Exposure to the Insecticide Sulfoxaflor Affects Behaviour and Biomarkers Responses of Carcinus maenas (Crustacea: Decapoda)
Abstract
:Simple Summary
Abstract
1. Introduction
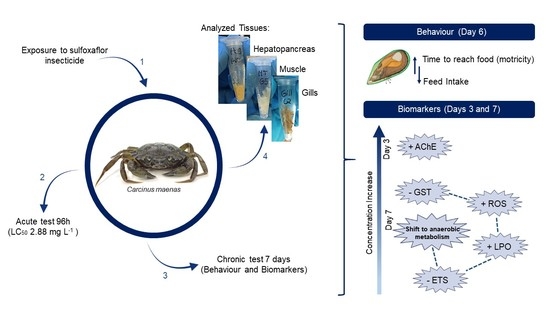
2. Materials and Methods
2.1. Ethical Experiments
2.2. Crab Sampling and Acclimation
2.3. Experimental Design
2.3.1. Material and Pesticide Stock Solution
2.3.2. Acute Toxicity Test
2.3.3. Sub-Lethal Toxicity Tests
2.4. Tissue Preparation and Biomarkers Analysis
2.4.1. Protein Quantification
2.4.2. Energy Metabolism Related Biomarkers
2.4.3. Detoxification and Oxidative Stress Related Biomarkers
