health Risk Manag. 2010, 6, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, D.; Van Zaane, B.; Looije, M.; Peelen, L.; Van Klei, W. Typical rise and fall of troponin in (peri-procedural) myocardial infarction: A systematic review. World J. Cardiol. 2016, 8, 293–301. [Google Scholar] [CrossRef]
- Zahler, S.; Massoudy, P.; Hartl, H.; Hähnel, C.; Meisner, H.; Becker, B.F. Acute cardiac inflammatory responses to postischemic reperfusion during cardiopulmonary bypass. Cardiovasc. Res. 1999, 41, 722–730. [Google Scholar] [CrossRef]
- Paparella, D.; Yau, T.; Young, E. Cardiopulmonary bypass induced inflammation: Pathophysiology and treatment. An update. Eur. J. Cardio-Thorac. Surg. 2002, 21, 232–244. [Google Scholar] [CrossRef]
- Schmid, F.-X.; Vudattu, N.; Floerchinger, B.; Hilker, M.; Eissner, G.; Hoenicka, M.; Holler, E.; Birnbaum, D.E. Endothelial apoptosis and circulating endothelial cells after bypass grafting with and without cardiopulmonary bypass. Eur. J. Cardio-Thorac. Surg. 2006, 29, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yan, G.; Ren, A.; You, B.; Liao, J.K. FHL2/SLIM3 Decreases Cardiomyocyte Survival by Inhibitory Interaction With Sphingosine Kinase-1. Circ. Res. 2006, 99, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Joashi, U.; Tibby, S.; Turner, C.; Mayer, A.; Austin, C.; Anderson, D.; Durward, A.; Murdoch, I. Soluble Fas may be a proinflammatory marker after cardiopulmonary bypass in children. J. Thorac. Cardiovasc. Surg. 2002, 123, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Aebert, H.; Kirchner, S.; Keyser, A.; Birnbaum, D.E.; Holler, E.; Andreesen, R.; Eissner, G. Endothelial apoptosis is induced by serum of patients after cardiopulmonary bypass. Eur. J. Cardio-Thorac. Surg. 2000, 18, 589–593. [Google Scholar] [CrossRef][Green Version]
- Ruifrok, W.T.; Westenbrink, B.D.; De Boer, R.A.; Hamer, I.J.D.; Erasmus, M.E.; Mungroop, H.E.; Epema, A.H.; Voors, A.A.; Van Veldhuisen, D.J.; Van Gilst, W.H. Apoptosis during CABG surgery with the use of cardiopulmonary bypass is prominent in ventricular but not in atrial myocardium. Neth. Heart J. 2010, 18, 236–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Collard, C.D.; Gelman, S. Pathophysiology, Clinical Manifestations, and Prevention of Ischemia-Reperfusion Injury. Anesthesiology 2001, 94, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shoorei, H.; Taheri, M. Non-coding RNAs participate in the ischemia-reperfusion injury. Biomed. Pharmacother. 2020, 129, 110419. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zheng, J.; Sun, Y.; Wu, Z.; Liu, Z.; Huang, G. MicroRNA-1 Regulates Cardiomyocyte Apoptosis by Targeting Bcl-2. Int. Heart J. 2009, 50, 377–387. [Google Scholar] [CrossRef]
- Bian, B.; Yu, X.-F.; Wang, G.-Q.; Teng, T.-M. Role of miRNA-1 in regulating connexin 43 in ischemia–reperfusion heart injury: A rat model. Cardiovasc. Pathol. 2017, 27, 37–42. [Google Scholar] [CrossRef]
- Lerner, D.L.; Yamada, K.A.; Schuessler, R.B.; Saffitz, J.E. Accelerated Onset and Increased Incidence of Ventricular Arrhythmias Induced by Ischemia in Cx43-Deficient Mice. Circulation 2000, 101, 547–552. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, X.; Ren, J.; Li, X.; Gao, X.; Lu, C.; Zhang, Y.; Sun, H.; Wang, Y.; Wang, H.; et al. miR-1 Exacerbates Cardiac Ischemia-Reperfusion Injury in Mouse Models. PLoS ONE 2012, 7, e50515. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.S.; Guo, W.; Zhu, J.N.; Tang, C.M.; Fu, Y.H.; Lin, Q.X.; Tan, N.; Shan, Z.X. Hsp90aa1: A novel target gene of miR-1 in cardiac ischemia/reperfusion injury. Sci. Rep. 2016, 6, 24498. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Tomaniak, M.; Błaszczyk, O.; Kołtowski, Ł.; Filipiak, K.J.; Sitkiewicz, D. Circulating microribonucleic acids miR-1, miR-21 and miR-208a in patients with symptomatic heart failure: Preliminary results. Arch. Cardiovasc. Dis. 2015, 108, 634–642. [Google Scholar] [CrossRef] [PubMed]
- D’Amours, D.; Sallmann, F.R.; Dixit, V.M. Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: Implications for apoptosis. J. Cell Sci. 2001, 114, 3771–3778. [Google Scholar] [CrossRef]
- Li, B.; Li, R.; Zhang, C.; Bian, H.-J.; Wang, F.; Xiao, J.; Liu, S.-W.; Yi, W.; Zhang, M.-X.; Wang, S.-X.; et al. MicroRNA-7a/b Protects against Cardiac Myocyte Injury in Ischemia/Reperfusion by Targeting Poly(ADP-Ribose) Polymerase. PLoS ONE 2014, 9, e90096. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ji, N.; Luo, G.; Ni, S.; Zong, J.; Chen, Z.; Bao, D.; Gong, X.; Fu, T. The Effects and Mechanism of miR-92a and miR-126 on Myocardial Apoptosis in Mouse Ischemia-Reperfusion Model. Cell Biophys. 2014, 70, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tao, Y.; Huang, Q. Effect and mechanism of miR-126 in myocardial ischemia reperfusion. Genet. Mol. Res. 2015, 14, 18990–18998. [Google Scholar] [CrossRef]
