Potential of Asparagopsis armata as a Biopesticide for Weed Control under an Invasive Seaweed Circular-Economy Framework
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Asparagospsis armata Collection and Preparation of Exudates
2.2. Thellungiella halophila Culture and Exposure
2.3. Plant Primary Photochemistry
2.4. Leaf Pigment Profile
2.5. Leaf Fatty Acid Profile
2.6. Leaf Oxidative Stress Biomarkers
2.7. Statistical Data Analysis
3. Results
3.1. Primary Photochemistry
3.2. Leaf Pigment Profile
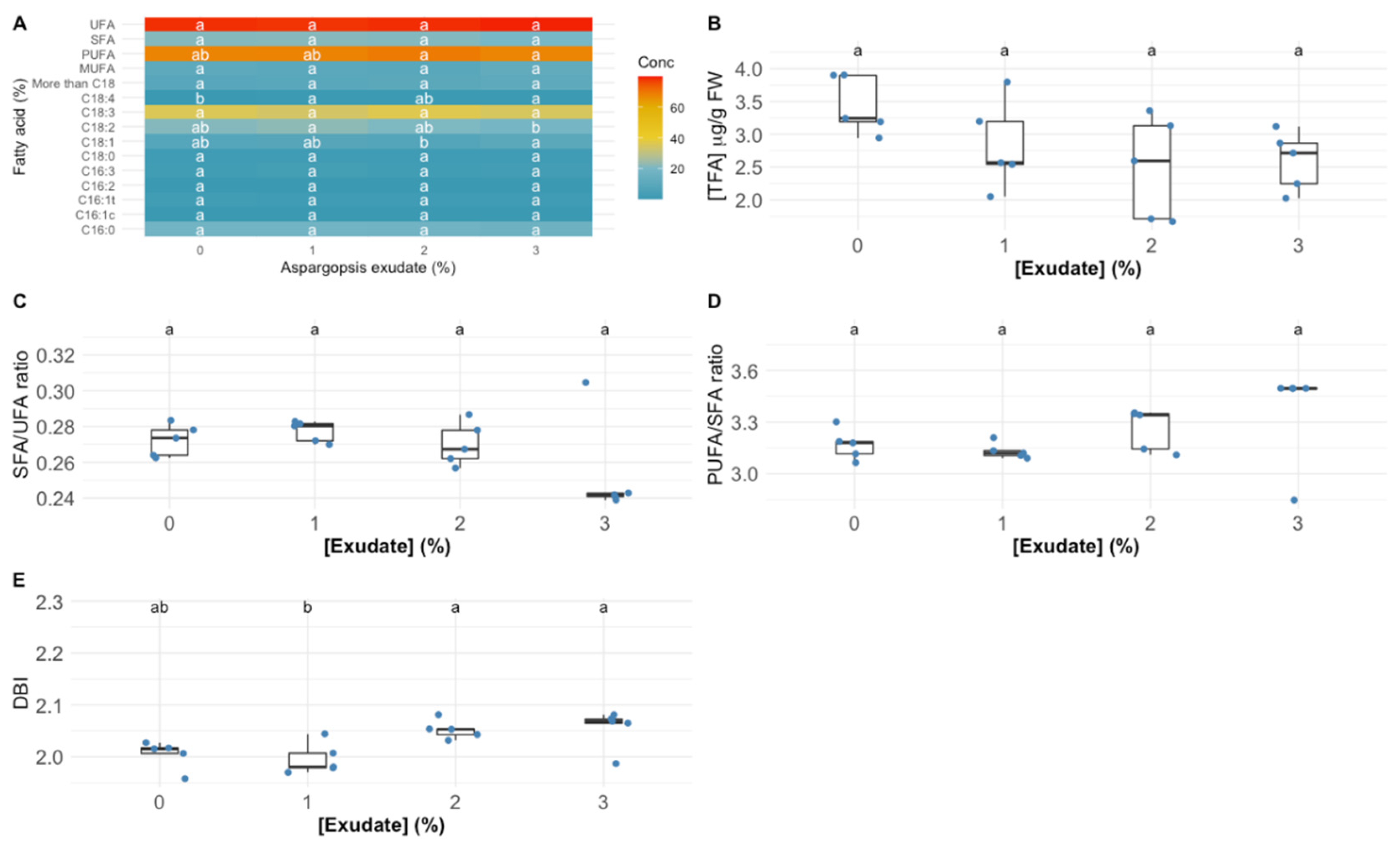
3.3. Leaf Fatty Acid Profile
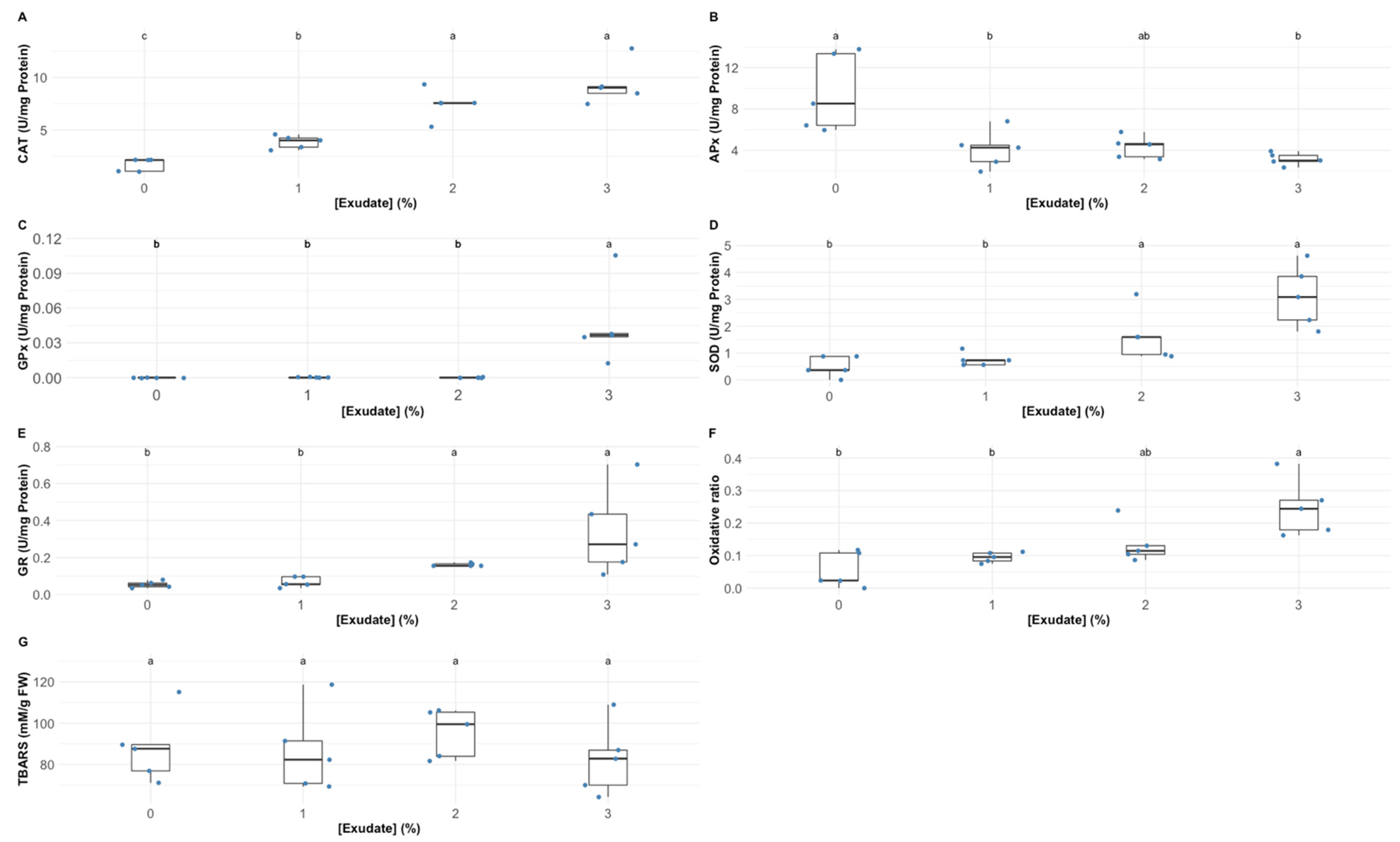
3.4. Oxidative Stress
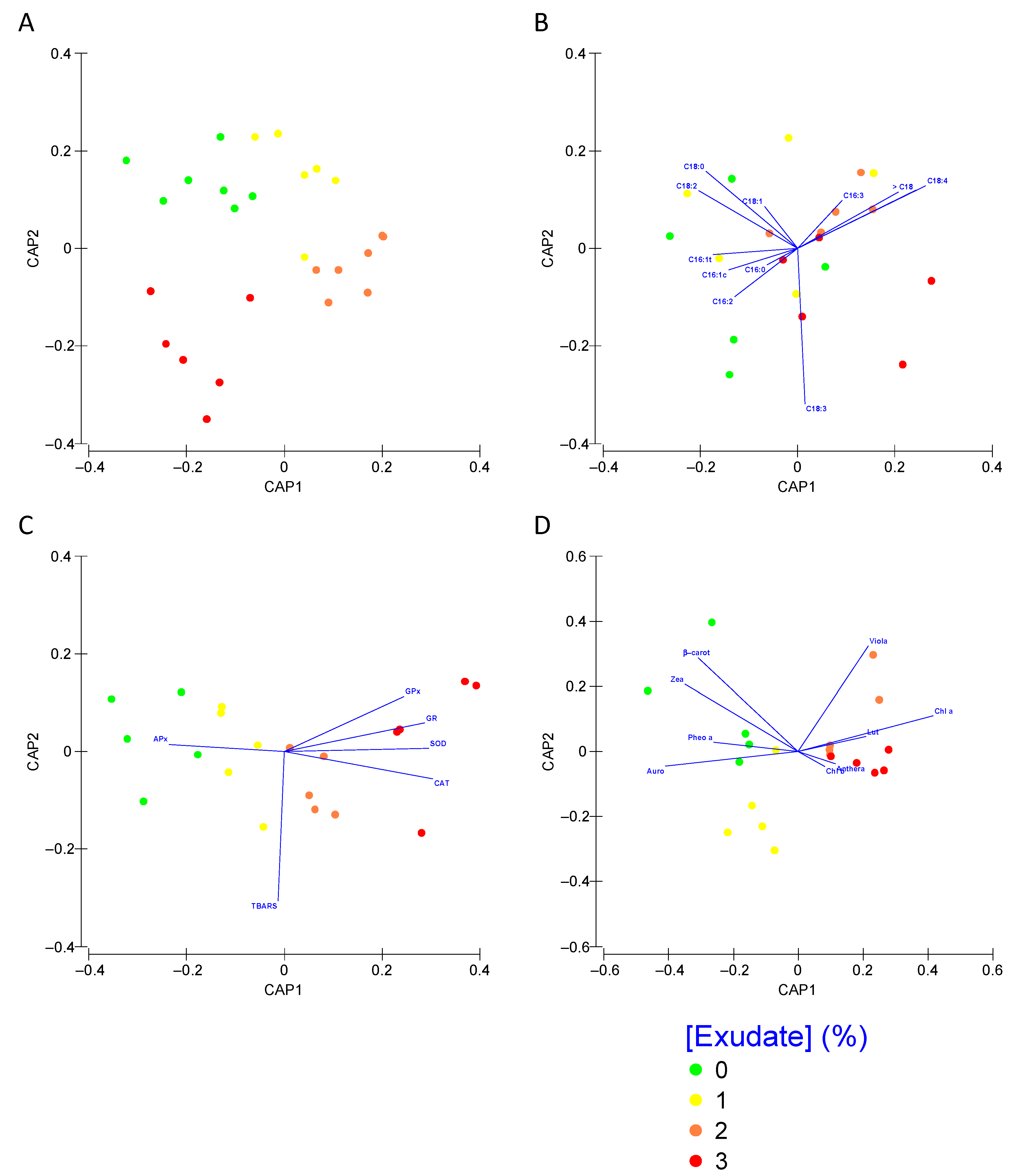
3.5. Biomarker Integrated Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- European Environment Agency. Late Lessons from Early Warnings: Science, Precaution, Innovation Summary; European Environment Agency: Luxembourg, 2013.
