Prevalence and Molecular Diversity of Plant-Parasitic Nematodes of Yam (Dioscorea spp.) in China, with Focus on Merlinius spp.
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction, Identification, and Morphological Characterization of Nematodes
sampled and also for pooled data of the two provinces
2.1.1. Morphological Characterization
2.1.2. Molecular Characterization
3. Results
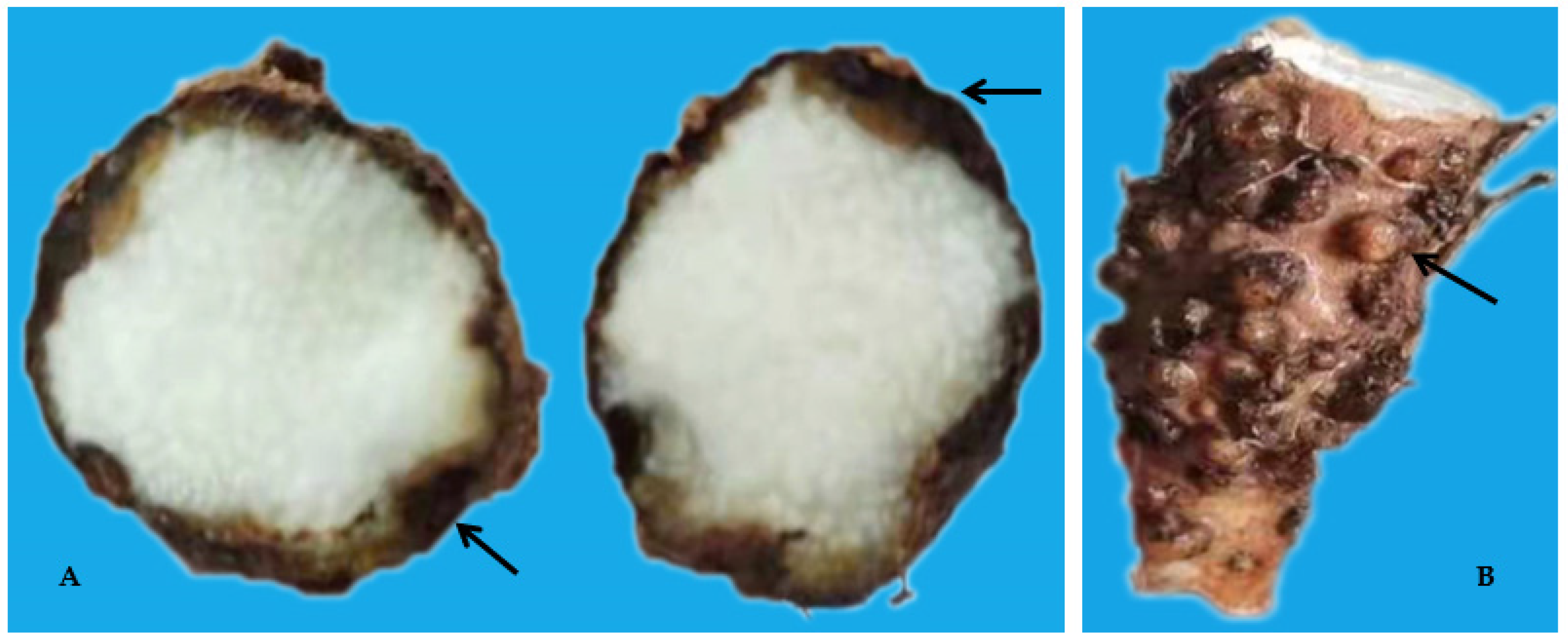
3.1. Survey
3.2. Morphological and Molecular Characterizations of Merlinius brevidens
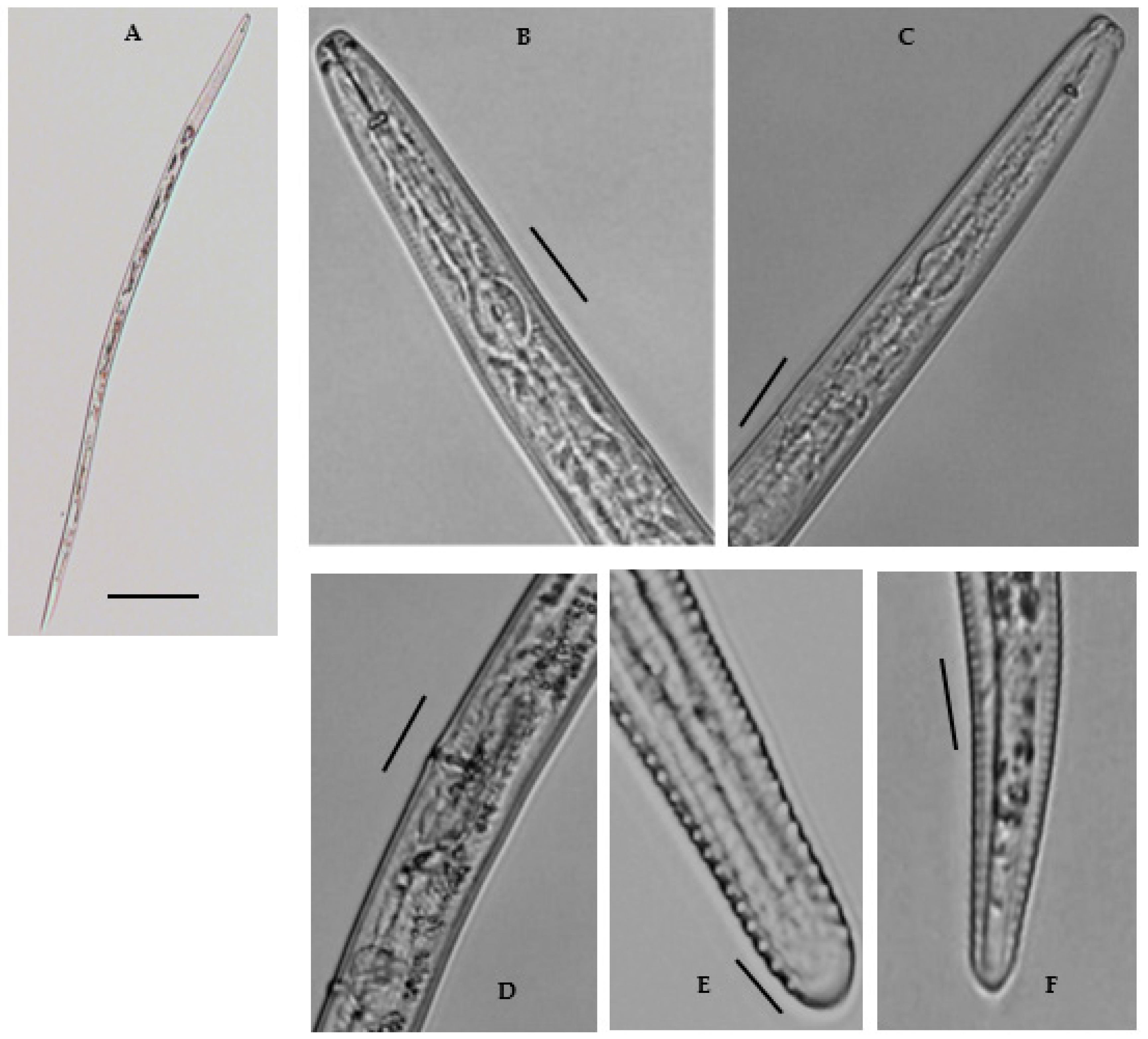
3.2.1. Morphology of Merlinius brevidens (Allen, 1955) Siddiqi, 1970 = Geocenamus brevidens (Allen, 1955)