- Chen, J.-J.; Zhou, S.-H. Mesenchymal stem cells overexpressing MiR-126 enhance ischemic angiogenesis via the AKT/ERK-related pathway. Cardiol. J. 2011, 18, 675–681. [Google Scholar] [CrossRef]
- Hu, X.; Van Marion, D.M.S.; Wiersma, M.; Zhang, D.; Brundel, B.J.J.M. The protective role of small heat shock proteins in cardiac diseases: Key role in atrial fibrillation. Cell Stress Chaperon 2017, 22, 665–674. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, Y.; Liang, P.; Li, Y.; Liu, Z.; Tong, Z.; Lv, Q.; Liu, M.; Xiao, X. MicroRNA-126a-5p enhances myocardial ischemia-reperfusion injury through suppressing Hspb8 expression. Oncotarget 2017, 8, 94172–94187. [Google Scholar] [CrossRef][Green Version]
- Zernecke, A.; Bidzhekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Denecke, B.; Hristov, M.; Köppel, T.; Jahantigh, M.N.; Lutgens, E.; et al. Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12-Dependent Vascular Protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef]
- Pordzik, J.; Eyileten-Postuła, C.; Jakubik, D.; Czajka, P.; Nowak, A.; De Rosa, S.; Gąsecka, A.; Cieślicka-Kapłon, A.; Sulikowski, P.; Filipiak, K.; et al. MiR-126 Is an Independent Predictor of Long-Term All-Cause Mortality in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. 2021, 10, 2371. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiao, F.-Y.; Shan, P.-R.; Su, L.; Chen, D.-L.; Ding, J.-Y.; Wang, Z.-Q. Overexpression of microRNA-133a inhibits ischemia-reperfusion-induced cardiomyocyte apoptosis by targeting DAPK2. J. Hum. Genet. 2015, 60, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zou, C.; Jia, Y.; Zhang, H.; Ma, X.; Zhang, J. Knockdown of circular RNA circMAT2B reduces oxygen-glucose deprivation-induced inflammatory injury in H9c2 cells through up-regulating miR-133. Cell Cycle 2020, 19, 2622–2630. [Google Scholar] [CrossRef]
- He, B.; Xiao, J.; Ren, A.-J.; Zhang, Y.-F.; Zhang, H.; Chen, M.; Xie, B.; Gao, X.-G.; Wang, Y.-W. Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J. Biomed. Sci. 2011, 18, 22. [Google Scholar] [CrossRef]
- Xu, C.; Hu, Y.; Hou, L.; Ju, J.; Li, X.; Du, N.; Guan, X.; Liu, Z.; Zhang, T.; Qin, W.; et al. β-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. J. Mol. Cell. Cardiol. 2014, 75, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lu, Z. MicroRNA-144 attenuates cardiac ischemia/reperfusion injury by targeting FOXO1. Exp. Ther. Med. 2019, 17, 2152–2160. [Google Scholar] [CrossRef]
- Gao, C.-K.; Liu, H.; Cui, C.-J.; Liang, Z.-G.; Yao, H.; Tian, Y. Role of microRNA-195 in cardiomyocyte apoptosis induced by myocardial ischaemia–reperfusion injury. J. Genet. 2016, 95, 99–108. [Google Scholar] [CrossRef]
- Gehmert, S.; Sadat, S.; Song, Y.-H.; Yan, Y.; Alt, E. The anti-apoptotic effect of IGF-1 on tissue resident stem cells is mediated via PI3-kinase dependent secreted frizzled related protein 2 (Sfrp2) release. Biochem. Biophys. Res. Commun. 2008, 371, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Liu, X.; Zheng, H.; Huang, X.; Wu, Y.; Huang, A.; Zhu, H.; Hu, Y.; Mai, W.; Huang, Y. IGF-1 enhances BMSC viability, migration, and anti-apoptosis in myocardial infarction via secreted frizzled-related protein 2 pathway. Stem Cell Res. Ther. 2020, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, B.; Diao, H.-Y.; Shi, Y.-F.; Zhang, J.-C.; Li, Y.-X.; Liu, N.; Yu, Y.-P.; Wang, G.; Wang, J.-P.; et al. Down-regulation of microRNA-320 suppresses cardiomyocyte apoptosis and protects against myocardial ischemia and reperfusion injury by targeting IGF-1. Oncotarget 2016, 7, 39740–39757. [Google Scholar] [CrossRef]
- Tian, Z.-Q.; Jiang, H.; Lu, Z.-B. MiR-320 regulates cardiomyocyte apoptosis induced by ischemia–reperfusion injury by targeting AKIP1. Cell. Mol. Biol. Lett. 2018, 23, 41. [Google Scholar] [CrossRef]
- Ren, X.-P.; Wu, J.; Wang, X.; Sartor, M.A.; Qian, J.; Jones, K.; Nicolaou, P.; Pritchard, T.J.; Fan, G.-C. MicroRNA-320 Is Involved in the Regulation of Cardiac Ischemia/Reperfusion Injury by Targeting Heat-Shock Protein 20. Circulation 2009, 119, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Yim, A.P.; Tang, N.L.; Lee, T.W.; Wan, I.Y.; Izzat, M.B.; Wan, S. Avoiding cardiopulmonary bypass in multivessel CABG reduces cytokine response and myocardial injury. Ann. Thorac. Surg. 1999, 68, 52–56. [Google Scholar] [CrossRef]