- Köhler, H.-R.; Triebskorn, R. Wildlife Ecotoxicology of Pesticides: Can We Track Effects to the Population Level and Beyond? Science 2013, 341, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Cruz de Carvalho, R.; Feijão, E.; Matos, A.R.; Cabrita, M.T.; Novais, S.C.; Lemos, M.F.L.; Caçador, I.; Marques, J.C.; Reis-Santos, P.; Fonseca, V.F.; et al. Glyphosate-Based Herbicide Toxicophenomics in Marine Diatoms: Impacts on Primary Production and Physiological Fitness. Appl. Sci. 2020, 10, 7391. [Google Scholar] [CrossRef]
- Copping, L.G.; Menn, J.J. Biopesticides: A Review of Their Action, Applications and Efficacy. Pest Manag. Sci. 2000, 56, 651–676. [Google Scholar] [CrossRef]
- Samada, L.H.; Tambunan, U.S.F. Biopesticides as Promising Alternatives to Chemical Pesticides: A Review of Their Current and Future Status. OnLine J. Biol. Sci. 2020, 20, 66–76. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- Trincone, A.; Kusaykin, M.; Ermakova, S. Editorial: Marine Biomolecules. Front. Chem. 2015, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.M.; Abd El-Rhman, A.A.; Abdel-Raouf, N.; Ibraheem, I.B.M. Role of Marine Macroalgae in Plant Protection & Improvement for Sustainable Agriculture Technology. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 104–110. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Neugebauer, A.; Silva, J.; Thomas, O.P.; Botana, L.M.; Gaspar, H.; Pedrosa, R. Marine Invasive Macroalgae: Turning a Real Threat into a Major Opportunity—The Biotechnological Potential of Sargassum muticum and Asparagopsis armata. Algal Res. 2018, 34, 217–234. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Biological Pollution in the Mediterranean Sea: Invasive versus Introduced Macrophytes. Mar. Pollut. Bull. 2002, 44, 32–38. [Google Scholar] [CrossRef]
- Silva, C.O.; Lemos, M.F.L.; Gaspar, R.; Gonçalves, C.; Neto, J.M. The Effects of the Invasive Seaweed Asparagopsis Armata on Native Rock Pool Communities: Evidences from Experimental Exclusion. Ecol. Indic. 2021, 125, 107463. [Google Scholar] [CrossRef]
- Silva, C.O.; Simões, T.; Félix, R.; Soares, A.M.V.M.; Barata, C.; Novais, S.C.; Lemos, M.F.L. Asparagopsis armata Exudate Cocktail: The Quest for the Mechanisms of Toxic Action of an Invasive Seaweed on Marine Invertebrates. Biology 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.O.; Novais, S.C.; Soares, A.M.V.M.; Barata, C.; Lemos, M.F.L. Impacts of the Invasive Seaweed Asparagopsis Armata Exudate on Energetic Metabolism of Rock Pool Invertebrates. Toxins 2021, 13, 15. [Google Scholar] [CrossRef]
- Burreson, B.J.; Moore, R.E.; Roller, P.P. Volatile Halogen Compounds in the Alga Asparagopsis taxiformis (Rhodophyta). J. Agric. Food Chem. 1976, 24, 856–861. [Google Scholar] [CrossRef]
- McConnell, O.; Fenical, W. Halogen Chemistry of the Red Alga Asparagopsis. Phytochemistry 1977, 16, 367–374. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. The Use of Invasive Algae Species as a Source of Secondary Metabolites and Biological Activities: Spain as Case-Study. Mar. Drugs 2021, 19, 178. [Google Scholar] [CrossRef]
- Félix, R.; Dias, P.; Félix, C.; Cerqueira, T.; Andrade, P.B.; Valentão, P.; Lemos, M.F.L. The Biotechnological Potential of Asparagopsis armata: What Is Known of Its Chemical Composition, Bioactivities and Current Market? Algal Res. 2021, 60, 102534. [Google Scholar] [CrossRef]
- European Economic and Social Committee. European Comission Innovation in the Blue Economy: Realising the Potential of Our Seas and Oceans for Jobs and Growth; European Economic and Social Committee: Brussels, Belgium, 2014.
