Effect of Coriander Plants on Human Emotions, Brain Electrophysiology, and Salivary Secretion
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Coriander Plant Material
2.3. Collection and Detection of VOCs from Coriander Plants
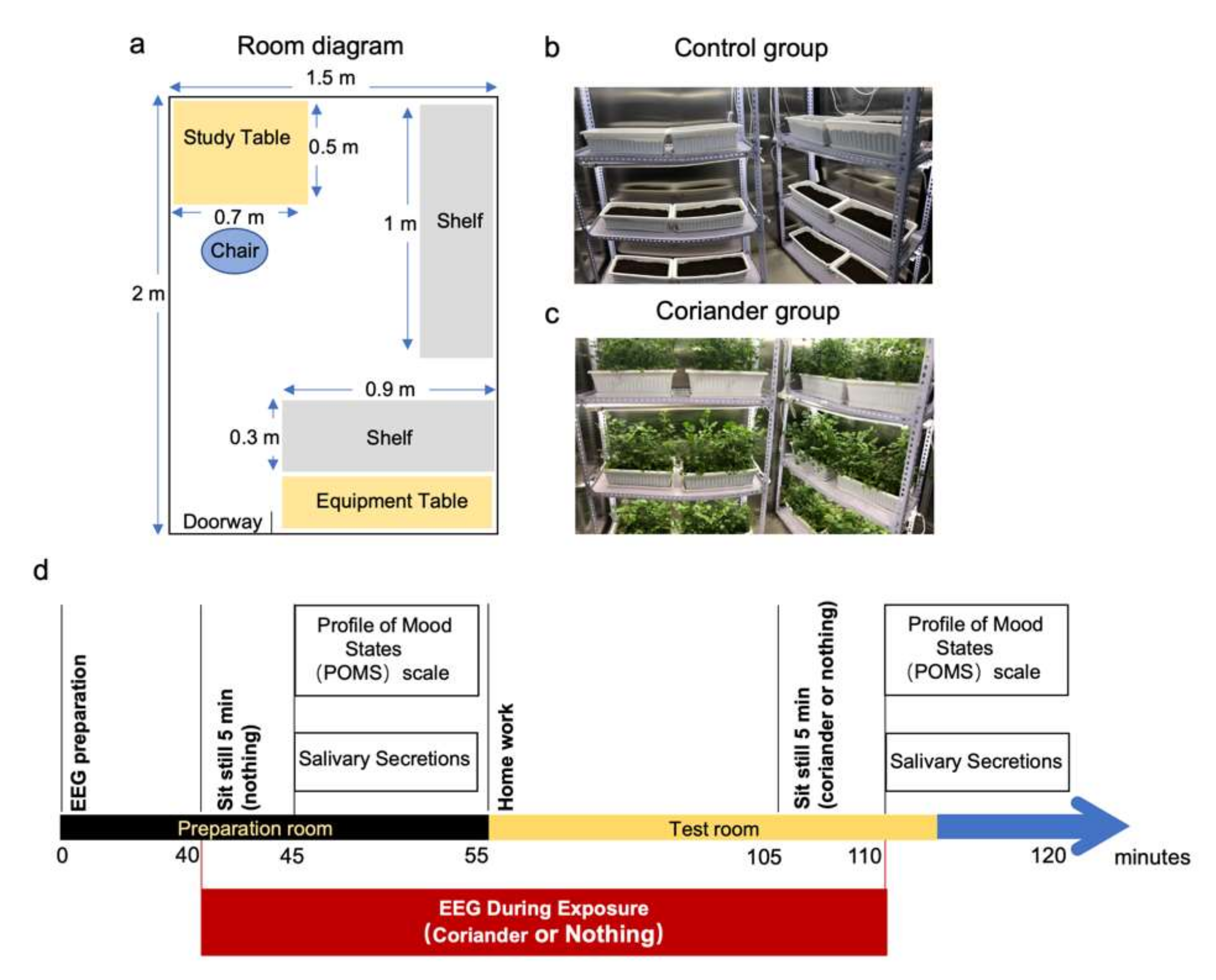
2.4. Protocol
2.5. Subjective Evaluation
2.6. Measurement of Salivary Alpha-Amylase and Cortisol
2.7. EEG Date Collection and Analysis
2.8. Salivary Amino Acid Determination
2.9. Data Analysis
3. Results
3.1. Main VOCs of Coriander Plants
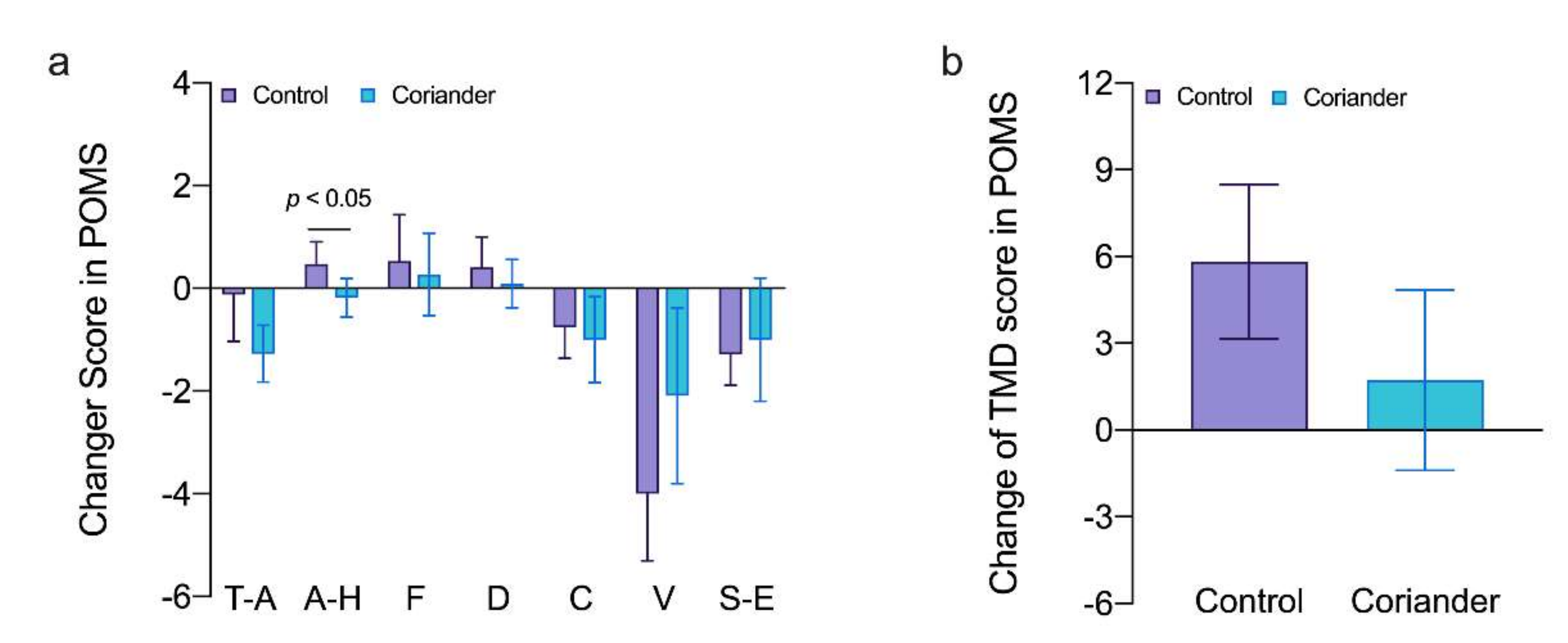
3.2. Effects of Coriander Plants on Subjective Emotion
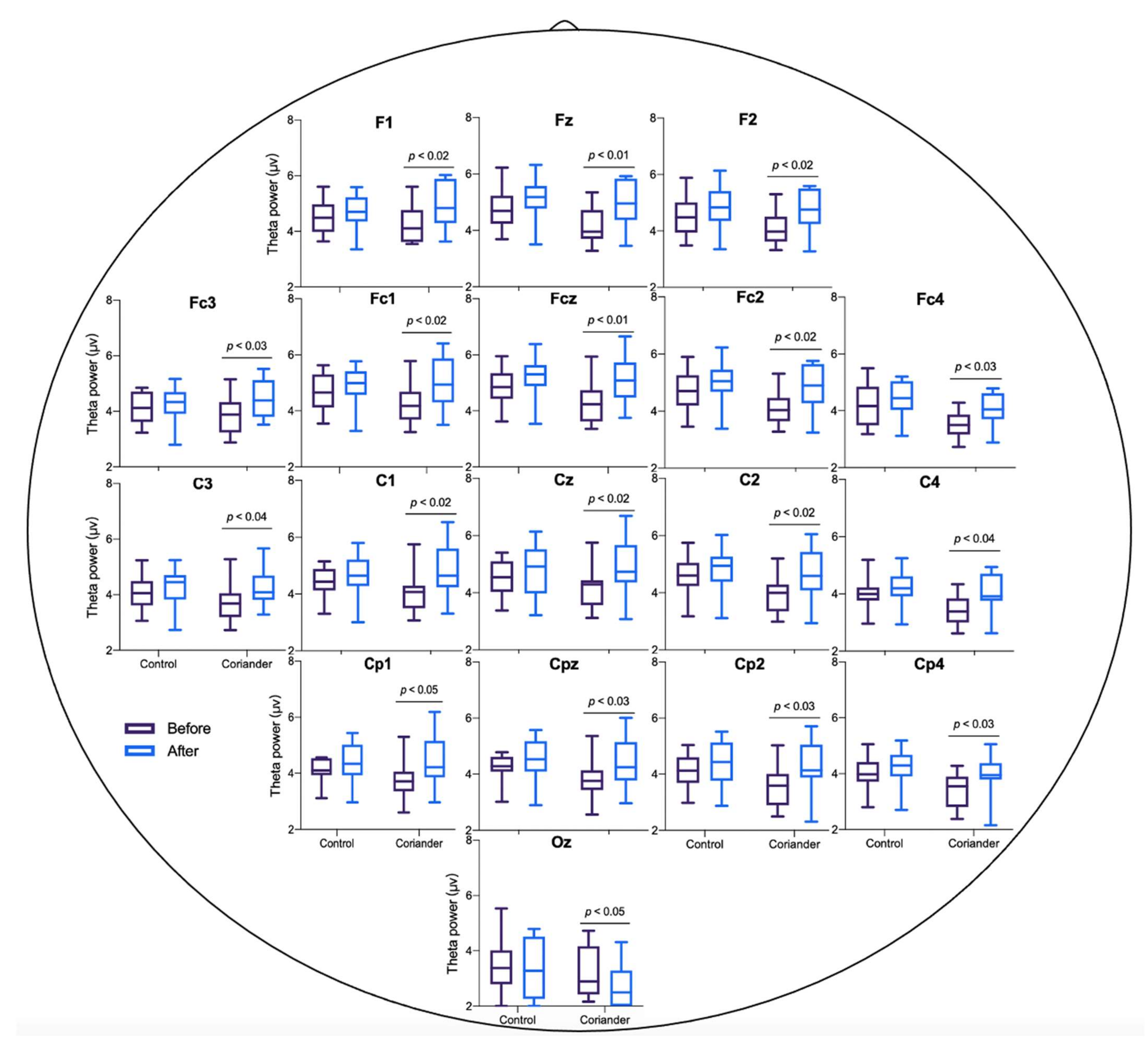
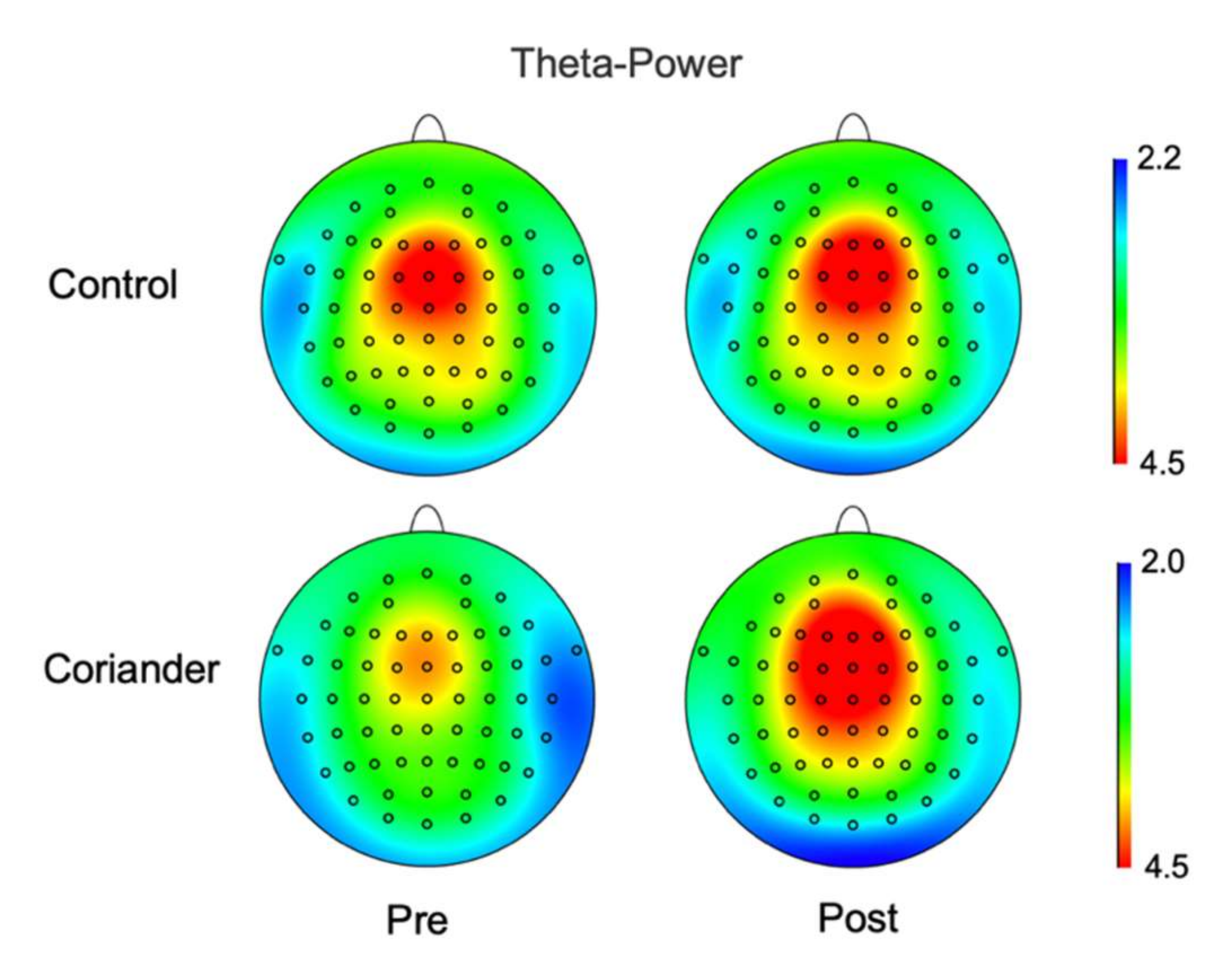
3.3. Effects of Coriander Plants on EEG
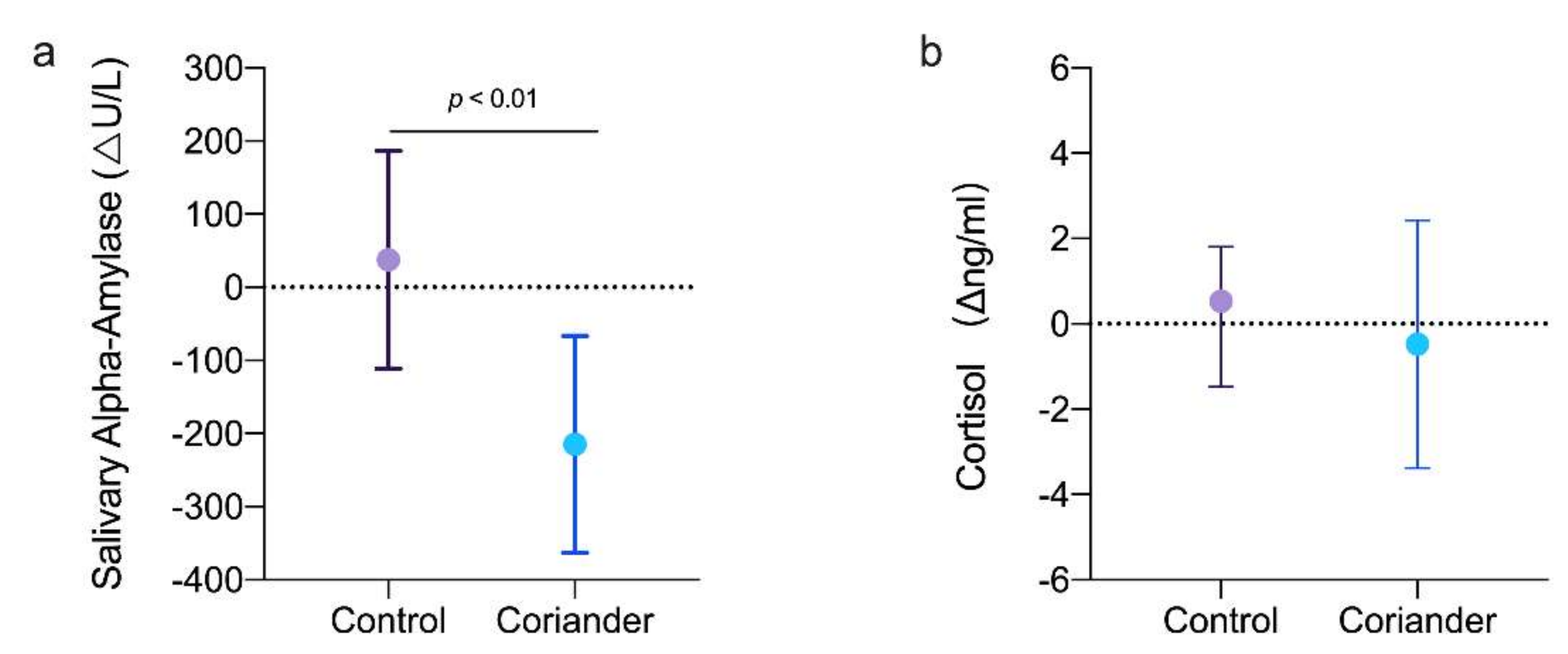
3.4. Effects of Coriander on Salivary Alpha-Amylase and Cortisol
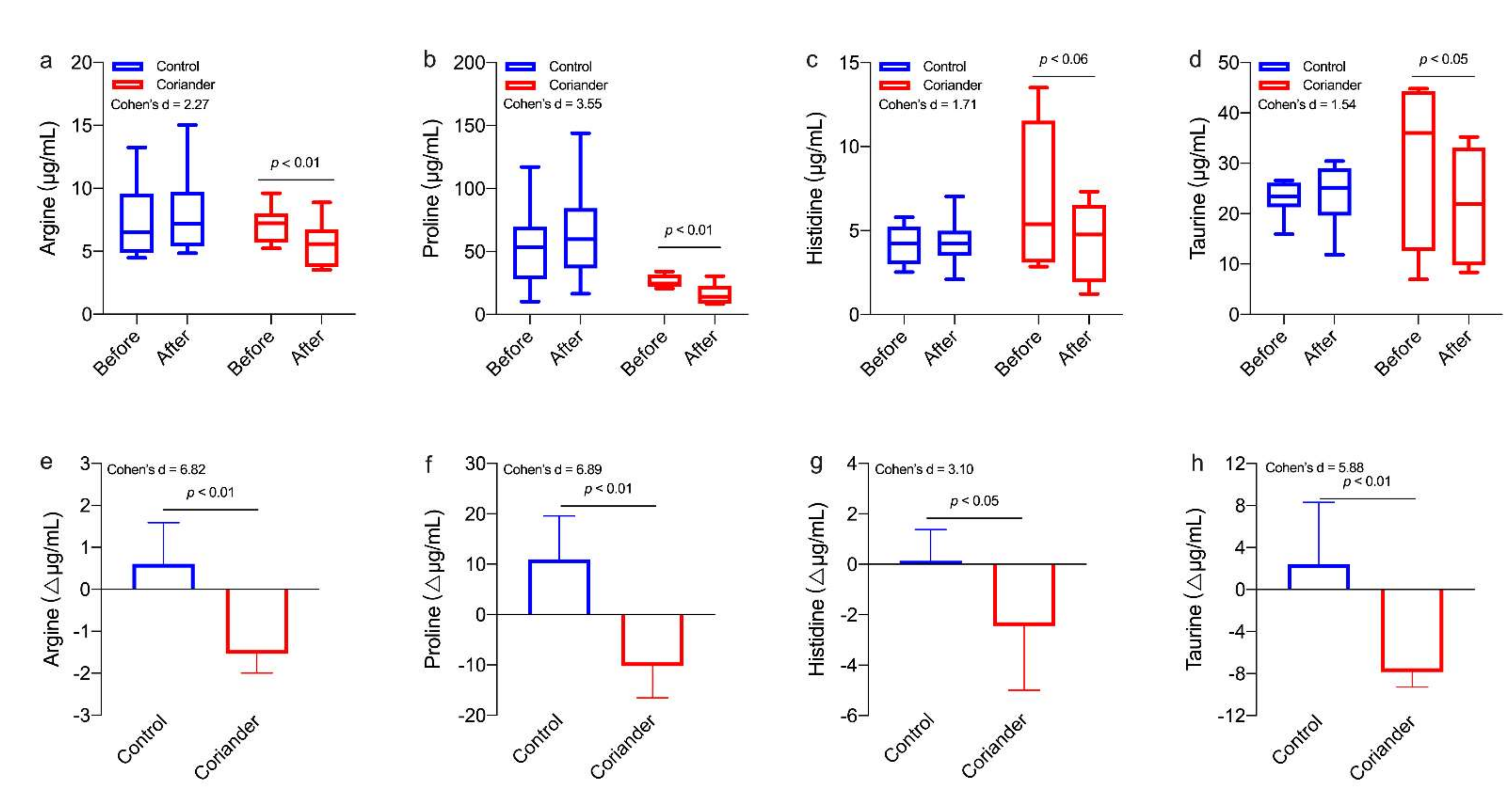
3.5. Effects of Coriander on Salivary Amino Acids
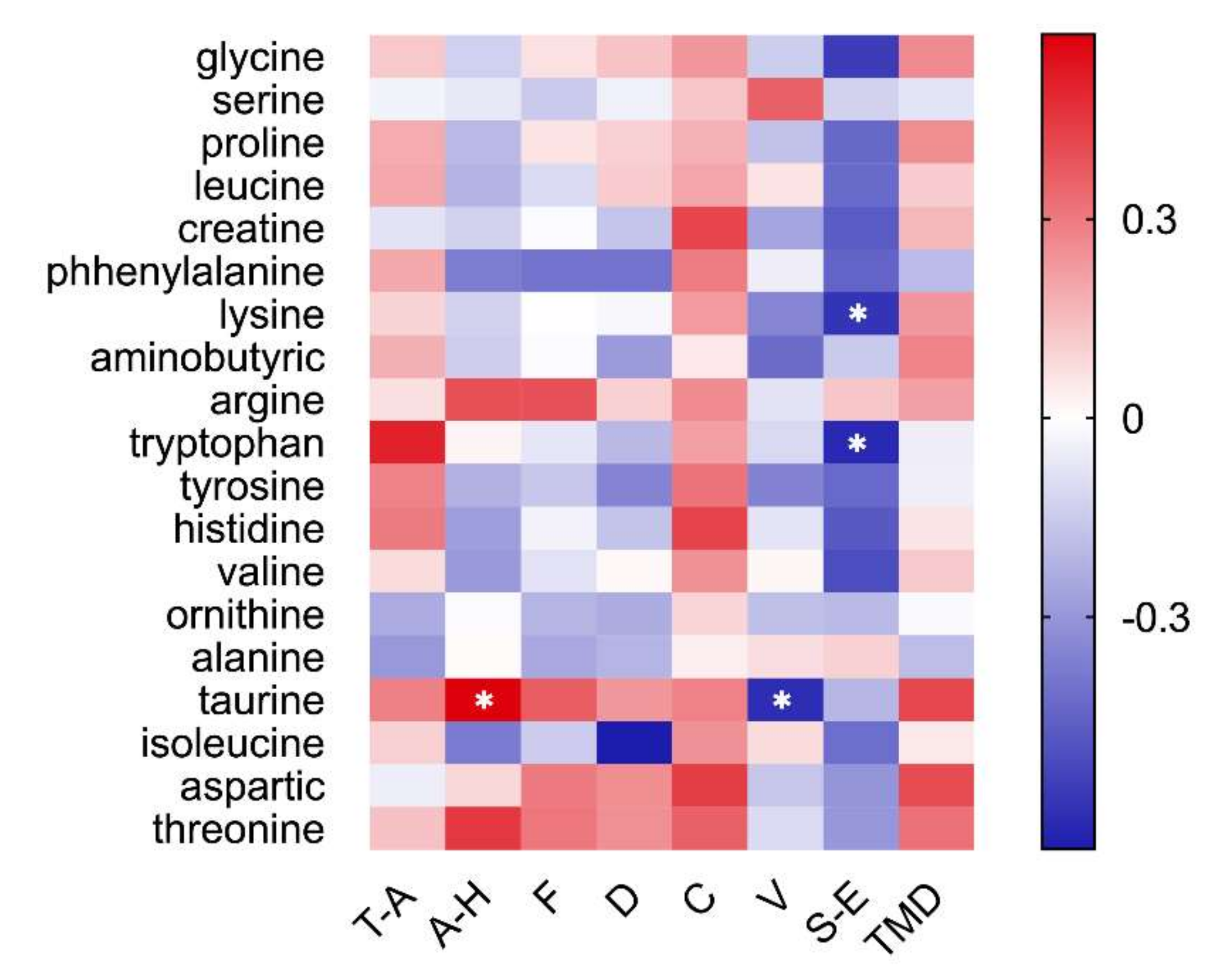
3.6. Emotion Correlates with Salivary Amino Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoo, M.; Lee, E.-H. The Impact of Modulized Interior Landscape on Office Workers’ Psychological Wellbeing—A Pilot Study of Focused on the Office Wall. Korean Inst. Inter. Des. J. 2014, 23, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Peng, G.; Tian, J.; Gao, X.; Zhou, Y.; Qin, X. Research on the Pathological Mechanism and Drug Treatment Mechanism of Depression. Curr. Neuropharmacol. 2015, 13, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Miret, M.; Ayuso-Mateos, J.L.; Sanchez-Moreno, J.; Vieta, E. Depressive disorders and suicide: Epidemiology, risk factors, and burden. Neurosci. Biobehav. Rev. 2013, 37, 2372–2374. [Google Scholar] [CrossRef]