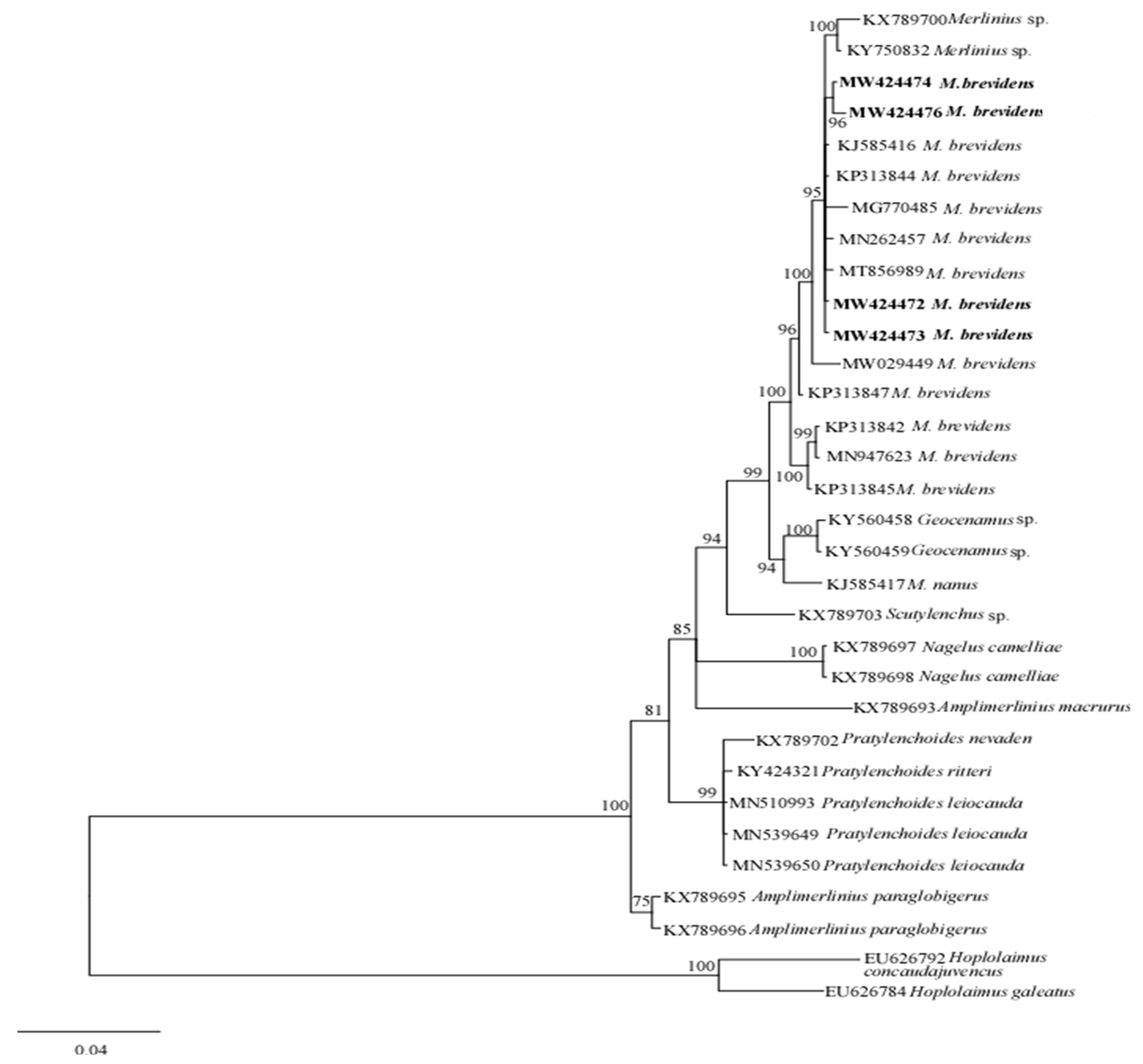
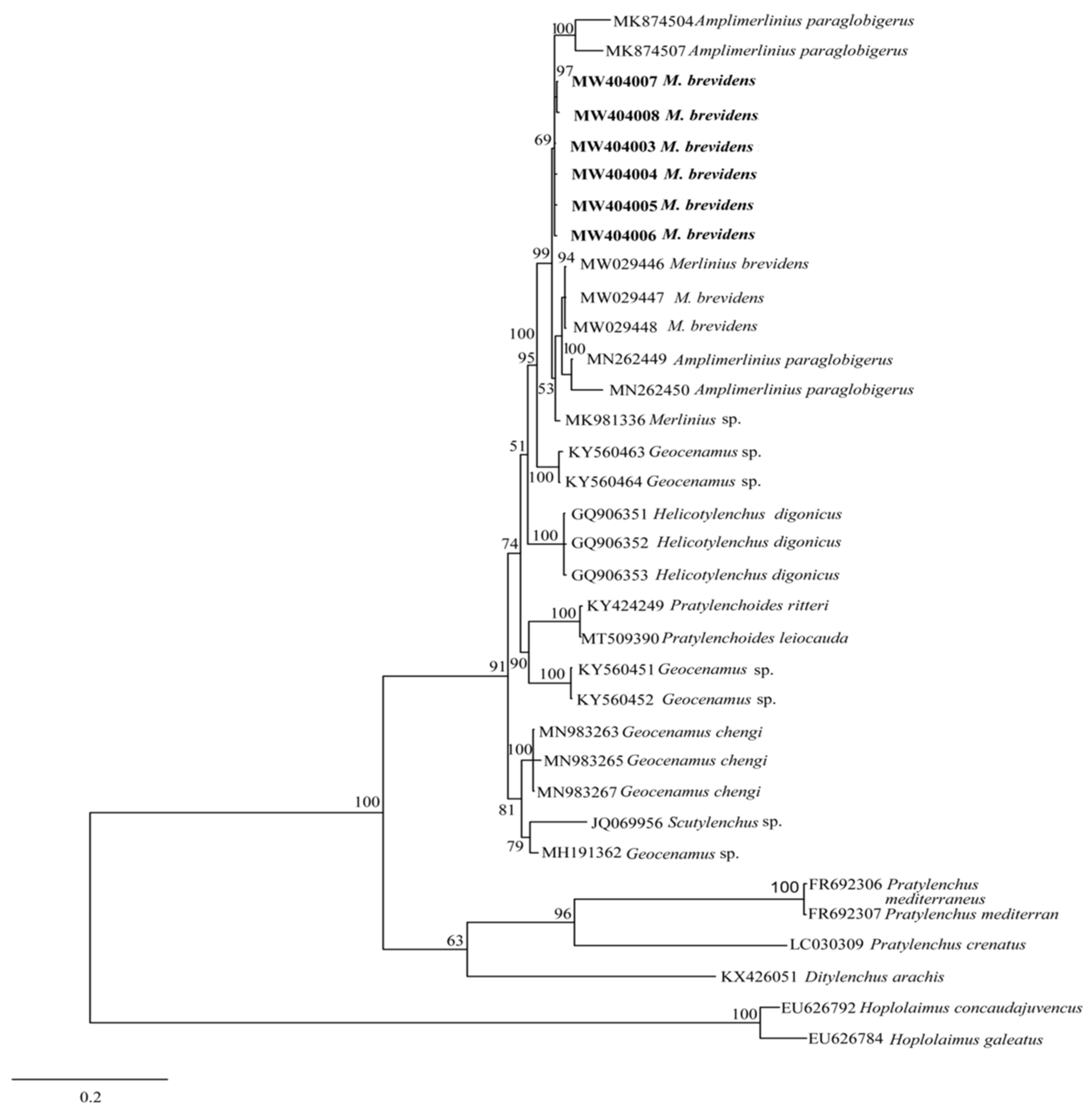
3.2.2. Molecular Characterization and Phylogenetic Analysis of Merlinius brevidens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govaerts, R.; Wilkin, P.; Saunders, R.M.K. World Checklist of Dioscoreales, Yams and Their Allies; Kew Publishing: Kew, UK, 2007. [Google Scholar]
- Darkwa, K.; Olasanmi, B.; Asiedu, R.; Asfaw, A. Review of empirical and emerging breeding methods and tools for yam (Dioscorea spp.) improvement: Status and prospects. Plant Breed. 2020, 139, 474–497. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, J.N. Yam Nutrition: Nutrient Disorders and Soil Fertility Management; ACIAR Monograph Canberra: Canberra, Australia, 2010. [Google Scholar]
- Coyne, D.L.; Affokpon, A. Nematode parasites of tropical root and tuber crops (excluding potatoes). In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; Luc, M., Sikora, R.A., Bridge, J., Eds.; CABI: Wallingford, UK, 2018; pp. 258–272. [Google Scholar]
- Wu, W.; Chena, C.; Zhanga, Q.; Ahmeda, Z.J.; Xua, Y.; Huanga, X.; Xiea, J.; Xiaa, W.; Huanga, D. A comparative assessment of diversity of greater yam (Dioscorea alata) in China. Sci. Hortic. 2019, 243, 116–124. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT, 2017. Rome. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 28 April 2019).