- Amtmann, A.; Bohnert, H.J.; Bressan, R.A. Abiotic Stress and Plant Genome Evolution. Search for New Models. Plant Physiol. 2005, 138, 127–130. [Google Scholar] [CrossRef]
- Volkov, V.; Amtmann, A. Thellungiella Halophila, a Salt-Tolerant Relative of Arabidopsis thaliana, Has Specific Root Ion-Channel Features Supporting K+/Na+ Homeostasis under Salinity Stress. Plant J. 2006, 48, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Matos, A.R.; Caçador, I. Photobiological and Lipidic Responses Reveal the Drought Tolerance of Aster Tripolium Cultivated under Severe and Moderate Drought: Perspectives for Arid Agriculture in the Mediterranean. Plant Physiol. Biochem. 2020, 154, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.G.; Govindjee; Baker, N.R.; DeSturler, E.; Ort, D.R.; Long, S.P. Chlorophyll a Fluorescence Induction Kinetics in Leaves Predicted from a Model Describing Each Discrete Step of Excitation Energy and Electron Transfer Associated with Photosystem II. Planta 2005, 223, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Küpper, H.; Seibert, S.; Parameswaran, A. Fast, Sensitive, and Inexpensive Alternative to Analytical Pigment HPLC: Quantification of Chlorophylls and Carotenoids in Crude Extracts by Fitting with Gauss Peak Spectra. Anal. Chem. 2007, 79, 7611–7627. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Carreiras, J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Matos, A.R.; Marques, J.C.; Caçador, I. Halophyte Fatty Acids as Biomarkers of Anthropogenic-Driven Contamination in Mediterranean Marshes: Sentinel Species Survey and Development of an Integrated Biomarker Response (IBR) Index. Ecol. Indic. 2018, 87, 86–96. [Google Scholar] [CrossRef]
- Duarte, B.; Carreiras, J.; Feijão, E.; Reis-Santos, P.; Caçador, I.; Matos, A.R.; Fonseca, V.F. Fatty Acid Profiles of Estuarine Macroalgae Are Biomarkers of Anthropogenic Pressures: Development and Application of a Multivariate Pressure Index. Sci. Total Environ. 2021, 788, 147817. [Google Scholar] [CrossRef] [PubMed]