2.4.4. Neuromuscular Toxicity
2.5. Statistical Analysis
3. Results
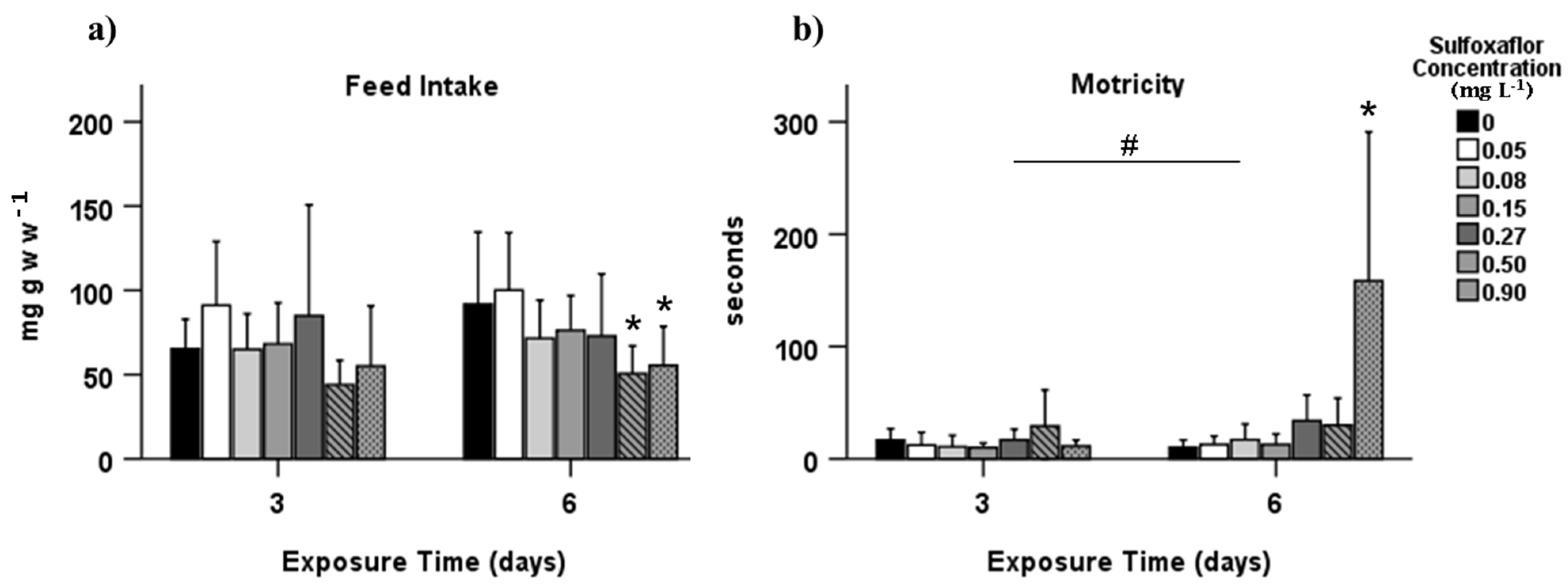
3.1. Behavioural Effects
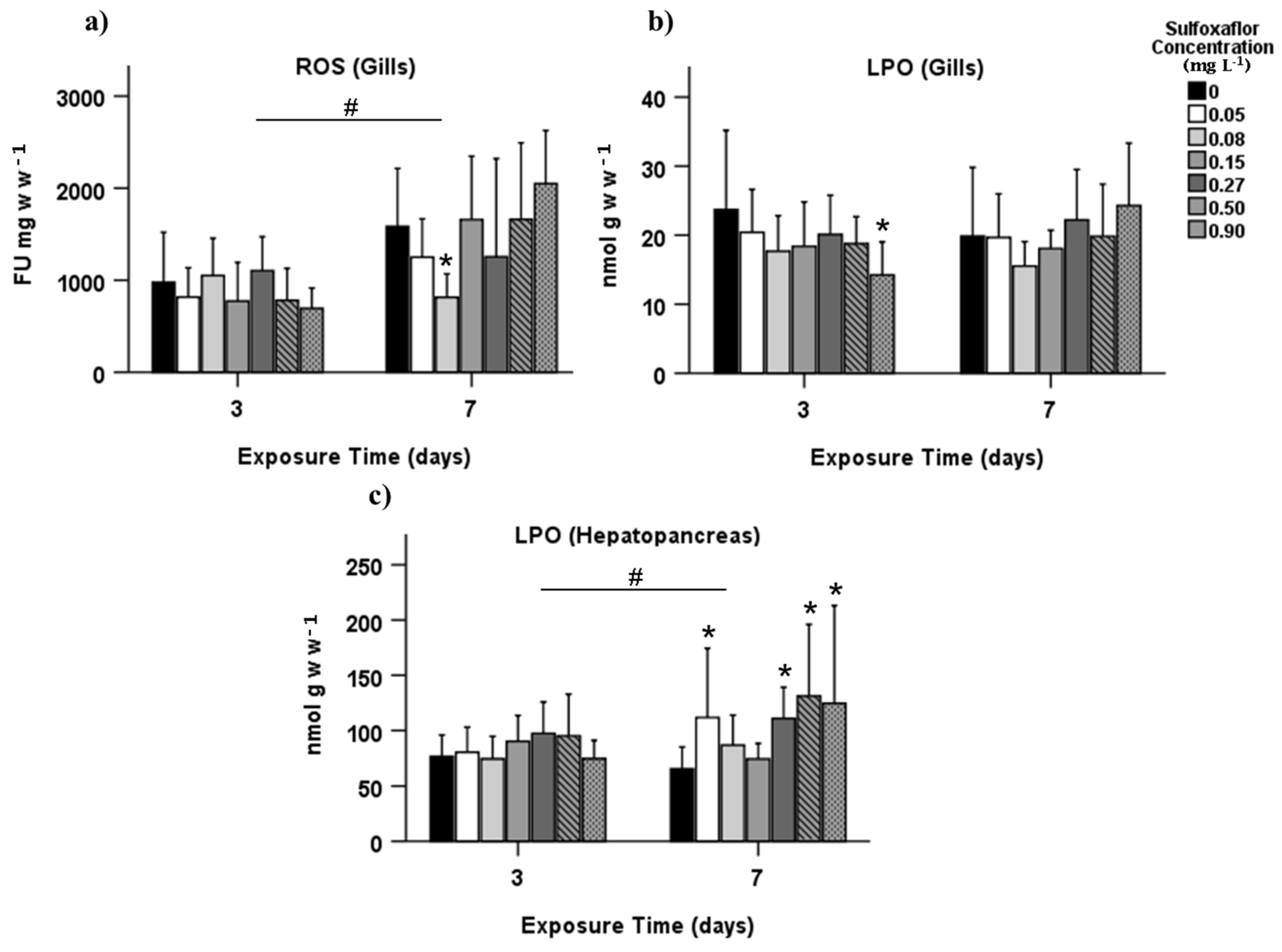
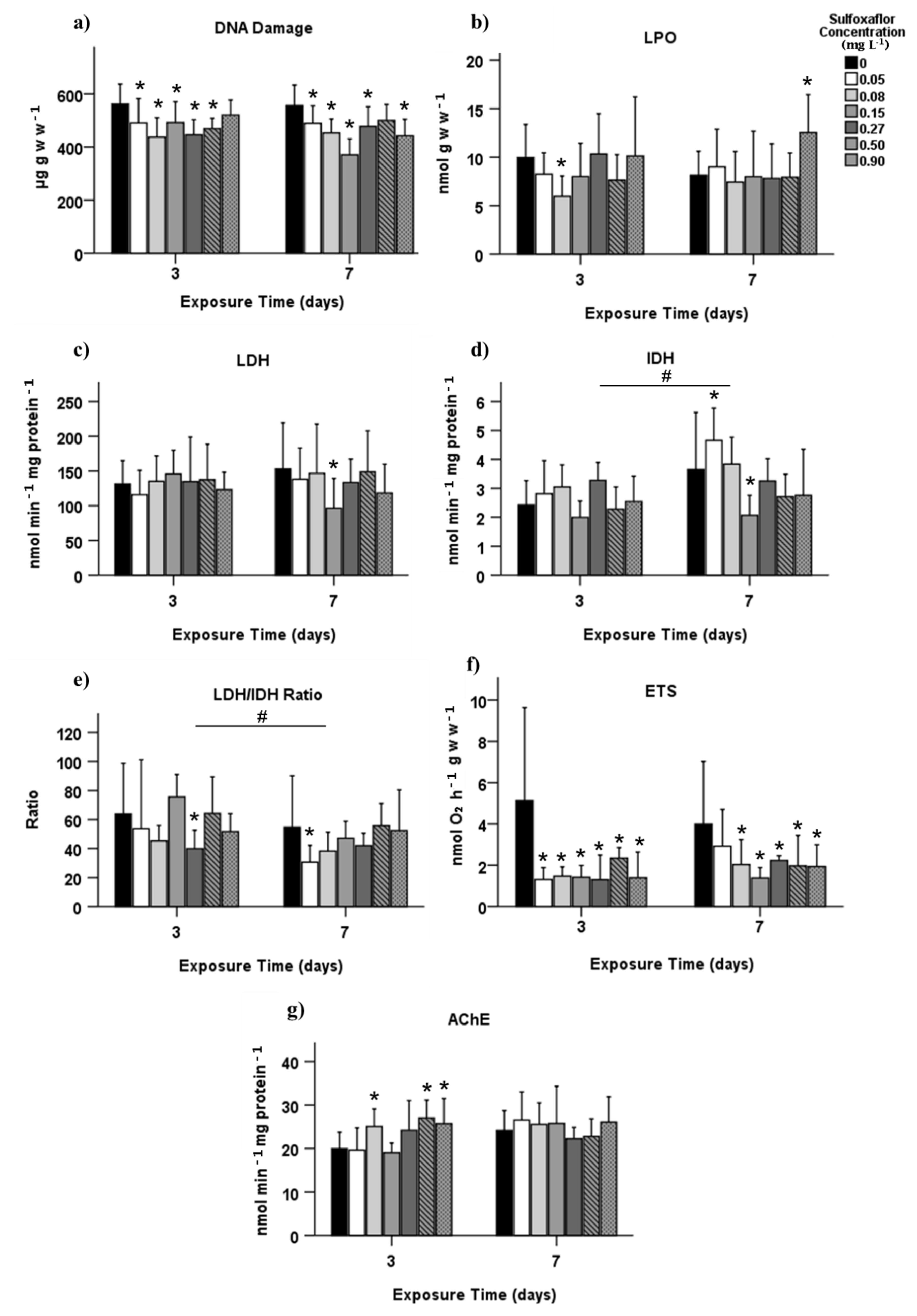
3.2. Biochemical Biomarkers
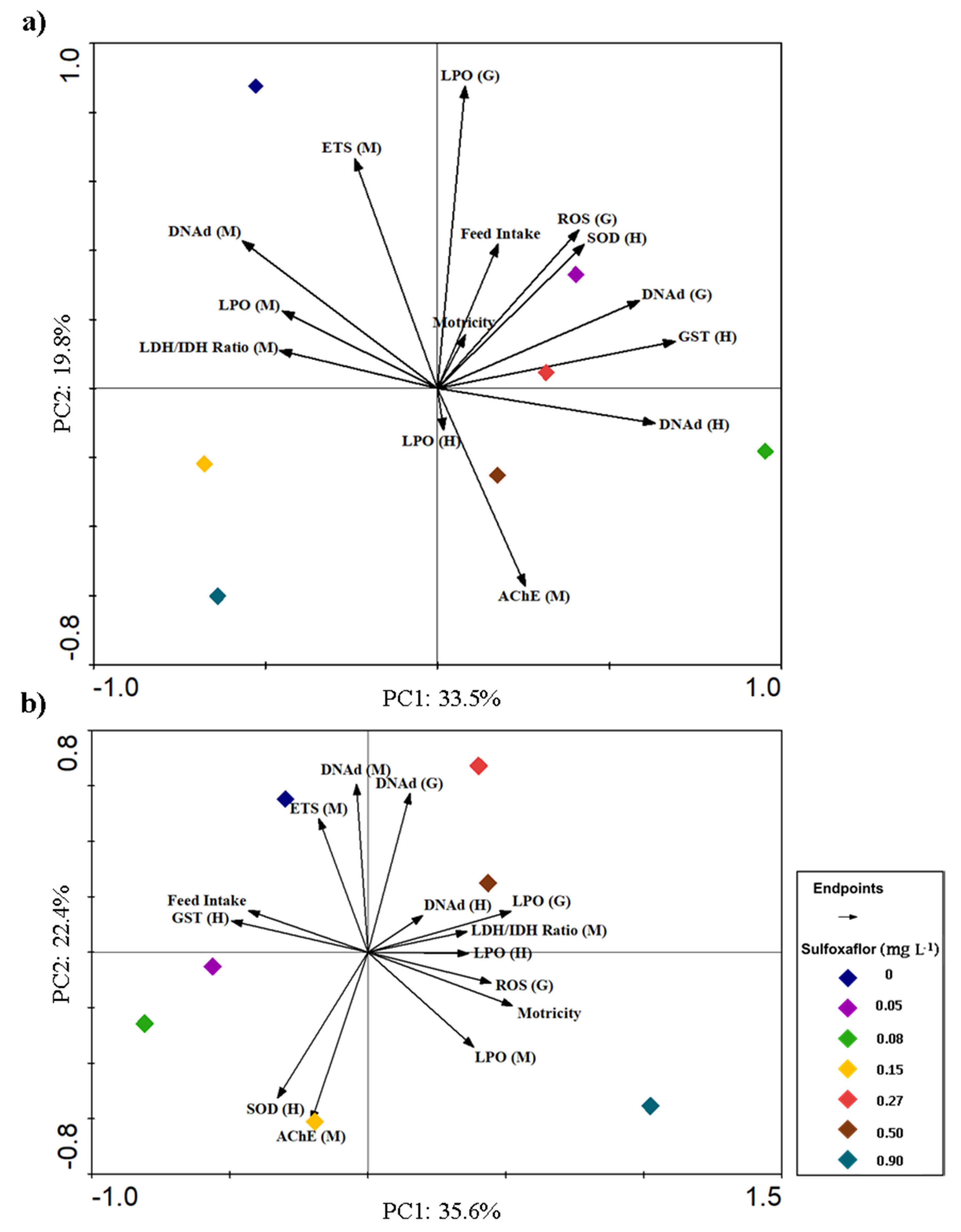
3.3. Integrative Analysis of Behavioural and Biochemical Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mateo-Sagasta, J.; Zadeh, S.M.; Turral, H.; Burke, J. Water Pollution from Agriculture: A Global Review; FAO: Rome, Italy; IWMI: Colombo, Sri Lanka, 2017; Available online: https://www.fao.org/3/i7754e/i7754e.pdf (accessed on 12 May 2021).