- Wu, Z.G.; Jiang, W.; Nitin, M.; Bao, X.Q.; Chen, S.L.; Tao, Z.M. Characterizing diversity based on nutritional and bioactive compositions of yam germplasm (Dioscorea spp.) commonly cultivated in China. J. Food Drugs Anal. 2016, 24, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, D.; Zhong, H.; Hu, B.; Tian, Z.; Sun, L.; Fischer, G.; Wang, X.; Jiang, Z. Agro-ecological suitability assessment of Chinese Medicinal Yam under future climate change. Environ. Geochem. Health 2019, 42, 987–1000. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.J.; Gao, Q.; Liu, C.; Li, D.; Liu, C.; Xue, Y. Comparison of volatile components in 11 Chinese yams (Dioscorea spp.) varieties. Food Biosci. 2020, 34, 100531. [Google Scholar] [CrossRef]
- Korada, R.; Naskar, S.K.; Edison, S. Insect pests and their management in yam production and storage: A world review. Int. J. Pest Manag. 2010, 56, 337–349. [Google Scholar] [CrossRef]
- Bridge, J.; Coyne, D.L.; Kwoseh, C.K. Nematode parasites of tropical root and tuber crops (excluding potatoes). In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd ed.; Luc, M., Sikora, R.A., Bridge, J., Eds.; CABI Publisher: Wallingford, UK, 2005; pp. 221–258. [Google Scholar]
- Kolombia, A.Y.; Ogundero, O.; Olajide, E.; Viaene, N.; Kumar, L.P.; Coyne, L.D.; Bert, W. Morphological and molecular characterization of Pratylenchus species from Yam (Dioscorea spp.) in West Africa. J. Nematol. 2020, 52, e2020-126. [Google Scholar] [CrossRef] [PubMed]
- Bharti, L.; Bhat, H.A.; Chaubey, K.A.; Abolafia, J. Morphological and molecular characterization of Merlinius brevidens (Allen, 1955) Siddiqi, 1970 (Nematoda: Rhabditida: Merlinidae) from India. J. Nat. Hist. 2020, 54, 1477–1498. [Google Scholar] [CrossRef]
- Allen, M.W. A review of the nematode genus Tylenchorhynchus. Univ. Calif. Publ. Zool. 1955, 61, 129–166. [Google Scholar]
- Abdulsalam, S.; Peng, H.; Huang, W.; Liu, S.; Kong, L.; Peng, D. Molecular and morphological characterization of stunt nematodes of wheat, maize, and rice in the savannahs f northern Nigeria. J. Integr. Agric. 2021, 20, 2–11. [Google Scholar]
- Smiley, R.W.; Whittaker, R.G.; Gourlie, J.A.; Easley, S.A. Geocenamus brevidens associated with reduced yield of no-till annual spring wheat in Oregon. Plant Dis. 2006, 90, 885–890. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, M.R. Taxonomy of the plant nematode subfamily Merliniinae Siddiqi, 1970, with descriptions of Merlinius processus n. sp., M. loofi n. sp. and Amplimerlinius globigerus n. sp. from Europe. Syst. Biol. 1979, 1, 43–59. [Google Scholar]
- Handoo, Z.A.; Khan, A.; Islam, S. A key and diagnostic compendium to the species of the genus Merlinius Siddiqi, 1970 (Nematoda: Tylenchida) with description of Merlinius khuzdarensis n. sp. Associated with Date Palm. Nematology 2007, 9, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Kolombia, A.Y. Diversity and Characterization of Plant-Parasitic Nematodes Associated with Yam (Dioscorea spp.) in West Africa and a Novel Approach for Rapid Resistance Screening. Ph.D. Thesis, Gent University, Ghent, Belgium, 2017. [Google Scholar]
- Hooper, D.J.; Hallmann, J.; Subbotin, S.A. Methods for Extraction, Processing, and Detection of Plant and Soil Nematodes. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; Luc, M., Sikora, R.A., Bridge, J., Eds.; CAB International: Wallingford, UK, 2018; pp. 53–86. [Google Scholar]