- Tiryakioglu, M.; Eker, S.; Ozkutlu, F.; Husted, S.; Cakmak, I. Antioxidant Defense System and Cadmium Uptake in Barley Genotypes Differing in Cadmium Tolerance. J. Trace Elem. Med. Biol. 2006, 20, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, Y.; Tanaka, A.; Osumi, M.; Fukui, S.; Osumi, M. Catalase Activities of Hydrocarbon-Utilizing Candida Yeasts. Agric. Biol. Chem. 1974, 38, 1213–1220. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Gawehn, K.; Grassl, M. Lactatedehydrogenase, UV-Assay with Pyruvate and NADH. In Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Marklund, S.; MARKLUND, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.G.; Hess, J.L. Responses of Superoxide Dismutase and Glutathione Reductase Activities in Cotton Leaf Tissue Exposed to an Atmosphere Enriched in Oxygen. Plant Physiol. 1980, 66, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Taiyun. Taiyun/Corrplot; Taiyun Tech Co., Ltd: Guangzhou, China, 2021. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Use R! Springer: New York, NY, USA, 2009; ISBN 978-0-387-98141-3. [Google Scholar]
- De Mendiburu, F.; Simon, R. Agricolae—Ten Years of an Open Source Statistical Tool for Experiments in Breeding, Agriculture and Biology. PeerJ PrePrints 2015, 3, e1404v1. [Google Scholar]
- Duarte, B.; Durante, L.; Marques, J.C.; Reis-Santos, P.; Fonseca, V.F.; Caçador, I. Development of a Toxicophenomic Index for Trace Element Ecotoxicity Tests Using the Halophyte Juncus Acutus: Juncus-TOX. Ecol. Indic. 2021, 121, 107097. [Google Scholar] [CrossRef]
- Pires, V.L.; Novais, S.C.; Lemos, M.F.L.; Fonseca, V.F.; Duarte, B. Evaluation of Multivariate Biomarker Indexes Application in Ecotoxicity Tests with Marine Diatoms Exposed to Emerging Contaminants. Appl. Sci. 2021, 11, 3878. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; 192p. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Silva, J.; Pedrosa, R. The Marine Invasive Seaweeds Asparagopsis Armata and Sargassum Muticum as Targets for Greener Antifouling Solutions. Sci. Total Environ. 2021, 750, 141372. [Google Scholar] [CrossRef]
- Félix, R.; Félix, C.; Januário, A.P.; Carmona, A.M.; Baptista, T.; Gonçalves, R.A.; Sendão, J.; Novais, S.C.; Lemos, M.F.L. Tailoring Shrimp Aquafeed to Tackle Acute Hepatopancreatic Necrosis Disease by Inclusion of Industry-Friendly Seaweed Extracts. Aquaculture 2020, 529, 735661. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Simões, M.; Alves, C.; Silva, J.; Gaspar, H.; Martins, A.; Rodrigues, A.; Pedrosa, R. Marine Invasive Species for High-Value Products’ Exploration—Unveiling the Antimicrobial Potential of Asparagopsis armata against Human Pathogens. Algal Res. 2020, 52, 102091. [Google Scholar] [CrossRef]