- Tian, J.S.; Shi, B.Y.; Xiang, H.; Gao, S.; Qin, X.M.; Du, G.H. 1H-NMR-Based Metabonomic Studies on the Anti-Depressant Effect of Genipin in the Chronic Unpredictable Mild Stress Rat Model. PLoS ONE 2013, 8, e75721. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Battagliese, G.; Feige, B.; Spiegelhalder, K.; Nissen, C.; Voderholzer, U.; Lombardo, C.; Riemann, D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 2011, 135, 10–19. [Google Scholar] [CrossRef]
- Liu, D.; Gong, L.; Shen, J.; Lu, T. Discussion on the value of core self-evaluation for the prevention and treatment of depression. Diet Health 2021, 16, 102. [Google Scholar]
- Soga, M.; Gaston, K.J.; Yamaura, Y. Gardening is beneficial for health: A meta-analysis. Prev. Med. Rep. 2017, 5, 92–99. [Google Scholar] [CrossRef]
- Song, C.; Joung, D.; Ikei, H.; Igarashi, M.; Aga, M.; Park, B.J.; Miwa, M.; Takagaki, M.; Miyazaki, Y. Physiological and psychological effects of walking on young males in urban parks in winter. J. Physiol. Anthropol. 2013, 32, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.J.; Tsunetsugu, Y.; Lee, J.; Kagawa, T.; Miyazaki, Y. Effect of the forest environment on physiological relaxation using the results of field tests at 35 sites throughout Japan. For. Med. 2012, 57–67. [Google Scholar]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Suzuki, H.; Li, Y.J.; Wakayama, Y.; et al. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2008, 21, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Sofo, A. Converting Home Spaces into Food Gardens at the Time of Covid-19 Quarantine: All the Benefits of Plants in this Difficult and Unprecedented Period. Hum. Ecol. 2020, 48, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.; Li, Z.; Liu, H. Synergistic effects of edible plants with light environment on the emotion and sleep of humans in long-duration isolated environment. Life Sci. Space Res. 2020, 24, 42–49. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Nelson, R.J. Influence of the modern light environment on mood. Mol. Psychiatry 2013, 18, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Duesenberg, M.; Weber, J.; Schulze, L.; Schaeuffele, C.; Roepke, S.; Hellmann-Regen, J.; Otte, C.; Wingenfeld, K. Does cortisol modulate emotion recognition and empathy? Psychoneuroendocrinology 2016, 66, 221–227. [Google Scholar] [CrossRef]