- Singh, B.; Kaur, A. Control of insect pests in crop plants and stored food grains using plant saponins: A review. LWT-Food Sci. Technol. 2018, 87, 93–101. [Google Scholar] [CrossRef]
- Gonçalves, A.M.M.; Rocha, C.P.; Marques, J.C.; Gonçalves, F.J.M. Enzymes as useful biomarkers to assess the response of freshwater communities to pesticide exposure—A review. Ecol. Indic. 2021, 122, 107303. [Google Scholar] [CrossRef]
- Zhu, Y.; Loso, M.R.; Watson, G.B.; Sparks, T.C.; Rogers, R.B.; Huang, J.X.; Gerwick, B.C.; Babcock, J.M.; Kelley, D.; Hegde, V.B.; et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 2011, 59, 2950–2957. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, V.; Unnikrishnan, P.; Anu, G.; Nair, S.M. Distribution of organophosphorus pesticides in the bed sediments of a backwater system located in an agricultural watershed: Influence of seasonal intrusion of seawater. Arch. Environ. Contam. Toxicol. 2011, 60, 597–609. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Dow AgroSciences. Isoclast™ active Technical Bulletin; Dow AgroSciences: Indianapolis, IN, USA, 2014; Available online: https://www.corteva.com/content/dam/dpagco/corteva/global/corporate/general/files/active-ingredients/DF_Isoclast-Active-TechBulletin_LO7_2019.pdf (accessed on 15 April 2021).
- Babcock, J.M.; Gerwick, C.B.; Huang, J.X.; Loso, M.R.; Nakamura, G.; Nolting, S.P.; Rogers, R.B.; Sparks, T.C.; Thomas, J.; Watson, G.B.; et al. Biological characterization of sulfoxaflor, a novel insecticide. Pest Manag. Sci. 2010, 67, 328–334. [Google Scholar] [CrossRef]
- Sparks, T.C.; Watson, G.B.; Loso, M.R.; Geng, C.; Babcock, J.M.; Thomas, J.D. Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pestic. Biochem. Physiol. 2013, 107, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Watson, G.B.; Loso, M.R.; Babcock, J.M.; Hasler, J.M.; Letherer, T.J.; Young, C.D.; Zhu, Y.; Casida, J.E.; Sparks, T.C. Novel nicotinic action of the sulfoximine insecticide sulfoxaflor. Insect Biochem. Mol. Biol. 2011, 41, 432–439. [Google Scholar] [CrossRef]
- Siviter, H.; Brown, M.J.F.; Leadbeater, E. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 2018, 561, 109–112. [Google Scholar] [CrossRef]
- Gauthier, J.R.; Mabury, S.A. Sulfoximine Insecticide Sulfoxaflor and its Photodegradate Demonstrates Acute Toxicity to Non-targeted Invertebrate Species Daphnia magna. Environ. Toxicol. Chem. 2021, 40, 2156–2164. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E. Pollution Biomarkers in Environmental and Human Biomonitoring. Open Biomark. J. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, E.T.; Pardal, M.Â. The crab Carcinus maenas as a suitable experimental model in ecotoxicology. Environ. Int. 2014, 70, 158–182. [Google Scholar] [CrossRef] [PubMed]
- Klassen, G.J.; Locke, A. A Biological Synopsis of the European Green Crab, Carcinus Maenas; Fisheries and Oceans Canada: Moncton, NB, Canada, 2007; pp. 1–82. [Google Scholar]
- Young, A.M.; Elliott, J.A. Life History and Population Dynamics of Green Crabs (Carcinus maenas). Fishes 2020, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H. Activation and Detoxification Enzymes: Functions and Implications, 1st ed.; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Liska, D.A.J. The detoxification enzyme systems. Altern. Med. Rev. 1998, 3, 187–198. [Google Scholar] [PubMed]
- Boyland, E.; Chasseaud, L.F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1969, 32, 173–219. [Google Scholar] [CrossRef]
- Di Giulio, R.T.; Washburn, P.C.; Wenning, R.J. Biochemical responses in aquatic animals: A review of determinants of oxidative stress. Environ. Toxicol. Chem. 1989, 8, 1103–1123. [Google Scholar] [CrossRef]
- Novais, S.C.; Gomes, N.C.; Soares, A.M.V.M.; Amorim, M.J.B. Antioxidant and neurotoxicity markers in the model organism Enchytraeus albidus (Oligochaeta): Mechanisms of response to atrazine, dimethoate and carbendazim. Ecotoxicology 2014, 23, 1220–1233. [Google Scholar] [CrossRef]