- Long, G.; Wang, F.; Duan, Q.; Chen, F.; Yang, S.; Gong, W.; Wang, Y.; Chen, C.; Wang, D.W. Human Circulating MicroRNA-1 and MicroRNA-126 as Potential Novel Indicators for Acute Myocardial Infarction. Int. J. Biol. Sci. 2012, 8, 811–818. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, Y.; Wang, X.; Li, S.; Yu, T.; Duan, W.; Wu, J.; Wen, Z.; Jiao, Y.; Sun, Z.; et al. Circulating miR-1 as a potential predictor of left ventricular remodeling following acute ST-segment myocardial infarction using cardiac magnetic resonance. Quant. Imaging Med. Surg. 2020, 10, 1490–1503. [Google Scholar] [CrossRef] [PubMed]
- Grabmaier, U.; Clauss, S.; Gross, L.; Klier, I.; Franz, W.; Steinbeck, G.; Wakili, R.; Theiss, H.; Brenner, C. Diagnostic and prognostic value of miR-1 and miR-29b on adverse ventricular remodeling after acute myocardial infarction—The SITAGRAMI-miR analysis. Int. J. Cardiol. 2017, 244, 30–36. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Shen, J.; Tian, D.; Ji, Q.; Xia, L.; Lv, Q. Plasma microRNAs reflecting cardiac and inflammatory injury in coronary artery bypass grafting surgery. J. Surg. Res. 2018, 224, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Emanueli, C.; Shearn, A.I.U.; Laftah, A.; Fiorentino, F.; Reeves, B.C.; Beltrami, C.; Mumford, A.; Clayton, A.; Gurney, M.; Shantikumar, S.; et al. Coronary Artery-Bypass-Graft Surgery Increases the Plasma Concentration of Exosomes Carrying a Cargo of Cardiac MicroRNAs: An Example of Exosome Trafficking Out of the Human Heart with Potential for Cardiac Biomarker Discovery. PLoS ONE 2016, 11, e0154274. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhao, X.; Liu, Y.-Z.; Meng, Z.; Wang, D.; Yang, F.; Shi, Q.-W. Plasma MicroRNA-126-5p is Associated with the Complexity and Severity of Coronary Artery Disease in Patients with Stable Angina Pectoris. Cell. Physiol. Biochem. 2016, 39, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Mukaihara, K.; Yamakuchi, M.; Kanda, H.; Shigehisa, Y.; Arata, K.; Matsumoto, K.; Takenouchi, K.; Oyama, Y.; Koriyama, T.; Hashiguchi, T.; et al. Evaluation of VEGF-A in platelet and microRNA-126 in serum after coronary artery bypass grafting. Heart Vessel. 2021, 36, 1635–1645. [Google Scholar] [CrossRef]
- Pourrajab, F.; Velashani, F.T.; Khanaghaei, M.; Hekmatimoghaddam, S.; Rahaie, M.; Zare-Khormizi, M.R. Comparison of miRNA signature versus conventional biomarkers before and after off-pump coronary artery bypass graft. J. Pharm. Biomed. Anal. 2017, 134, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Bostjancic, E.; Zidar, N.; Štajer, D.; Glavač, D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 Are Dysregulated in Human Myocardial Infarction. Cardiology 2010, 115, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA Expression in Circulating Microvesicles Predicts Cardiovascular Events in Patients With Coronary Artery Disease. J. Am. Heart Assoc. 2014, 3, e001249. [Google Scholar] [CrossRef] [PubMed]
- Yamac, A.H.; Huyut, M.A.; Yilmaz, E.; Celikkale, I.; Bacaksiz, A.; Demir, Y.; Demir, A.R.; Erturk, M.; Bakhshaliyev, N.; Ozdemir, R.; et al. MicroRNA 199a Is Downregulated in Patients After Coronary Artery Bypass Graft Surgery and Is Associated with Increased Levels of Sirtuin 1 (SIRT 1) Protein and Major Adverse Cardiovascular Events at 3-Year Follow-Up. Med. Sci. Monit. 2018, 24, 6245–6254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-L.; Sun, Q.; Zhang, L.; Li, R. RETRACTED: miR-208b targets Bax to protect H9c2 cells against hypoxia-induced apoptosis. Biomed. Pharmacother. 2018, 106, 1751–1759. [Google Scholar] [CrossRef]
- Pinchi, E.; Frati, P.; Aromatario, M.; Cipolloni, L.; Fabbri, M.; La Russa, R.; Maiese, A.; Neri, M.; Santurro, A.; Scopetti, M.; et al. miR-1, miR-499 and miR-208 are sensitive markers to diagnose sudden death due to early acute myocardial infarction. J. Cell. Mol. Med. 2019, 23, 6005–6016. [Google Scholar] [CrossRef]
- Gidlof, O.; Smith, J.G.; Miyazu, K.; Gilje, P.; Spencer, A.; Blomquist, S.; Erlinge, D. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc. Disord. 2013, 13, 12. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Gao, L.; Li, Y.; Sun, Y.; Yao, H.-C. Plasma miR-208b and miR-499: Potential Biomarkers for Severity of Coronary Artery Disease. Dis. Markers 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Olivieri, F.; Antonicelli, R.; Lorenzi, M.; D’Alessandra, Y.; Lazzarini, R.; Santini, G.; Spazzafumo, L.; Lisa, R.; La Sala, L.; Galeazzi, R.; et al. Diagnostic potential of circulating miR-499-5p in elderly patients with acute non ST-elevation myocardial infarction. Int. J. Cardiol. 2013, 167, 531–536. [Google Scholar] [CrossRef]