- Coyne, D.L.; Nicol, J.M.; Claudius-Cole, B. Practical Plant Nematology: A Field and Laboratory Guide, 3rd ed.; International Institute of Tropical Agriculture (IITA): Nigeria, Ibadan, 2018; pp. 1–3. [Google Scholar]
- Bello, T.T.; Coyne, L.D.; Rashidifard, M.; Fourie, H. Abundance and diversity of plant-parasitic nematodes associated with watermelon in Nigeria, with focus on Meloidogyne spp. J. Nematol. 2020, 22, 781–797. [Google Scholar] [CrossRef]
- Htay, C.; Peng, H.; Huang, W.; Kong, L.; He, W.; Holgado, R.; Peng, D. The development and molecular characterization of a rapid detection method for rice root-knot nematode (Meloidogyne graminicola). Eur. J. Plant Pathol. 2016, 146, 281–291. [Google Scholar] [CrossRef]
- Nunn, G.B. Nematode Molecular Evolution. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 1992. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, X.W.; Wang, T.G. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Munawar, M.; Yevtushenko, P.D.; Castillo, P. Integrative taxonomy, distribution, and host associations of Geocenamus brevidens and Quinisulcius capitatus from southern Alberta, Canada. J. Nematol. 2021, 53, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.R. Studies on Tylenchorhynchus spp. (Nematoda: Tylenchida) from India. Z. Parasitenk. 1961, 21, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Geraert, E. The Dolichodoridae of the World; Identification of the Family Dolichodoridae Academia Press: Ghent, Belgium, 2011; p. 520. [Google Scholar]
- Geraert, E. On some Tylenchidae and Neotylenchidae from Belgium with the description of a new species, Tylenchorhynchus microdorus. Nematologica 1966, 12, 409–416. [Google Scholar] [CrossRef]
- Siddiqi, M.R. On the plant-parasitic nematode genera Merlinius gen. n. and Tylenchorhynchus Cobb and the classification of the families Dolichodoridae and Belonolaimidae n. rank. Proc. Helminthol. Soc. Wash. 1970, 37, 68–77. [Google Scholar]
- Alvani, S.; Mahdikhani-Moghadam, E.; Rouhani, H.; Mohammadi, A. Morphological and molecular characterization and phylogenetic position of a new record, Tylenchorhynchus zeae, and some known species of Telotylenchidae Siddiqi, 1960 and Merliniidae Siddiqi, 1971 from Iran. Turk. J. Zool. 2017, 41, 227–236. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Liu, Y.; Lu, Q.; Wang, K. Occurrence of soybean root rot caused by Pratylenchus coffeae in Henan province, China. Dis. Notes 2019, 103, 1435. [Google Scholar] [CrossRef]
- Peng, D.L.; Ye, X.W.; Peng, H.; Gu, C.X. First report of the cyst nematode (Heterodera filipjevi) on wheat in Henan Province, China. Plant. Dis. 2010, 94, 1262. [Google Scholar] [CrossRef]
- Zeng, Y.; Ye, W.; Kerns, J. First report and morphological and molecular characterization of Meloidogyne incognita from Radermachera sinica in China. Nematropica 2014, 44, 118–129. [Google Scholar]
- Zhang, F.; Wang, Y.; Zhan, X.; Dai, D.; Guo, G.; Guo, S.; Sun, M.; Zhang, J. First Report of Rotylenchulus reniformis on Tomato in Henan, China. Dis. Notes 2019, 103, 1044. [Google Scholar] [CrossRef]
- Humphreys-Pereira, D.A.; Flores-Chaves, L.; Salazar, L.; Gómez-Alpízar, L. Plant-parasitic nematodes associated with yams (Dioscorea spp.) and identification of Meloidogyne and Pratylenchus species in three yam-growing regions of Costa Rica. Nematropica 2017, 47, 120–134. [Google Scholar]
- Castillo, P.; Vovlas, N. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, Biology, Pathogenicity, and Management; Brill Academic Publishers: Leiden, The Netherlands, 2007. [Google Scholar]