- Schittenhelm, D.; Neuss-Radu, M.; Verma, N.; Pink, M.; Schmitz-Spanke, S. ROS and pentose phosphate pathway: Mathematical modelling of the metabolic regulation in response to xenobiotic-induced oxidative stress and the proposed Impact of the gluconate shunt. Free Radic. Res. 2019, 53, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [Green Version]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Holmblad, T.; Söderhäll, K. Cell adhesion molecules and antioxidative enzymes in a crustacean, possible role in immunity. Aquaculture 1999, 172, 111–123. [Google Scholar] [CrossRef]
- Mohankumar, K.; Ramasamy, P. White spot syndrome virus infection decreases the activity of antioxidant enzymes in Fenneropenaeus indicus. Virus Res. 2006, 115, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sancho, E.; Villarroel, M.J.; Andreu, E.; Ferrando, M.D. Disturbances in energy metabolism of Daphnia magna after exposure to tebuconazole. Chemosphere 2009, 74, 1171–1178. [Google Scholar] [CrossRef]
- Diamantino, T.C.; Almeida, E.; Soares, A.M.V.M.; Guillermino, L. Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna strauss. Chemosphere 2001, 45, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Lima, I.; Moreira, S.M.; Osten, J.R.V.; Soares, A.M.V.M.; Guilhermino, L. Biochemical responses of the marine mussel Mytilus galloprovincialis to petrochemical environmental contamination along the North-western coast of Portugal. Chemosphere 2007, 66, 1230–1242. [Google Scholar] [CrossRef]
- Tonn, N.; Novais, S.C.; Silva, C.S.E.; Morais, H.A.; Correia, J.P.S.; Lemos, M.F.L. Stress responses of the sea cucumber Holothuria forskali during aquaculture handling and transportation. Mar. Biol. Res. 2016, 12, 948–957. [Google Scholar] [CrossRef]
- De Coen, W.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV. Cellulst Energy Allocation: A new methodology to assess the energy budget of toxicant-stressed Daphinia populations. J. Aquat. Ecosyst. Stress Recover. 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Sturm, A.; Da Silva De Assis, H.C.; Hansen, P.D. Cholinesterases of marine teleost fish: Enzymological characterization and potential use in the monitoring of neurotoxic contamination. Mar. Environ. Res. 1999, 47, 389–398. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010, protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L276, 33–79.
- Decreto-Lei 113/2013, de 7 de agosto. Diário da República 1a série; Ministério da Agricultura, do Mar, do Ambiente e do Ordenamento do Território: Lisboa, Portugal, 2013; pp. 4709–4739.
- Durand, F.; Regnault, M. Nitrogen metabolism of two portunid crabs, Carcinus maenas and Necora puber, during prolonged air exposure and subsequent recovery: A comparative study. J. Exp. Biol. 1998, 201, 2515–2528. [Google Scholar] [CrossRef]
- ASTM International. Standard Guide for Conducting Acute Toxicity Tests on Tests Materials with Fishes, Macroinvertebrates, and Amphibians; ASTM International: West Conshohocken, PA, USA, 2002; Volume 96, pp. 1–22. [Google Scholar]
- MARETEC. Programa de Monitorização da Lagoa de Óbidos e do Emissário Submarino da Foz do Arelho. Caracterização da Situação de Referência: Qualidade da Água da Lagoa de Óbidos. 2004, p. 57. Available online: http://maretec.mohid.com/projects/fozdoarelho/Relatorios/2004-2005/Rel_02_Modela%C3%A7%C3%A3o%20da%20Qualidade%20da%20Agua.pdf. (accessed on 4 December 2020).
- Rodrigues, E.T.; Moreno, A.; Mendes, T.; Palmeira, C.; Pardal, M.Â. Biochemical and physiological responses of Carcinus maenas to temperature and the fungicide azoxystrobin. Chemosphere 2015, 132, 127–134. [Google Scholar] [CrossRef]
- Yue, S. The Welfare of Crustaceans at Slaughter. Impacts Farm Anim. 2008, 5, 10. Available online: https://animalstudiesrepository.org/hsus_reps_impacts_on_animals/4 (accessed on 23 April 2020).