- Li, P.; Li, S.-Y.; Liu, M.; Ruan, J.-W.; Wang, Z.-D.; Xie, W.-C. Value of the expression of miR-208, miR-494, miR-499 and miR-1303 in early diagnosis of acute myocardial infarction. Life Sci. 2019, 232, 116547. [Google Scholar] [CrossRef]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Wang, L.; Xu, H.; Lu, Z.; Xuan, Y.; Meng, W.; Ye, L.; Fang, D.; Zhou, Y.; et al. MicroRNA-126 suppresses the proliferation and migration of endothelial cells in experimental diabetic retinopathy by targeting polo-like kinase 4. Int. J. Mol. Med. 2020, 47, 151–160. [Google Scholar] [CrossRef]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.-F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D. miR-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Head, S.J.; Davierwala, P.M.; Serruys, P.W.; Redwood, S.R.; Colombo, A.; Mack, M.J.; Morice, M.-C.; Holmes, D.R.; Feldman, T.E.; Ståhle, E.; et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: Final five-year follow-up of the SYNTAX trial. Eur. Heart J. 2014, 35, 2821–2830. [Google Scholar] [CrossRef]
- Qu, Q.; Bing, W.; Meng, X.; Xi, J.; Bai, X.; Liu, Q.; Guo, Y.; Zhao, X.; Bi, Y. Upregulation of miR-126-3p promotes human saphenous vein endothelial cell proliferation in vitro and prevents vein graft neointimal formation ex vivo and in vivo. Oncotarget 2017, 8, 106790–106806. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arisato, T.; Hashiguchi, T.; Sarker, K.P.; Arimura, K.; Asano, M.; Matsuo, K.; Osame, M.; Maruyama, I. Highly accumulated platelet vascular endothelial growth factor in coagulant thrombotic region. J. Thromb. Haemost. 2003, 1, 2589–2593. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 789–791. [Google Scholar] [CrossRef]
- Grozovsky, R.; Giannini, S.; Falet, H.; Hoffmeister, K.M. Novel mechanisms of platelet clearance and thrombopoietin regulation. Curr. Opin. Hematol. 2015, 22, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shen, C.; Zhao, S.L.; Hu, Y.J.; Song, Y.; Zhong, Q.J. MicroRNA-126 affects cell apoptosis, proliferation, cell cycle and mod-ulates VEGF/ TGF-β levels in pulmonary artery endothelial cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3058–3069. [Google Scholar] [CrossRef]
- Miyamoto, S.; Usami, S.; Kuwabara, Y.; Horie, T.; Baba, O.; Hakuno, D.; Nakashima, Y.; Nishiga, M.; Izuhara, M.; Nakao, T.; et al. Expression Patterns of miRNA-423-5p in the Serum and Pericardial Fluid in Patients Undergoing Cardiac Surgery. PLoS ONE 2015, 10, e0142904. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jodati, A.; Pirouzpanah, S.M.; Maroufi, N.F.; Pezeshkian, M.; Safaie, N.; Bijanpour, H.; Khamaneh, A.M.; Mota, A.; Nouri, M. Different expression of Micro RNA-126, 133a and 145 in aorta and saphenous vein samples of patients undergoing coronary artery bypass graft surgery. J. Cardiovasc. Thorac. Res. 2019, 11, 43–47. [Google Scholar] [CrossRef]
- Navickas, R.; Gal, D.; Laucevičius, A.; Taparauskaitė, A.; Zdanytė, M.; Holvoet, P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: A systematic review. Cardiovasc. Res. 2016, 111, 322–337. [Google Scholar] [CrossRef]
- Izarra, A.; Moscoso, I.; Levent, E.; Cañón, S.; Cerrada, I.; Diez-Juan, A.; Blanca, V.; Núñez-Gil, I.-J.; Valiente, I.; Ruíz-Sauri, A.; et al. miR-133a Enhances the Protective Capacity of Cardiac Progenitors Cells after Myocardial Infarction. Stem Cell Rep. 2014, 3, 1029–1042. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Chen, W.; Xie, L.; Zhao, Z.-A.; Yang, J.; Chen, Y.; Lei, W.; Shen, Z. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res. Ther. 2017, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xin, J.; Song, L.; Chen, Y.; Ma, J.; Liu, L.; Qi, Z.; Pan, X.; Zhou, S. Serum miR-133 as a Potential Biomarker in Acute Cerebral Infarction Patients. Clin. Lab. 2020, 6, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Vegter, E.L.; Ovchinnikova, E.S.; Van Veldhuisen, D.J.; Jaarsma, T.; Berezikov, E.; Van Der Meer, P.; Voors, A.A. Low circulating microRNA levels in heart failure patients are associated with atherosclerotic disease and cardiovascular-related rehospitalizations. Clin. Res. Cardiol. 2017, 106, 598–609. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, L.; Lou, J.; Lu, M.; Gao, C.; Zhao, S.; Li, B.; Liang, S.; Li, Y.; Li, D.; Liu, M. Suppression of miR-199a maturation by HuR is crucial for hypoxia-induced glycolytic switch in hepatocellular carcinoma. EMBO J. 2015, 34, 2671–2685. [Google Scholar] [CrossRef]