- Budiman, A.; Mulyadisastra, S.; Giyanto, G. Morphological and Molecular Characteristics of Pratylenchus coffeae from the Origin of Robusta Coffee Plantation in Malang, East Java. J. Perlindungan Tanam. Indones. 2019, 23, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Acosta, N.; Ayala, N. Pathogenicity of Pratylenchus coffeae, Scutellonema bradys, Meloidogyne incognita, and Rotylenchulus reniformis on Dioscorea rotundata. J. Nematol. 1975, 7, 1–6. [Google Scholar]
- Gao, Q.K.; Zhao, H.H.; Xu, M.E.; Liu, W.Z. Preliminary study on the root-knot nematode disease of yam. Acta Phytopathol. Sin. 2000, 30, 162–165. [Google Scholar]
- Xu, J.; Liu, P.; Meng, Q.; Long, H. Characterisation of Meloidogyne species from China using isozyme phenotypes and amplified mitochondrial DNA restriction fragment length polymorphism. Eur. J. Plant Pathol. 2004, 110, 309–315. [Google Scholar] [CrossRef]
- Palomares-Rius, J.E.; Cantalapiedra-Navarrete, C.; Archidona-Yuste, A.; Tzortzakakis, A.E.; Birmpilis, G.I.; Vovlas, N.; Subbotin, S.A.; Castillo, P. Prevalence and molecular diversity of reniform nematodes of the genus Rotylenchulus (Nematoda: Rotylenchulinae) in the Mediterranean Basin. Eur. J. Plant Pathol. 2018, 150, 439–455. [Google Scholar] [CrossRef]
- Chen, D.Y.; Ni, H.F.; Yen, J.H.; Chen, R.S.; Tsay, T.T. Distribution of rice root nematode Hirschmanniella oryzae and a new recorded H. mucronata (Nematoda: Pratylenchidae) in Taiwan. Plant Pathol. Bull. 2006, 15, 197–210. [Google Scholar]
- Tzortzakakis, E.A.; Cantalapiedra-Navarrete, C.; Kormpi, M.; Lazanaki, M.S.; Castillo, P.; Archidona-Yuste, A. First Report of Bitylenchus hispaniensis, Pratylenchoides alkani, and Helicotylenchus vulgaris in association with cultivated and wild olives in Crete, Greece and molecular identification of Helicotylenchus microlobus and Merlinius brevidens. J. Nematol. 2018, 50, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Handoo, Z.A.; Palomares-Rius, J.; Cantalapiedra-Navarrete, E.; Liébanas, C.; Subbotin, G.S.A.; Castillo, P. Integrative taxonomy of the stunt nematodes of the genera Bitylenchus and Tylenchorhynchus (Nematoda, Telotylenchidae) with description of two new species and molecular phylogeny. Zool. J. Linn. Soc. 2014, 172, 231–264. [Google Scholar] [CrossRef]
| Code | City/County | Province | Soil Type | Species | GenBank Accession Number | |
|---|---|---|---|---|---|---|
| 28S | ITS | |||||
| 360826/THE | Taihe | Jiangxi | Sandy clay | Pratylenchus coffeae, | MW082090, | MW042905 |
| Rotylenchulus reniformis, | MW082086 | MW042913 | ||||
| Helicotylenchus dihystera | MW082085, | MW042910 | ||||
| Merlinius brevidens | MW424473, | MW404014 | ||||
| Meloidogyne incognita | MW424479, | MW404001 | ||||
| Ditylenchus | - | MW042916 | ||||
| Hirschmanniella mucronata, | >MW424469, | MW042911 | ||||
| Tylenchorhynchus annulatus | MW424463 | MW042920 | ||||
| 360827/SCN | Suichun | Jiangxi | Sandy clay | P. coffeae | MW082091 | MW042906 |
| R. reniformis, | MW082086 | MW042914 | ||||
| M. brevidens | - | MW404009 | ||||
| M. incognita | - | MW403996 | ||||
| Ditylenchus | - | MW042917 | ||||
| H. mucronata | MW424470 | MW042912 | ||||
| 360881/JGS | jingganshan | Jiangxi | Clay | P. coffeae | - | MW042907 |
| R. reniformis | MW082088 | - | ||||
| M. incognita | - | MW403997 | ||||
| M. brevidens | - | MW404008 | ||||
| Aphelenchus avenae | - | MW042919 | ||||
| Tylenchorhynchus zeae | MW082095 | - | ||||
| 360481/RCG | Ruichang | Jiangxi | Sandy loam | P. coffeae | - | MW042908 |
| R. reniformis | MW082089, | MW042915 | ||||