- Bringslimark, T.; Hartig, T.; Patil, G.G. The psychological benefits of indoor plants: A critical review of the experimental literature. J. Environ. Psychol. 2009, 29, 422–433. [Google Scholar] [CrossRef]
- Cohen-Mansfield, J.; Werner, P. Outdoor wandering parks for persons with dementia: A survey of characteristics and use. Alzheimer Dis. Assoc. Disord. 1999, 13, 109–117. [Google Scholar] [CrossRef]
- Sahoo, S.; Brijesh, S. Anxiolytic activity of Coriandrum sativum seeds aqueous extract on chronic restraint stressed mice and effect on brain neurotransmitters. J. Funct. Foods 2020, 68, 103884. [Google Scholar] [CrossRef]
- Bendifallah, L.; Louadi, K.; Doumandji, S. Bee fauna potential visitors of coriander flowers Coriandrum sativum L. (Apiaceae) in the Mitidja area (Algeria). J. Apic. Sci. 2013, 57, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.; Schneider, S.V.; Kluge, M.; Kessler, M.; Hamburger, M. Epilepsy in the Renaissance: A survey of remedies from 16th and 17th century German herbals. J. Ethnopharmacol. 2012, 143, 1–13. [Google Scholar] [CrossRef]
- Padalia, K.; Bargali, K.; Bargali, S.S. How does traditional home-gardens support ethnomedicinal values in Kumaun Himalayan Bhabhar belt, India? African J. Tradit. Complement. Altern. Med. 2015, 12, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26. [Google Scholar] [CrossRef]
- Yuan, R.; Liu, Z.; Zhao, J.; Wang, Q.-Q.; Zuo, A.; Huang, L.; Gao, H.; Xu, Q.; Khan, I.A.; Yang, S. Novel compounds in fruits of coriander (Coşkuner & Karababa) with anti-inflammatory activity. J. Funct. Foods 2020, 73, 104145. [Google Scholar] [CrossRef]