- Kido, M.; Du, L.; Sullivan, C.C.; Li, X.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Hypoxia-Inducible Factor 1-Alpha Reduces Infarction and Attenuates Progression of Cardiac Dysfunction After Myocardial Infarction in the Mouse. J. Am. Coll. Cardiol. 2005, 46, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Rane, S.; He, M.; Sayed, D.; Vashistha, H.; Malhotra, A.; Sadoshima, J.; Vatner, D.E.; Vatner, S.F.; Abdellatif, M. Downregulation of MiR-199a Derepresses Hypoxia-Inducible Factor-1α and Sirtuin 1 and Recapitulates Hypoxia Preconditioning in Cardiac Myocytes. Circ. Res. 2009, 104, 879–886. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, Y.; Hu, H.; Cui, W. Atorvastatin Protects Myocardium Against Ischemia-Reperfusion Injury Through Inhibiting miR-199a-5p. Cell. Physiol. Biochem. 2016, 39, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Takahashi, R.; Hiura, Y.; Hirokawa, G.; Fukushima, Y.; Iwai, N. Plasma miR-208 as a Biomarker of Myocardial Injury. Clin. Chem. 2009, 55, 1944–1949. [Google Scholar] [CrossRef]
- Montgomery, R.L.; Hullinger, T.G.; Semus, H.M.; Dickinson, B.A.; Seto, A.G.; Lynch, J.M.; Stack, C.; Latimer, P.A.; Olson, E.N.; Van Rooij, E. Therapeutic Inhibition of miR-208a Improves Cardiac Function and Survival During Heart Failure. Circulation 2011, 124, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Su, T.; Li, H.; Huang, Q.; Wu, D.; Yang, C.; Han, Z. Circulating miR-499 are novel and sensitive biomarker of acute myocardial infarction. J. Thorac. Dis. 2015, 7, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Agiannitopoulos, K.; Pavlopoulou, P.; Tsamis, K.; Bampali, K.; Samara, P.; Nasioulas, G.; Mertzanos, G.; Babalis, D.; Lamnissou, K. Expression of miR-208b and miR-499 in Greek Patients with Acute Myocardial Infarction. In Vivo 2018, 32, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yang, C.; Han, Z. Circulating miR-499 as a potential biomarker for acute myocardial infarction. Ann. Transl. Med. 2016, 4, 135. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Z.; Zhao, T.; Cao, W.; Zhang, L.; Li, H.; Xie, Q.; Tian, Y.; Wang, B. Plasma miR-1, miR-208, miR-499 as potential predictive biomarkers for acute myocardial infarction: An independent study of Han population. Exp. Gerontol. 2015, 72, 230–238. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Fichtlscherer, S.; Lehmann, R.; Assmus, B.; Dimmeler, S.; Zeiher, A.M. Transcoronary Concentration Gradients of Circulating MicroRNAs. Circulation 2011, 124, 1936–1944. [Google Scholar] [CrossRef]
- Mathew, J.P.; Fontes, M.L.; Tudor, I.C.; Ramsay, J.; Duke, P.; Mazer, C.D.; Barash, P.G.; Hsu, P.H.; Mangano, D.T.; for the Investigators of the Ischemia Research and Education Foundation and the Multicenter Study of Perioperative Ischemia Research Group. A Multicenter Risk Index for Atrial Fibrillation After Cardiac Surgery. JAMA 2004, 291, 1720–1729. [Google Scholar] [CrossRef]
- Saxena, A.; Dinh, D.T.; Smith, J.A.; Shardey, G.C.; Reid, C.M.; Newcomb, A.E. Usefulness of Postoperative Atrial Fibrillation as an Independent Predictor for Worse Early and Late Outcomes After Isolated Coronary Artery Bypass Grafting (Multicenter Australian Study of 19,497 Patients). Am. J. Cardiol. 2012, 109, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mariscalco, G.; Musumeci, F.; Banach, M. Factors influencing post-coronary artery bypass grafting atrial fibrillation episodes. Kardiol. Polska 2013, 71, 1115–1120. [Google Scholar] [CrossRef]
- El-Essawi, A.; Abdelhalim, A.; Groeger, S.; Breitenbach, I.; Brouwer, R.; Kück, F.; Harringer, W. Predictors of postoperative atrial fibrillation persisting beyond hospital discharge after coronary artery bypass grafting. Perfusion 2020, 0267659120978647. [Google Scholar] [CrossRef]
- Phan, K.; Ha, H.S.; Phan, S.; Medi, C.; Thomas, S.P.; Yan, T.D. New-onset atrial fibrillation following coronary bypass surgery predicts long-term mortality: A systematic review and meta-analysis. Eur. J. Cardio-Thorac. Surg. 2015, 48, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Thorén, E.; Wernroth, M.-L.; Christersson, C.; Grinnemo, K.-H.; Jidéus, L.; Ståhle, E. Compared with matched controls, patients with postoperative atrial fibrillation (POAF) have increased long-term AF after CABG, and POAF is further associated with increased ischemic stroke, heart failure and mortality even after adjustment for AF. Clin. Res. Cardiol. 2020, 109, 1232–1242. [Google Scholar] [CrossRef]
- Mazzone, C.; Cioffi, G.; Di Nora, C.; Barbati, G.; Guidetti, F.; Faggiano, P.; Gaibazzi, N.; Faganello, G.; Borca, E.C.; Di Lenarda, A. Prognostic role of cardiac calcifications in primary prevention: A powerful marker of adverse outcome highly dependent on underlying cardiac rhythm. Int. J. Cardiol. 2018, 258, 262–268. [Google Scholar] [CrossRef]