- Oliveira, D.N.; Christofoletti, R.A.; Barreto, E.R. Feeding behavior of a crab according to cheliped number. PLoS ONE 2015, 10, e0145121. [Google Scholar] [CrossRef] [Green Version]
- Van den Brink, A.; Hutting, S. Clash of the crabs: Interspecific, inter-cohort competition between the native European green crab, Carcinus maenas and the exotic brush clawed crab Hemigrapsus takanoi on artificial oyster reefs. J. Sea Res. 2017, 128, 41–51. [Google Scholar] [CrossRef]
- Abreu, I.O.; Monteiro, C.; Rocha, A.C.S.; Reis-Henriques, M.A.; Teixeira, C.; Basto, M.C.P.; Ferreira, M.; Almeida, C.M.R.; Oliva-Teles, L.; Guimarães, L. Multibiomarker interactions to diagnose and follow-up chronic exposure of a marine crustacean to Hazardous and Noxious Substances (HNS). Environ. Pollut. 2018, 242, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ellis, G.; Goldberg, D.M. An improved manual and semi-automatic assay for NADP-dependent isocitrate dehydrogenase activity, with a description of some kinetic properties of human liver and serum enzyme. Clin. Biochem. 1971, 2, 175–185. [Google Scholar] [CrossRef]
- Vassault, A.; Wahlfold, A.W.; Deneke, U. Lactate Dehydrogenasein in Methods of Enzymatic Analysis, 3rd ed.; Bergmeyer, H.U., Bergmeyer, J., GraB l, M., Moss, D.W., Eds.; Verlag Chemie: Weinheim, Germany, 1983; Volume 3, pp. 118–126. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases—First enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 7130–7139. [Google Scholar] [CrossRef]
- Socci, D.J.; Bjugstad, K.B.; Jones, H.C.; Pattisapu, J.V.; Arendash, G.W. Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp. Neurol. 1999, 155, 109–117. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Bird, R.P.; Draper, A.H. Comparative studies on different methods of malonaldehyde determination. Methods Enzym. 1984, 105, 299–305. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Torres, M.A.; Testa, C.P.; Gáspari, C.; Masutti, M.B.; Panitz, C.M.N.; Curi-Pedrosa, R.; De Almeida, E.A.; Di Mascio, P.; Filho, D.W. Oxidative stress in the mussel Mytella guyanensis from polluted mangroves on Santa Catarina Island, Brazil. Mar. Pollut. Bull. 2002, 44, 923–932. [Google Scholar] [CrossRef]
- Olive, P.L. DNA precipitation assay: A rapid and simple method for detecting DNA damage in mammalian cells. Environ. Mol. Mutagen 1988, 11, 487–495. [Google Scholar] [CrossRef] [PubMed]
- De Lafontaine, Y.; Gagné, F.; Blaise, C.; Costan, G.; Gagnon, P.; Chan, H.M. Biomarkers in zebra mussels (Dreissena polymorpha) for the assessment and monitoring of water quality of the St Lawrence River (Canada). Aquat. Toxicol. 2000, 50, 51–71. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D., Jr. Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 2, 88–95. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soares, A.M.V.M. Inhibition of acetylcholinesterase activity as effect criterion in acute tests with juvenile Daphinia magna. Chemosphere 1996, 32, 727–738. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson: Upper Saddle River, NJ, USA, 2010; ISBN 9780131008465. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analysing Ecological Data; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9780387459677. [Google Scholar]
- Ho, R. Multivariate Data Analysis with IBM SPSS; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9781439890226. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, NY, USA, 2003; ISBN 9780521814096. [Google Scholar]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Gibbons, D.; Morrissey, C.; Mineau, P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. 2015, 22, 103–118. [Google Scholar] [CrossRef] [Green Version]
- Siviter, H.; Horner, J.; Brown, M.J.F.; Leadbeater, E. Sulfoxaflor exposure reduces egg laying in bumblebees Bombus terrestris. J. Appl. Ecol. 2020, 57, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Niesen, M.; Sappington, K.; Ruhman, M.; Mroz, R.; Leader, R.A.P.; Housenger, J.; Chief, B. Ecological Risk Assessment for the Registration Review of Sulfoxaflor; United States Environmental Protection Agency: Washington, DC, USA, 2019; pp. 1–286.