- Black, C.C. Effects of Herbicides on Photosynthesis. In Weed Physiology; CRC Press: Boca Raton, FL, USA, 1985; ISBN 978-1-351-07773-6. [Google Scholar]
- Hassannejad, S.; Lotfi, R.; Ghafarbi, S.P.; Oukarroum, A.; Abbasi, A.; Kalaji, H.M.; Rastogi, A. Early Identification of Herbicide Modes of Action by the Use of Chlorophyll Fluorescence Measurements. Plants 2020, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Hess, F.D. Light-Dependent Herbicides: An Overview. Weed Sci. 2000, 48, 160–170. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ohki, S.; Koizumi, K.; Tanaka, A.; Watanabe, H.; Kohno, H.; van Rensen, J.J.S.; Böger, P.; Wakabayashi, K. Binding Site of Novel 2-Benzylamino-4-Methyl-6-Trifluoromethyl-1,3,5- Triazine Herbicides in the D1 Protein of Photosystem II. Photosynth. Res. 2003, 77, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Lim, S.H.; Kim, J.W.; Nah, G.; Fischer, A.; Kim, D.S. Leaf Chlorophyll Fluorescence Discriminates Herbicide Resistance in Echinochloa Species. Weed Res. 2016, 56, 424–433. [Google Scholar] [CrossRef]
- de Souza, J.M.; Fazolo, B.R.; Lacerda, J.W.F.; de Moura, M.S.; dos Santos, A.C.R.; de Vasconcelos, L.G.; de Sousa Junior, P.T.; Dall’Oglio, E.L.; Ali, A.; Sampaio, O.M.; et al. Rational Design, Synthesis and Evaluation of Indole Nitrogen Hybrids as Photosystem II Inhibitors. Photochem. Photobiol. 2020, 96, 1233–1242. [Google Scholar] [CrossRef]
- Darwish, M.; Vidal, V.; Lopez-Lauri, F.; Alnaser, O.; Junglee, S.; El Maataoui, M.; Sallanon, H. Tolerance to Clomazone Herbicide Is Linked to the State of LHC, PQ-Pool and ROS Detoxification in Tobacco (Nicotiana tabacum L.). J. Plant Physiol. 2015, 175, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.L.; Geider, R.J.; Flynn, K.J. A Mechanistic Model of Photoinhibition. New Phytol. 2000, 145. [Google Scholar] [CrossRef]
- Havurinne, V.; Tyystjärvi, E. Action Spectrum of Photoinhibition in the Diatom Phaeodactylum tricornutum. Plant Cell Physiol. 2017, 58, 2217–2225. [Google Scholar] [CrossRef]
- Chiang, Y.-J.; Wu, Y.-X.; Chiang, M.-Y.; Wang, C.-Y. Role of Antioxidative System in Paraquat Resistance of Tall Fleabane (Conyza sumatrensis). Weed Sci. 2008, 56, 350–355. [Google Scholar] [CrossRef]