- Tiru, M.; Kadado, A.J.; Rastegar, V.; Shah, K.; Joshi, K.K.; Lindenauer, P.; Lagu, T.; Stefan, M.S. An observational study of the management practices and outcomes of patients with new onset atrial fibrillation in non-cardiothoracic surgeries. Heart Lung 2020, 49, 304–308. [Google Scholar] [CrossRef]
- Lowres, N.; Freedman, B.; Gallagher, R.; Kirkness, A.; Marshman, D.; Orchard, J.; Neubeck, L. Identifying postoperative atrial fibrillation in cardiac surgical patients posthospital discharge, using iPhone ECG: A study protocol. BMJ Open 2015, 5, e006849. [Google Scholar] [CrossRef]
- Guenancia, C.; Pujos, C.; Debomy, F.; Malapert, G.; Laurent, G.; Bouchot, O. Incidence and Predictors of New-Onset Silent Atrial Fibrillation after Coronary Artery Bypass Graft Surgery. BioMed Res. Int. 2015, 2015, 703685. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitrega, K.; Lip, G.; Sredniawa, B.; Sokal, A.; Streb, W.; Przyludzki, K.; Zdrojewski, T.; Wierucki, L.; Rutkowski, M.; Bandosz, P.; et al. Predicting Silent Atrial Fibrillation in the Elderly: A Report from the NOMED-AF Cross-Sectional Study. J. Clin. Med. 2021, 10, 2321. [Google Scholar] [CrossRef] [PubMed]
- Dobreanu, D.; Svendsen, J.H.; Lewalter, T.; Hernández-Madrid, A.; Lip, G.Y.; Blomström-Lundqvist, C. Current practice for diagnosis and management of silent atrial fibrillation: Results of the European Heart Rhythm Association survey. Europace 2013, 15, 1223–1225. [Google Scholar] [CrossRef]
- ASalam, Z.A.; Nammas, W. Incidence and predictors of atrial fibrillation after coronary artery bypass surgery: Detection by event loop recorder monitoring from a contemporary multicentre cohort. Acta Cardiol. 2017, 72, 311–317. [Google Scholar] [CrossRef]
- Feldman, A.; Moreira, D.A.R.; Gun, C.; Wang, H.L.; Hirata, M.H.; Germano, J.D.F.; Leite, G.G.S.; Farsky, P. Analysis of Circulating miR-1, miR-23a, and miR-26a in Atrial Fibrillation Patients Undergoing Coronary Bypass Artery Grafting Surgery. Ann. Hum. Genet. 2017, 81, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Hussain, S.-R.A.; Biswas, S.; Azad, A.; Rink, C.; Gnyawali, S.; Shilo, S.; Nuovo, G.J.; Sen, C.K. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc. Res. 2009, 82, 21–29. [Google Scholar] [CrossRef]
- Krzywińska, O.; Bracha, M.; Jeanniere, C.; Recchia, E.; Kornatowska, K.K.; Kozakiewicz, M. Meta-Analysis of the Potential Role of miRNA-21 in Cardiovascular System Function Monitoring. BioMed Res. Int. 2020, 2020, 4525410. [Google Scholar] [CrossRef] [PubMed]
- Pernigo, M.; Benfari, G.; Geremia, G.; Noni, M.; Borio, G.; Mazzali, G.; Zamboni, M.; Onorati, F.; Faggian, G.; Vassanelli, C.; et al. Atrial Function as an Independent Predictor of Postoperative Atrial Fibrillation in Patients Undergoing Aortic Valve Surgery for Severe Aortic Stenosis. J. Am. Soc. Echocardiogr. 2017, 30, 956–965.e1. [Google Scholar] [CrossRef]
- Frustaci, A.; Chimenti, C.; Bellocci, F.; Morgante, E.; Russo, M.A.; Maseri, A. Histological Substrate of Atrial Biopsies in Patients With Lone Atrial Fibrillation. Circulation 1997, 96, 1180–1184. [Google Scholar] [CrossRef]
- Goette, A.; Juenemann, G.; Peters, B.; Klein, H.U.; Roessner, A.; Huth, C.; Röcken, C. Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc. Res. 2002, 54, 390–396. [Google Scholar] [CrossRef]
- Swartz, M.F.; Fink, G.W.; Sarwar, M.F.; Hicks, G.L.; Yu, Y.; Hu, R.; Lutz, C.J.; Taffet, S.M.; Jalife, J. Elevated Pre-Operative Serum Peptides for Collagen I and III Synthesis Result in Post-Surgical Atrial Fibrillation. J. Am. Coll. Cardiol. 2012, 60, 1799–1806. [Google Scholar] [CrossRef]
- Neuberger, H.-R.; Cacciatore, A.; Reil, J.-C.; Gräber, S.; Schäfers, H.-J.; Ukena, C.; Böhm, M.; Mewis, C. Procollagen propeptides: Serum markers for atrial fibrosis? Clin. Res. Cardiol. 2012, 101, 655–661. [Google Scholar] [CrossRef]
- Rizvi, F.; Mirza, M.; Olet, S.; Albrecht, M.; Edwards, S.; Emelyanova, L.; Kress, D.; Ross, G.R.; Holmuhamedov, E.; Tajik, A.J.; et al. Noninvasive biomarker-based risk stratification for development of new onset atrial fibrillation after coronary artery bypass surgery. Int. J. Cardiol. 2020, 307, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chiang, D.Y.; Zhang, M.; Voigt, N.; Alsina, K.M.; Jakob, H.; Martin, J.F.; Dobrev, D.; Wehrens, X.H.; Li, N. Identification of microRNA–mRNA dysregulations in paroxysmal atrial fibrillation. Int. J. Cardiol. 2015, 184, 190–197. [Google Scholar] [CrossRef]