- Sparkman, O.D. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy Robert P. Adams. J. Am. Soc. Mass Spectrom. 2005, 16, 1902–1903. [Google Scholar] [CrossRef] [Green Version]
- Chan, H.Y.; Ho, R.C.-M.; Mahendran, R.; Ng, K.S.; Tam, W.W.-S.; Rawtaer, I.; Tan, C.H.; Larbi, A.; Feng, L.; Sia, A.; et al. Effects of horticultural therapy on elderly’ health: Protocol of a randomized controlled trial. BMC Geriatr. 2017, 17, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zung, W.W.K. A Self-Rating Depression Scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zung, W.W.K. A Rating Instrument For Anxiety Disorders. Psychosomatics 1971, 12, 371–379. [Google Scholar] [CrossRef]
- Zhu, B. Brief introduction of poms scale and its model for China. J. Tianjin Inst. Phys. Educ. 1995, 10, 35–37. [Google Scholar]
- Windmann, S.; Hill, H. Dissociating electrophysiological correlates of subjective, objective, and correct memory in investigating the emotion-induced recognition bias. Conscious. Cogn. 2014, 29, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Grassini, S.; Revonsuo, A.; Castellotti, S.; Petrizzo, I.; Benedetti, V.; Koivisto, M. Processing of natural scenery is associated with lower attentional and cognitive load compared with urban ones. J. Environ. Psychol. 2019, 62, 1–11. [Google Scholar] [CrossRef]
- Jang, H.S.; Kim, J.; Kim, K.S.; Pak, C.H. Human Brain Activity and Emotional Responses to Plant Color Stimuli. Hortic. Abstr. 2014, 39, 307–316. [Google Scholar] [CrossRef]
- Vecchiato, G.; Tieri, G.; Jelic, A.; De Matteis, F.; Maglione, A.G.; Babiloni, F. Electroencephalographic correlates of sensorimotor integration and embodiment during the appreciation of virtual architectural environments. Front. Psychol. 2015, 6, 1944. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Brown, D.K.; Barton, J.L.; Gladwell, V.F. Viewing Nature Scenes Positively Affects Recovery of Autonomic Function Following Acute-Mental Stress. Environ. Sci. Technol. 