- Duarte, B.; Goessling, J.W.; Marques, J.C.; Caçador, I. Ecophysiological Constraints of Aster Tripolium under Extreme Thermal Events Impacts: Merging Biophysical, Biochemical and Genetic Insights. Plant Physiol. Biochem. 2015, 97, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, M.; Ruban, A.V.; Horton, P. Chlorophyll Fluorescence Quenching in Isolated Light Harvesting Complexes Induced by Zeaxanthin. FEBS Lett. 2000, 471, 71–74. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Bleaching Herbicides: Action Mechanism in Carotenoid Biosynthesis, Structural Requirements and Engineering of Resistance. In Herbicide Classes in Development: Mode of Action, Targets, Genetic Engineering, Chemistry; Böger, P., Wakabayashi, K., Hirai, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 43–57. ISBN 978-3-642-59416-8. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Trémolières, A.; Roche, O.; Dubertret, G.; Guyon, D.; Garnier, J. Restoration of Thylakoid Appression by Δ3-Trans-Hexadecenoic Acid-Containing Phosphatidylglycerol in a Mutant of Chlamydomonas Reinhardtii. Relationships with the Regulation of Excitation Energy Distribution. BBA Bioenerg. 1991, 1059, 286–292. [Google Scholar] [CrossRef]
- Duarte, B.; Matos, A.R.; Marques, J.C.; Caçador, I. Leaf Fatty Acid Remodeling in the Salt-Excreting Halophytic Grass Spartina Patens along a Salinity Gradient. Plant Physiol. Biochem. 2018, 124, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Cabrita, M.T.; Gameiro, C.; Matos, A.R.; Godinho, R.; Marques, J.C.; Caçador, I. Disentangling the Photochemical Salinity Tolerance in Aster Tripolium L.: Connecting Biophysical Traits with Changes in Fatty Acid Composition. Plant Biol. J. 2017, 19, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.-U.; Lem, N.W.; Chandorkar, K.R.; Williams, J.P. Effects of Substituted Pyridazinones (San 6706, San 9774, San 9785) on Glycerolipids and Their Associated Fatty Acids in the Leaves of Vicia Faba and Hordeum vulgare. Plant Physiol. 1979, 64, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.M.; Batista, J.E.; Mariano, P.; Fonseca, V.; Duarte, B.; Silva, S. Artificial Intelligence Meets Marine Ecotoxicology: Applying Deep Learning to Bio-Optical Data from Marine Diatoms Exposed to Legacy and Emerging Contaminants. Biology 2021, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Feijão, E.; Cruz de Carvalho, R.; Franzitta, M.; Carlos Marques, J.; Caçador, I.; Teresa Cabrita, M.; Fonseca, V.F. Unlocking Kautsky’s Dark Box: Development of an Optical Toxicity Classification Tool (OPTOX Index) with Marine Diatoms Exposed to Emerging Contaminants. Ecol. Indic. 2021, 131, 108238. [Google Scholar] [CrossRef]