| M. brevidens | - | MW404006 | ||||
| M. incognita | MW812359 | MW404000 | ||||
| T. zeae | MW082096 | - | ||||
| Coslenchus rafiqi | MW082097 | - | ||||
| 360724/SYO | Shanyou | Jiangxi | Sandy loam | P. coffeae | - | MW042909 |
| R. reniformis | MW082087 | - | ||||
| H. dihystera, | MW424464 | - | ||||
| M. incognita | - | MW403998 | ||||
| M. brevidens | - | MW404003 | ||||
| Filenchus vulgaris | MW082093 | - | ||||
| 360825/YFX | Yongfeng | Jiangxi | Sandy clay | P. coffeae | - | MW404011 |
| R. reniformis | MW812367 | - | ||||
| H. dihystera | MW424466 | - | ||||
| M. incognita | MW812358 | MW403999 | ||||
| F. vulgaris | MW082092 | - | ||||
| 3714/DZS | Dezhou | Shandong | Sandy loam | P. coffeae | MW424468 | MW404010 |
| R. reniformis | MW812368 | - | ||||
| H. dihystera | MW424465 | MW812365 | ||||
| Merlinius brevidens | MW424472 | MW404007 | ||||
| M. incognita | MW424478 | MW404002 | ||||
| Heterodera filipjevi | MW424477 | MW403994 | ||||
| Ditylenchus | - | MW042918 | ||||
| M. hispanica | MW829784 | MW812366 | ||||
| M. ethiopica | MW424480 | - | ||||
| 3707/WEF | Weifang | Shandong | Sandy loam | P. coffeae | - | MW404012 |
| R. reniformis | - | - | ||||
| M. brevidens | MW424474 | - | ||||
| M. incognita | - | MW404005 | ||||
| H. filipjevi | MW812362 | MW403993 | ||||
| Ditylenchus | - | MW404013 | ||||
| M. hispanica | MW829785 | - | ||||
| M.ethiopica | MZ352771 | - | ||||
| 3705/DYG | Dongying | Shandong | Sandy | P. coffeae | - | - |
| R. reniformis | - | - | ||||
| M. brevidens | MW424476 | - | ||||
| M. incognita | MW812357 | MW404004 | ||||
| H. filipjevi | MW812361 | MW403995 | ||||
| M. hispanica | MZ352769 | - | ||||
| Province | |||||
|---|---|---|---|---|---|
| Jiangxi (n = 65) | Shandong (n = 45) | ||||
| MPD | FO (%) | MPD | FO (%) | ||
| Nematode species | |||||
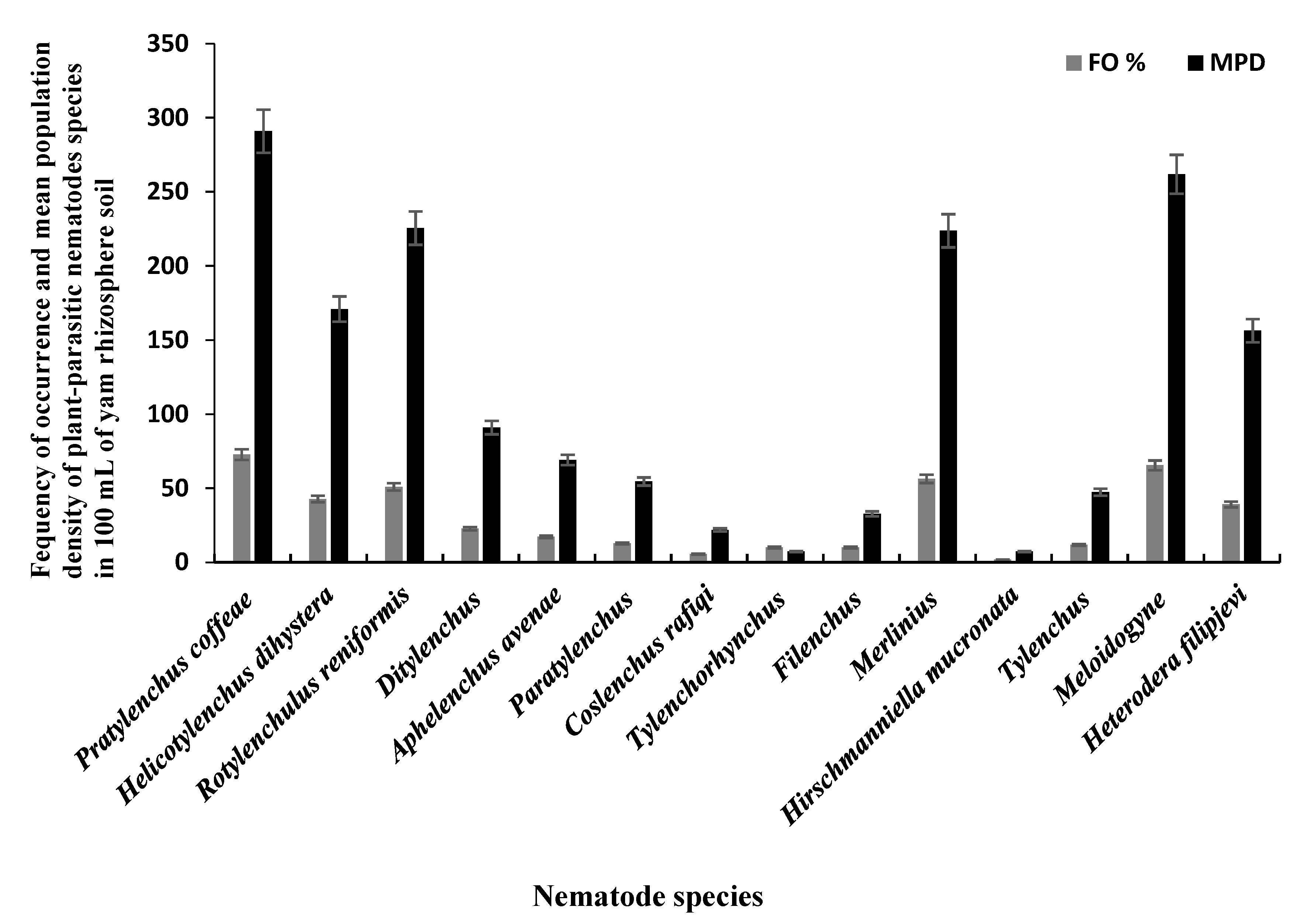
| Pratylenchus coffeae | 302 | 75 | 276 | 69 | |
| Meloidogyne species (M. incognita, M. ethiopica, M. hispanica) | 166 | 42 | 400 | 100 | |
| Rotylenchulus reniformis | 218 | 55 | 382 | 82 | |
| Helicotylenchus dihystera | 191 | 48 | 142 | 36 | |
| Merlinius brevidens | 117 | 29 | 231 | 58 | |
| Aphelenchus avenae | 74 | 18 | 62 | 16 | |
| Ditylenchus spp. | 92 | 23 | 89 | 22 | |
| Tylenchorhyncus (T. annulatus, T. zeae) | 55 | 14 | 18 | 4 | |
| Filenchus vulgaris | 55 | 14 | 18 | 4 | |
| Tylenchus spp. | 68 | 17 | 18 | 4 | |
| Coslenchus spp. | 37 | 9 | |||
| Paratylenchus spp. | 68 | 15 | 36 | 9 | |
| Heterodera filipjevi | - | - | 382 | 96 | |
| Hirschmanniella mucronata | 12 | 3 | - | - | |
| Province | |||||
|---|---|---|---|---|---|
| Jiangxi (n = 28) | Shandong (n = 20) | ||||
| MPD | FO (%) | MPD | FO (%) | ||
| Nematode species | |||||
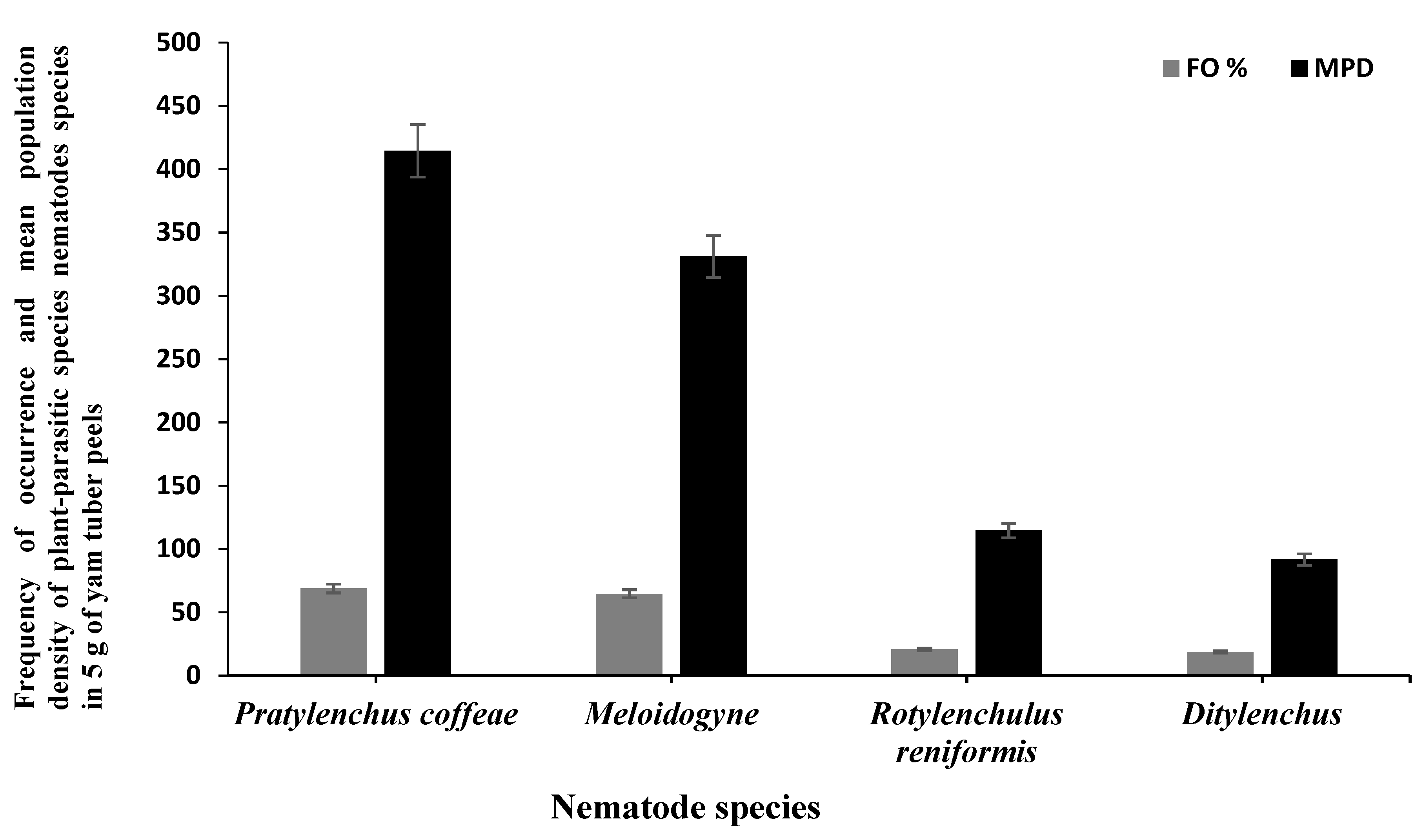
| Pratylenchus coffeae | 607 | 93 | 145 | 35 | |
| Meloidogyne species (M. incognita, M. ethiopica, M. hispanica) | 264 | 54 | 325 | 80 | |
| Rotylenchulus reniformis | 115 | 21 | 110 | 25 | |
| Ditylenchus spp. | 96 | 18 | 35 | 10 | |
| Character | Merlinius brevidens | M. brevidens | M. brevidens | M. brevidens | M. brevidens | M. brevidens | M. brevidens | M. microdorus | M. nanus |
|---|---|---|---|---|---|---|---|---|---|
| Reference | Present study (China) | Canada: Munawar et al. (2021) | India: Bharti et al. (2020) | Greece: Tzortzakakis et al. (2018) | Iran: Alvani et al. (2017) | UK: Bridge (1971) | USA: Allen (1955) | Belgium: Geraert (1966) | USA: Allen (1955) |
| N | 14 | 15 | 10 | 3 | 8 | 10 | 11 | 14 | 8 |