2013, 47, 5562–5569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herz, R.S.; Eliassen, J.; Beland, S.; Souza, T. Neuroimaging evidence for the emotional potency of odor-evoked memory. Neuropsychologia 2004, 42, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Cioanca, O.; Hritcu, L.; Mihasan, M.; Hancianu, M. Cognitive-enhancing and antioxidant activities of inhaled coriander volatile oil in amyloid β(1-42) rat model of Alzheimer’s disease. Physiol. Behav. 2013, 120, 193–202. [Google Scholar] [CrossRef]
- Linck, V.M.; da Silva, A.L.; Figueiró, M.; Caramão, E.B.; Moreno, P.R.H.; Elisabetsky, E. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Z.-H.; Leng, P.-S.; Zhang, H.-X.; Cheng, F.-Y. Fragrance Composition in Six Tree Peony Cultivars. Korean J. Hortic. Sci. Technol. 2012, 30, 617–625. [Google Scholar] [CrossRef]
- Cuevas-Glory, L.; Sosa-Moguel, O.; Ortiz-Vázquez, E.; Sauri-Duch, E.; Pino, J.A. Volatile constituents of tzitzilché flower (Gymnopodium floribundum Rolfe) from Yucatan Peninsula, Mexico. J. Essent. Oil Res. 2012, 24, 359–361. [Google Scholar] [CrossRef]
- Guzmán-Gutiérrez, S.L.; Bonilla-Jaime, H.; Gómez-Cansino, R.; Reyes-Chilpa, R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015, 128, 24–29. [Google Scholar] [CrossRef]
- Koriem, K.M.M. Lavandulae aetheroleum oil: A review on phytochemical screening, medicinal applications, and pharmacological effects. Biointerface Res. Appl. Chem. 2021, 11, 9836–9847. [Google Scholar] [CrossRef]
- Kutlu, A.K.; Yilmaz, E.; Çeçen, D. Effects of aroma inhalation on examination anxiety. Teach. Learn. Nurs. 2008, 3, 125–130. [Google Scholar] [CrossRef]
- Sayorwan, W.; Siripornpanich, V.; Piriyapunyaporn, T.; Hongratanaworakit, T.; Kotchabhakdi, N.; Ruangrungsi, N. The effects of lavender oil inhalation on emotional states, autonomic nervous system, and brain electrical activity. J. Med. Assoc. Thail. 2012, 95, 598–606. [Google Scholar]