- Sun, X.L.; Bu, P.L.; Liu, J.N.; Wang, X.; Wu, X.N.; Zhao, L.X. Expression of SIRT1 in right auricle tissues and the relationship with oxidative stress in patients with atrial fibrillation. Chin. J. Cell. Mol. Immunol. 2012, 28, 972–974. [Google Scholar]
- Yamac, A.H.; Kucukbuzcu, S.; Ozansoy, M.; Gok, O.; Oz, K.; Erturk, M.; Yilmaz, E.; Ersoy, B.; Zeybek, R.; Goktekin, O.; et al. Altered expression of micro-RNA 199a and increased levels of cardiac SIRT1 protein are associated with the occurrence of atrial fibrillation after coronary artery bypass graft surgery. Cardiovasc. Pathol. 2016, 25, 232–236. [Google Scholar] [CrossRef]
- Harling, L.; Lambert, J.; Ashrafian, H.; Darzi, A.; Gooderham, N.; Athanasiou, T. Elevated serum microRNA 483-5p levels may predict patients at risk of post-operative atrial fibrillation. Eur. J. Cardio-Thorac. Surg. 2016, 51, 73–78. [Google Scholar] [CrossRef]
- Bolkier, Y.; Nevo-Caspi, Y.; Salem, Y.; Vardi, A.; Mishali, D.; Paret, G. Micro-RNA-208a, -208b, and -499 as Biomarkers for Myocardial Damage After Cardiac Surgery in Children. Pediatr. Crit. Care Med. 2016, 17, e193–e197. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Xia, L.; Xu, Q.; Ji, Q.; Yao, Z.; Lv, Q. MiR-223 levels predicting perioperative bleeding in off-pump coronary artery bypass grafting. Ann. Transl. Med. 2020, 8, 1341. [Google Scholar] [CrossRef]
- Danowski, N.; Manthey, I.; Jakob, H.G.; Siffert, W.; Peters, J.; Frey, U.H. Decreased Expression of miR-133a but Not of miR-1 is Associated with Signs of Heart Failure in Patients Undergoing Coronary Bypass Surgery. Cardiology 2013, 125, 125–130. [Google Scholar] [CrossRef]
- Stoica, S.C.; Dorobantu, D.M.; Vardeu, A.; Biglino, G.; Ford, K.; Bruno, V.D.; Zakkar, M.; Mumford, A.; Angelini, G.D.; Caputo, M.; et al. MicroRNAs as potential biomarkers in congenital heart surgery. J. Thorac. Cardiovasc. Surg. 2019, 159, 1532–1540.e7. [Google Scholar] [CrossRef]
- Kapoor, R.; So, J.B.; Zhu, F.; Too, H.-P.; Yeoh, K.-G.; Yoong, J.S.-Y. Evaluating the Use of microRNA Blood Tests for Gastric Cancer Screening in a Stratified Population-Level Screening Program: An Early Model-Based Cost-Effectiveness Analysis. Value Health 2020, 23, 1171–1179. [Google Scholar] [CrossRef]
- Kapoor, R.; Zhu, F.; So, J.; Zou, R.; Zhou, L.; Too, H.; Yeoh, K.; Yik-Ying, T.; Yoong, J. Cost-Effectiveness analysis of using mirna biomarker panel as a screen before endoscopy for gastric cancer diagnosis. Value Health 2016, 19, A304. [Google Scholar] [CrossRef]
- Velmanickam, L.; Baines, M.; Fondakowski, M.; Dorsam, G.P.; Nawarathna, D. iLluminate-miRNA: Paradigm for high-throughput, low-cost, and sensitive miRNA detection in serum samples at point-of-care. J. Phys. D Appl. Phys. 2018, 52, 055401. [Google Scholar] [CrossRef]
| miRNA | Patient Group | Control Group | Outcome | Ref. |
|---|---|---|---|---|
| mir-1 | 17 AMI patients | 25 healthy adults | miR-1 expression increased in AMI patients and correlated with cTnI. | [85] |
| 80 STEMI patients: 22 with adverse LV remodeling, 58 without adverse LV remodeling | n/a | miR-1 predicts LV remodeling in patients with STEMI alone and shows incremental prediction value compared to clinical and magnetic resonance imaging. | [86] | |
| 44 AMI patients | 18 healthy adults | miR-1 increased in AMI patients and was inversely correlated with infarct volume (CMR marker of adverse ventricular remodeling). | [87] | |
| 29 CABG patients with ACS | n/a | miR-1 increased after CABG surgery, did not correlate with cTnT. | [88] | |
| 21 CABG patients | n/a | Plasma and exosome miR-1 increased after a CABG procedure. Exosomal but no plasma-circulating miR-1 positively correlated with cTnI. | [89] | |
| miR-126 | 140 CAD patients | 40 patients without CAD | The miR-126 level was lower in patients with multivessel CAD and higher SYNTAX score but not dramatically downregulated in patients with one vessel CAD and low SYNTAX score compared with the control. | [90] |
| 67 CABG patients | n/a | miR-126-3p level in serum increased rapidly after CABG and then decreased below preoperative levels. Seven days after CABG surgery, miR-126-3p level was higher in patients with peripheral artery disease (PAD), compared with patients without PAD. | [91] | |
| 70 off-pump CABG patients | n/a | miR-126 was downregulated 4 days after CABG surgery and correlated strongly with the level of uric acid. | [92] | |
| miR-133 | 50 patients with MI | 8 healthy adults and 9 fetuses | miR-133a was downregulated in patients with MI compared to control. | [93] |