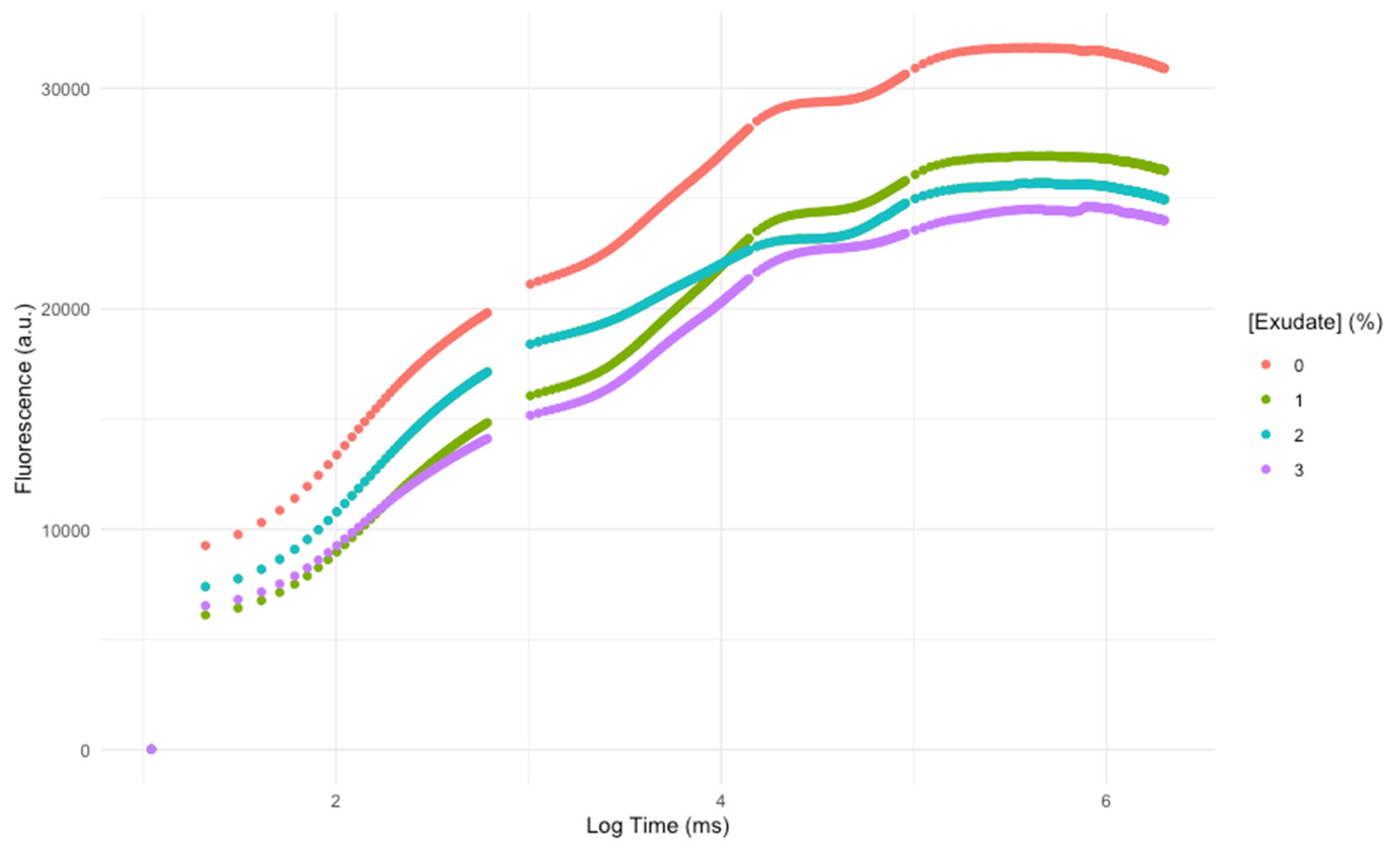
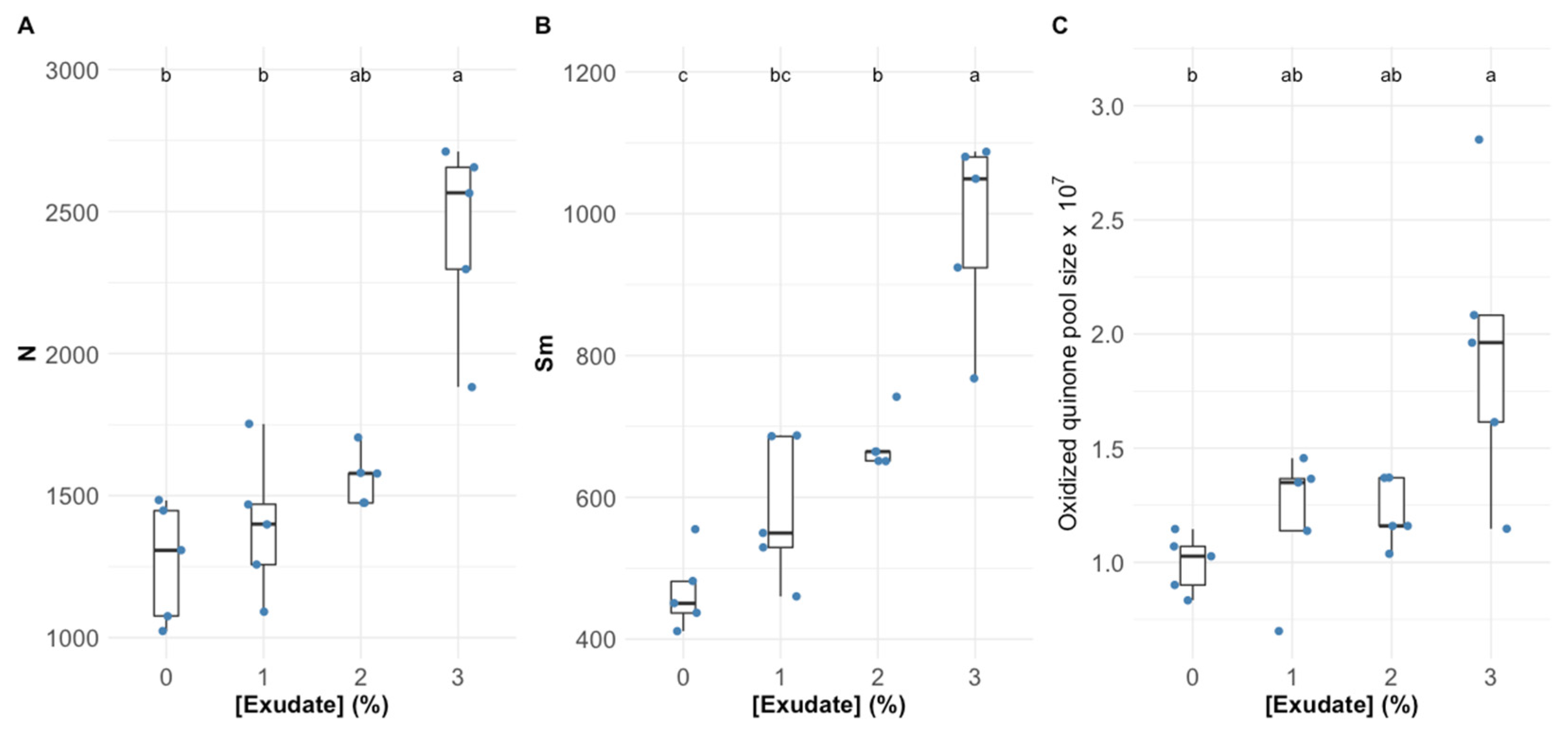
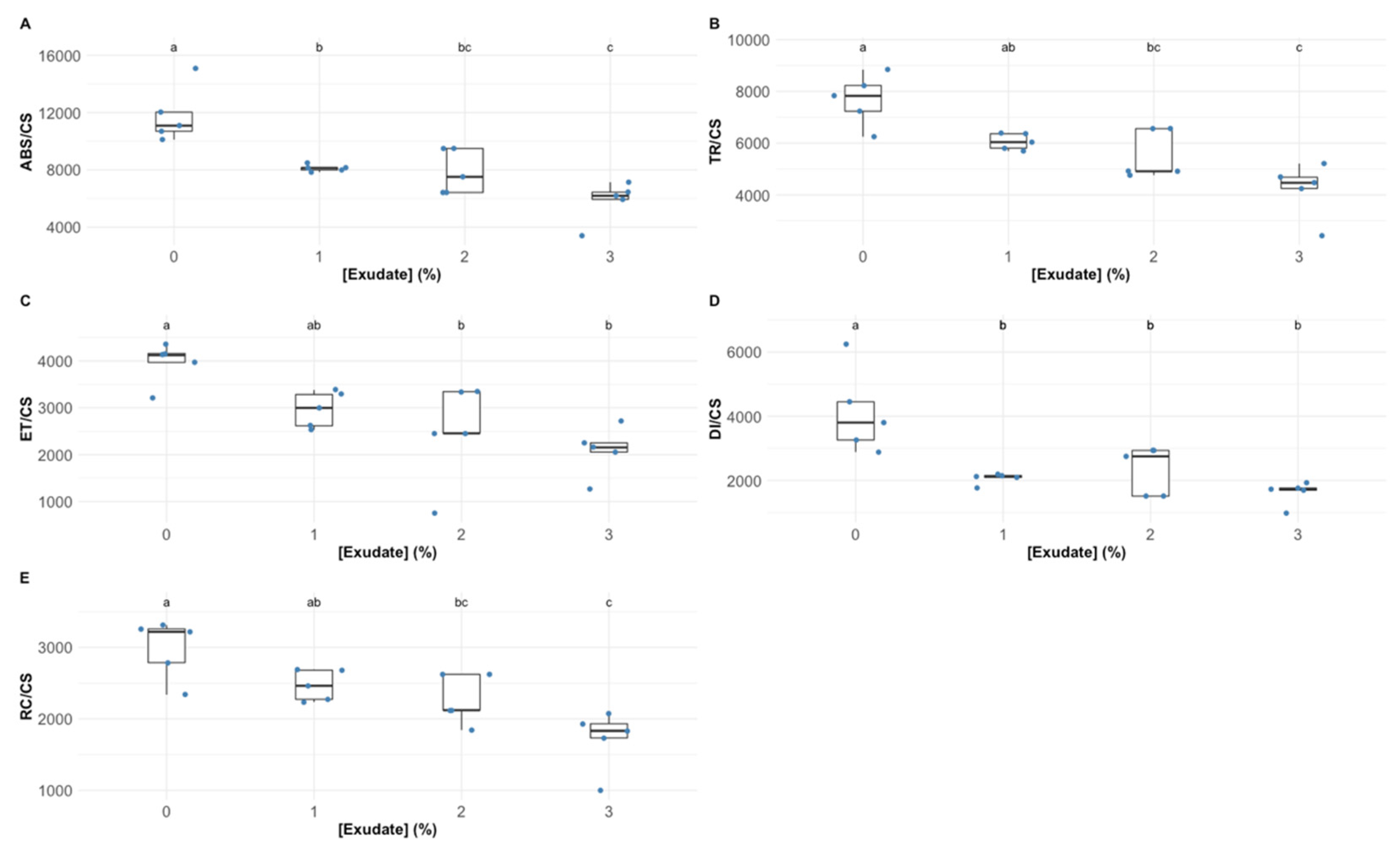
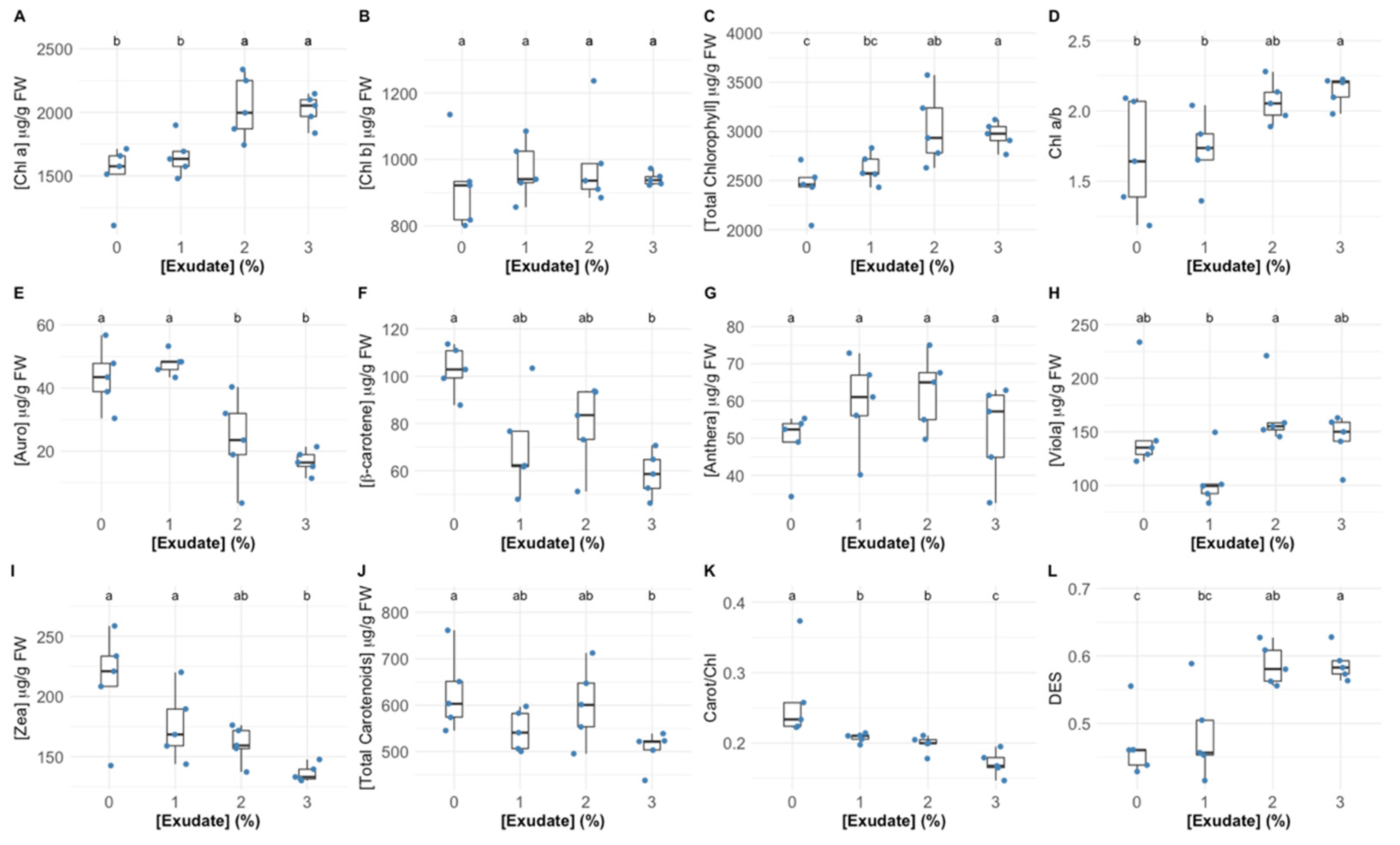

| Variable | Definition |
|---|---|
| Area | Corresponds to the oxidized quinone pool size available for reduction and is a function of the area above the Kautsky plot. |
| N | Reaction centre turnover rate. |
| SM | Corresponds to the energy needed to close all reaction centres. |
| ABS/CS | Absorbed energy flux per cross-section. |
| TR/CS | Trapped energy flux per cross-section |
| ET/CS | Electron transport energy flux per cross-section. |
| DI/CS | Dissipated energy flux per cross-section. |
| RC/CS | The number of available reaction centres per cross-section. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, B.; Carreiras, J.; Feijão, E.; de Carvalho, R.C.; Matos, A.R.; Fonseca, V.F.; Novais, S.C.; Lemos, M.F.L. Potential of Asparagopsis armata as a Biopesticide for Weed Control under an Invasive Seaweed Circular-Economy Framework. Biology 2021, 10, 1321. https://doi.org/10.3390/biology10121321
Duarte B, Carreiras J, Feijão E, de Carvalho RC, Matos AR, Fonseca VF, Novais SC, Lemos MFL. Potential of Asparagopsis armata as a Biopesticide for Weed Control under an Invasive Seaweed Circular-Economy Framework. Biology. 2021; 10(12):1321. https://doi.org/10.3390/biology10121321
Chicago/Turabian StyleDuarte, Bernardo, João Carreiras, Eduardo Feijão, Ricardo Cruz de Carvalho, Ana Rita Matos, Vanessa F. Fonseca, Sara C. Novais, and Marco F. L. Lemos. 2021. "Potential of Asparagopsis armata as a Biopesticide for Weed Control under an Invasive Seaweed Circular-Economy Framework" Biology 10, no. 12: 1321. https://doi.org/10.3390/biology10121321
APA StyleDuarte, B., Carreiras, J., Feijão, E., de Carvalho, R. C., Matos, A. R., Fonseca, V. F., Novais, S. C., & Lemos, M. F. L. (2021). Potential of Asparagopsis armata as a Biopesticide for Weed Control under an Invasive Seaweed Circular-Economy Framework. Biology, 10(12), 1321. https://doi.org/10.3390/biology10121321