- Lee, Y.K.; Za’aba, A.; Madzhi, N.K.; Ahmad, A. A study into salivary-based measurement of human stress subjected to ellestad stress test protocol. In Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine (EMBC 2009), Minneapolis, MN, USA, 3–6 September 2009; pp. 765–768. [Google Scholar]
- Takai, N.; Yamaguchi, M.; Aragaki, T.; Eto, K.; Uchihashi, K.; Nishikawa, Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch. Oral Biol. 2004, 49, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.; Bright, J. Stress: Myth, Theory and Research; Prentice Hall/Pearson Education: Upper Saddle River, NJ, USA, 2001; ISBN 0-130-41189-2. [Google Scholar]
- Kim, E.; Mattson, R.H. Human Stress Recovery during Exposure to Geranium Visual Stimuli. Hortscience A Public Am. Soc. Hortic. Sci. 1998, 33, 564. [Google Scholar] [CrossRef]
- Hendrickx, H.; McEwen, B.S.; Ouderaa, F. Van Der. Metabolism, mood and cognition in aging: The importance of lifestyle and dietary intervention. Neurobiol. Aging 2005, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jia, Z.; Gao, P.; Kong, H.; Li, X.; Lu, X.; Wu, Y.; Xu, G. Metabonomics study of urine and plasma in depression and excess fatigue rats by ultra fast liquid chromatography coupled with ion trap-time of flight mass spectrometry. Mol. Biosyst. 2010, 6, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yu, M.; Lu, X.; Huo, T.; Ge, L.; Yang, J.; Wu, C.; Li, F. Urinary metabonomic study on biochemical changes in chronic unpredictable mild stress model of depression. Clin. Chim. Acta 2010, 411, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Gao, H.C.; Li, Q.; Shao, W.H.; Zhang, M.L.; Cheng, K.; Yang, D.Y.; Fan, S.H.; Chen, L.; Fang, L.; et al. Plasma metabonomics as a novel diagnostic approach for major depressive disorder. J. Proteome Res. 2012, 11, 1741–1748. [Google Scholar] [CrossRef]