| 30 on-pump CABG patients | n/a | miR-133b level increased after declamping in CABG patients. Moreover, miR-133a was found to reflect the extent of myocardial injury. miR-133 was 89.3% sensitive and 67.4% specific for the identification of PMI compared to cTnI, which had a sensitivity of 64.3% and specificity of 86.5% for a cutoff value of 2.98. | [46] | |
| 27 ACS patients undergoing CABG surgery | n/a | After CABG surgery, miR-133a level increased significantly and was associated with cTnT. | [88] | |
| 21 CABG patients | n/a | Plasma and exosomal miR-133a and miR-133b increased after CABG surgery. Exosomal but not plasma-circulating miR-133a and mir-133b correlated positively with cTnI. | [89] | |
| miR-199 | 181 patients with stable CAD | n/a | Increased expression of microvesicles containing miR-199a but not freely circulating miR-199a was significantly associated with a lower major adverse cardiovascular event rate. | [94] |
| 68 CABG surgery patients | 34 patients undergoing heart valve surgery | The level of miR-199a in CABG patients was significantly reduced compared to the control group. Patients with a major adverse cardiac event had a significantly lower level of miR-199a than uneventful patients. | [95] | |
| miR-208 | 62 MI patients | 18 cases of traumatic death without cardiac pathology | miR-208 was highly expressed in AMI patients. | [96] |
| 19 cases of death due to AMI 25 cases of sudden cardiac death | n/a | miR-208 presented high accuracy in discriminating patients who died suddenly due to AMI from those who succumbed without pathological cardiac involvement. | [97] | |
| 424 patients with suspected ASC | n/a | miR-208 was higher in MI patients and correlated with LVEF. The increased miR-208 level was strongly associated with an increased risk of mortality or heart failure within 30 days. | [98] | |
| 195 CAD patients | n/a | High plasma levels of miR-208 were positively associated with the severity of CAD and plasma miR-208b could act as a potential biomarker for estimating the severity of CAD. | [99] | |
| 27 ACS patients undergoing CABG surgery | n/a | miR-208 significantly increased after CABG surgery and was found to be associated with cTnT, CK-MB and IL-6. | [88] | |
| miR-499 | 92 NSTEMI patients, 81 patients with CHF without AMI | 99 healthy patients | The diagnostic accuracy of miR-499 was superior to that of cardiac TnT in elderly, NSTEMI patients. | [100] |
| 41 AMI patients, 32 SAP patients | 10 healthy patients | Serum miR-499 expression level at different time points was significantly higher in the AMI group than in the SAP group and control group. miR-499 was not superior to hs-cTnI as myocardial marker in the diagnosis of early AMI. | [101] | |
| 30 on-pump CABG patients, 30 off-pump CABG patients and a prospective cohort of 120 on-pump CABG patients | n/a | miR-499 had higher sensitivity and specificity than cTnI for identifying PMI in cardiac surgery and is a novel, early biomarker for identifying perioperative myocardial infarction in cardiac surgery. | [46] | |
| 70 off-pump CABG patients | n/a | A strong positive correlation between miR-499 and plasma concentration of cTnI and miR-499 and ventricle contractility (EF%) was observed. | [92] | |
| 29 ACS patients undergoing CABG surgery | n/a | The level of miR-499 was not increased after CABG surgery in ACS patients. | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błażejowska, E.; Urbanowicz, T.; Gąsecka, A.; Olasińska-Wiśniewska, A.; Jaguszewski, M.J.; Targoński, R.; Szarpak, Ł.; Filipiak, K.J.; Perek, B.; Jemielity, M. Diagnostic and Prognostic Value of miRNAs after Coronary Artery Bypass Grafting: A Review. Biology 2021, 10, 1350. https://doi.org/10.3390/biology10121350
Błażejowska E, Urbanowicz T, Gąsecka A, Olasińska-Wiśniewska A, Jaguszewski MJ, Targoński R, Szarpak Ł, Filipiak KJ, Perek B, Jemielity M. Diagnostic and Prognostic Value of miRNAs after Coronary Artery Bypass Grafting: A Review. Biology. 2021; 10(12):1350. https://doi.org/10.3390/biology10121350
Chicago/Turabian StyleBłażejowska, Ewelina, Tomasz Urbanowicz, Aleksandra Gąsecka, Anna Olasińska-Wiśniewska, Miłosz J. Jaguszewski, Radosław Targoński, Łukasz Szarpak, Krzysztof J. Filipiak, Bartłomiej Perek, and Marek Jemielity. 2021. "Diagnostic and Prognostic Value of miRNAs after Coronary Artery Bypass Grafting: A Review" Biology 10, no. 12: 1350. https://doi.org/10.3390/biology10121350
APA StyleBłażejowska, E., Urbanowicz, T., Gąsecka, A., Olasińska-Wiśniewska, A., Jaguszewski, M. J., Targoński, R., Szarpak, Ł., Filipiak, K. J., Perek, B., & Jemielity, M. (2021). Diagnostic and Prognostic Value of miRNAs after Coronary Artery Bypass Grafting: A Review. Biology, 10(12), 1350. https://doi.org/10.3390/biology10121350