| L | 655.3 ± 25.5 (631.3–707.9) | (591–811) | (572–644) | (490–698) | (600–718.5) | (526–676) | (540–690) | (580–700) | (520–640) |
| a | 34.0 ± 2.0 (31.7–39.9) | (25.5–38.5) | (26.1–31.6) | (22.9–30.3) | (23.9–29.0) | (21.5–27) | (23–27) | (24.0–28.5) | (27–31) |
| b | 5.6 ± 0.4 (5.1–6.6) | (4.6–5.9) | (4.2–5.3) | (4.0–5.1) | (4.5–5.2) | (4.5–5.1) | (4.2–5.2) | (4.8–5.9) | (4.5–5.3) |
| b’ | 4.9 ± 0.3 (4.5–5.8) | - | (4.7–5.8) | - | - | - | - | - | - |
| c | 13.2 ± 1.9 (10.1–16.3) | (11.7–13.6) | (11.3–15.0) | (12.9–17.0) | (11.3–15.7) | (12–15) | (11–13) | 11.0–13.0 | (10–12) |
| c’ | 3.6 ± 0.7 (2.7–5.4) | (3.8–4.9) | (2.8–4.9) | (2.9–3.2) | (2.4–3.2) | (2.4) | (2.4) | (2.9) | - |
| MB | 53.1 ± 4.6 (45.2–61.1) | (46.2–54.3) | (46–57) | - | (41.1–46.5) | - | - | (41–51) | - |
| V | 59.0 ± 2.0 (54.7–62.4) | (53.8–61.0) | (54–58) | (52.0–58.0) | (54.5–57.0) | (53–58.5) | (52–58) | (55–59) | (52–57) |
| Lip height | 5.3 ± 0.4 (4.6–6.0) | (3.1–4.0) | (3–4) | - | - | - | - | - | - |
| Lip width | 9.9 ± 0.6 (9.0–11.0) | (6.1–7.7) | (6–8) | - | - | - | - | - | - |
| Stylet length | 18.8 ± 0.6 (17.4–19.5) | (15–17.5) | (13–14) | (13.0–16.0) | (16–16.5) | (14–15.5) | (14–16) | (12–14) | (12–15) |
| Stylet base diameter | 15.2 ± 0.5 (14.1–15.9) | - | (12–13) | - | - | - | - | - | - |
| Anterior to median bulb valve | 61.8 ± 3.2 (54.5–65.9) | - | (55–64) | - | - | - | - | - | - |
| Pharynx length | 116.8 ± 6.8 (107.2–124.6) | (120.3–144) | (107–123) | (123–136) | (118.5–141) | - | - | - | - |
| Anterior to end of pharyngeal glands | 134.5 ± 7.0 (123.1–142.0) | - | - | - | - | - | - | - | - |
| Anterior to excretory pore | 112.1 ± 2.6 (106.6–114.8) | - | (84–102) | - | (90.5–110) | - | - | - | - |
| Maximum body width | 19.3 ± 0.7 (17.7–20.5) | (15.5–21.2) | (19–24) | (19–23) | (21.5–30) | - | - | - | - |
| Anterior end to vulva | 386.8 ± 27.3 (345.1–439.1) | - | (313–361) | - | (327–392) | - | - | - | - |
| Body width at vulva | 22.3 ± 1.3 (20.1–24.6) | (16.4–22.3) | (17–24) | - | (21.5–30) | - | - | - | - |
| Tail length | 51 ± 7 (41–63) | (46–59.8) | (41–54) | (38–41) | (42–53) | (46) | - | (34–63) | - |
| Anal body diameter | 14.4 ± 1.4 (11.5–16.3) | (10.3–14.2) | (10–16) | (12–14) | (15.5–19) | (19) | - | (17) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulsalam, S.; Peng, H.; Yao, Y.; Fan, L.; Jiang, R.; Shao, H.; Zhang, Y.; Huang, W.; Kong, L.; Peng, D. Prevalence and Molecular Diversity of Plant-Parasitic Nematodes of Yam (Dioscorea spp.) in China, with Focus on Merlinius spp. Biology 2021, 10, 1299. https://doi.org/10.3390/biology10121299
Abdulsalam S, Peng H, Yao Y, Fan L, Jiang R, Shao H, Zhang Y, Huang W, Kong L, Peng D. Prevalence and Molecular Diversity of Plant-Parasitic Nematodes of Yam (Dioscorea spp.) in China, with Focus on Merlinius spp. Biology. 2021; 10(12):1299. https://doi.org/10.3390/biology10121299
Chicago/Turabian StyleAbdulsalam, Sulaiman, Huan Peng, Yingjuan Yao, Linjuan Fan, Ru Jiang, Hudie Shao, Yingdong Zhang, Wenkun Huang, Ling’an Kong, and Deliang Peng. 2021. "Prevalence and Molecular Diversity of Plant-Parasitic Nematodes of Yam (Dioscorea spp.) in China, with Focus on Merlinius spp." Biology 10, no. 12: 1299. https://doi.org/10.3390/biology10121299
APA StyleAbdulsalam, S., Peng, H., Yao, Y., Fan, L., Jiang, R., Shao, H., Zhang, Y., Huang, W., Kong, L., & Peng, D. (2021). Prevalence and Molecular Diversity of Plant-Parasitic Nematodes of Yam (Dioscorea spp.) in China, with Focus on Merlinius spp. Biology, 10(12), 1299. https://doi.org/10.3390/biology10121299