- Ding, X.; Yang, S.; Li, W.; Liu, Y.; Li, Z.; Zhang, Y.; Li, L.; Liu, S. The potential biomarker panels for identification of Major Depressive Disorder (MDD) patients with and without Early Life Stress (ELS) by metabonomic analysis. PLoS ONE 2014, 9, e97479. [Google Scholar] [CrossRef] [Green Version]
- Mitani, H.; Shirayama, Y.; Yamada, T.; Maeda, K.; Ashby, C.R.; Kawahara, R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1155–1158. [Google Scholar] [CrossRef]
- Ma, W.; Song, J.; Wang, H.; Shi, F.; Zhou, N.; Jiang, J.; Xu, Y.; Zhang, L.; Yang, L.; Zhou, M. Chronic paradoxical sleep deprivation-induced depression like behavior, energy metabolism and microbial changes in rats. Life Sci. 2019, 225, 88–97. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Control Group | Coriander Group | p-Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Number | 13 | - | 13 | - | - |
| Female | 7 | - | 7 | - | - |
| Male | 6 | - | 6 | - | - |
| Age (years) | 20.16 | 0.42 | 22.72 | 1.05 | 0.27 |
| Height (cm) | 169.75 | 4.71 | 171.45 | 3.01 | 0.60 |
| Body weight (kg) | 62.23 | 4.68 | 60.18 | 4.31 | 0.56 |
| BMI (kg/m2) | 21.58 | 1.12 | 20.18 | 0.98 | 0.22 |
| SAS | 38.53 | 2.02 | 38.50 | 2.96 | 0.98 |
| SDS | 41.46 | 2.09 | 40.92 | 1.96 | 0.73 |
| Temperature (°C) | 20.22 | 0.62 | 20.13 | 0.56 | 0.71 |
| Relative humidity (%) | 50.77 | 3.83 | 52.45 | 2.09 | 0.13 |
| CO2 concentration (ppm) | 769.44 | 31.13 | 737.66 | 38.96 | 0.10 |
| Compounds | Chemical Formula | RT (min) | RI | RC (%) | CAS |
|---|---|---|---|---|---|
| Longifolene | C15H24 | 36.46 | 1417.58 | 2.20 | 475-20-7 |
| 1,3,5-Benzetriol, 3TMS derivative | C15H30O3Si3 | 32.49 | 1265.55 | 1.66 | 10586-12-6 |
| 2-Ethylhexyl acrylate | C11H20O2 | 30.82 | 1146.97 | 1.59 | 103-11-7 |
| α-Terpineol | C10H18O | 29.88 | 1136.51 | 4.45 | 98-55-5 |
| Dimethyl glutarate | C7H12O4 | 27.86 | 1114.01 | 5.03 | 1119-40-0 |
| Lsophorone | C9H14O | 27.59 | 1110.99 | 6.06 | 78-59-1 |
| 1,2,3,5-Tetramethylbenzene | C10H14 | 27.37 | 1108.63 | 2.82 | 527-53-7 |
| 3-Hydroxymandelic acid, 3TMS derivative | C17H32O4Si3 | 27.22 | 1106.86 | 2.69 | 68595-69-7 |
| Linalool | C10H18O | 26.66 | 1100.66 | 0.60 | 78-70-6 |
| Acetophenone | C8H8O | 25.58 | 987.55 | 1.95 | 98-86-2 |
| γ-Terpinene | C10H16 | 25.27 | 983.73 | 1.10 | 99-85-4 |
| Benzyl alcohol | C7H8O | 24.36 | 972.51 | 6.16 | 100-51-6 |
| Eucalyptol | C10H18O | 24.30 | 971.76 | 8.87 | 470-82-6 |
| D-Limonene | C10H16 | 24.16 | 870.37 | 9.58 | 5989-27-5 |
| 2-Ethyl-1-hexanol | C8H18O | 24.07 | 968.90 | 15.60 | 104-76-7 |
| α-Pinene | C10H16 | 20.17 | 930.96 | 3.17 | 80-56-8 |
| Styrene | C8H8 | 18.11 | 805.88 | 3.92 | 100-42-5 |
| Furfural | C5H4O2 | 15.14 | 639.22 | 0.75 | 98-01-1 |
| Methyl methacrylate | C5H8O2 | 9.18 | 613.16 | 3.20 | 80-62-6 |
| 1-Butanol | C4H10O | 7.59 | 692.01 | 1.45 | 71-36-3 |
| 1,2-Ethanediol, diformate | C4H6O4 | 6.35 | 598.03 | 6.18 | 629-15-2 |
| Ethyl Acetate | C4H8O2 | 6.61 | 618.02 | 2.54 | 141-78-6 |
| Other volatile components | - | - | t | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, Z.; Wang, L.; Liu, H.; Liu, H. Effect of Coriander Plants on Human Emotions, Brain Electrophysiology, and Salivary Secretion. Biology 2021, 10, 1283. https://doi.org/10.3390/biology10121283
Zhang W, Li Z, Wang L, Liu H, Liu H. Effect of Coriander Plants on Human Emotions, Brain Electrophysiology, and Salivary Secretion. Biology. 2021; 10(12):1283. https://doi.org/10.3390/biology10121283
Chicago/Turabian StyleZhang, Wenzhu, Zhaoming Li, Lingshan Wang, Hui Liu, and Hong Liu. 2021. "Effect of Coriander Plants on Human Emotions, Brain Electrophysiology, and Salivary Secretion" Biology 10, no. 12: 1283. https://doi.org/10.3390/biology10121283
APA StyleZhang, W., Li, Z., Wang, L., Liu, H., & Liu, H. (2021). Effect of Coriander Plants on Human Emotions, Brain Electrophysiology, and Salivary Secretion. Biology, 10(12), 1283. https://doi.org/10.3390/biology10121283