- Benli, P.P.; Çelik, M. Glutathione and its dependent enzymes’ modulatory responses to neonicotinoid insecticide sulfoxaflor induced oxidative damage in zebrafish in vivo. Sci. Prog. 2021, 104, 368504211028361. [Google Scholar] [CrossRef]
- Benli, P.P.; Çelik, M. In Vivo effects of neonicotinoid-sulfoximine insecticide sulfoxaflor on acetylcholinesterase activity in the tissues of Zebrafish (Danio Rerio). Toxics 2021, 9, 73. [Google Scholar] [CrossRef]
- Watts, A.J.R.; Urbina, M.A.; Corr, S.; Lewis, C.; Galloway, T.S. Ingestion of Plastic Microfibers by the Crab Carcinus maenas and Its Effect on Food Consumption and Energy Balance. Environ. Sci. Technol. 2015, 49, 14597–14604. [Google Scholar] [CrossRef]
- Dissanayake, A.; Piggott, C.; Baldwin, C.; Sloman, K.A. Elucidating cellular and behavioural effects of contaminant impact (polycyclic aromatic hydrocarbons, PAHs) in both laboratory-exposed and field-collected shore crabs, Carcinus maenas (Crustacea: Decapoda). Mar. Environ. Res. 2010, 70, 368–373. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.P.; Lehtonen, K.K.; Guilhermino, L.; Guimarães, L. Exposure of Carcinus maenas to waterborne fluoranthene: Accumulation and multibiomarker responses. Sci. Total Environ. 2013, 443, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.L.N.; Venugopal, N.B.R.K. Effect of fluoride on acetylcholinesterase activity and oxygen consumption in a freshwater field crab, Barytelphusa guerini. Bull. Environ. Contam. Toxicol. 1990, 45, 760–766. [Google Scholar] [CrossRef]
- Silva, C.O.; Simões, T.; Félix, R.; Soares, A.M.V.M.; Barata, C.; Novais, S.C.; Lemos, M.F.L. Asparagopsis armata exudate cocktail: The quest for the mechanisms of toxic action of an invasive seaweed on marine invertebrates. Biology 2021, 10, 223. [Google Scholar] [CrossRef]
- Qi, S.; Wang, D.; Zhu, L.; Teng, M.; Wang, C.; Xue, X.; Wu, L. Neonicotinoid insecticides imidacloprid, guadipyr, and cycloxaprid induce acute oxidative stress in Daphnia magna. Ecotoxicol. Environ. Saf. 2018, 148, 352–358. [Google Scholar] [CrossRef]
- Bacci, L.; Convertini, S.; Rossaro, B. A review of sulfoxaflor, a derivative of biological acting substances as a class of insecticides with a broad range of action against many insect pests. J. Entomol. Acarol. Res. 2018, 50, 51–71. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, D.R. Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Rev. Med. Vet. 2003, 154, 427–430. [Google Scholar]
- Shukla, S.; Jhamtani, R.C.; Dahiya, M.S.; Agarwal, R. Oxidative injury caused by individual and combined exposure of neonicotinoid, organophosphate and herbicide in zebrafish. Toxicol. Rep. 2017, 4, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.E.D.; Pérez, M.R.; Acayaba, R.D.A.; Raimundo, C.C.M.; dos Reis Martinez, C.B. DNA damage and oxidative stress induced by imidacloprid exposure in different tissues of the Neotropical fish Prochilodus lineatus. Chemosphere 2018, 195, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.H.; Wang, J.H.; Zhu, L.S.; Chen, A.M.; Wang, J. Thiamethoxam induces oxidative stress and antioxidant response in zebrafish (Danio Rerio) livers. Environ. Toxicol. 2016, 31, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Stolze, K.; Nohl, H. Effect of xenobiotics on the respiratory activity of rat heart mitochondria and the concomitant formation of superoxide radicals. Environ. Toxicol. Chem. 1994, 13, 499–502. [Google Scholar] [CrossRef]
- Choi, J.; Roche, H.; Caquet, T. Hypoxia, hyperoxia and exposure to potassium dichromate or fenitrothion alter the energy metabolism in Chironomus riparius Mg. (Diptera: Chironomidae) larvae. Comp. Biochem. Physiol.-C Toxicol. Pharmacol. 2001, 130, 11–17. [Google Scholar] [CrossRef]
- Depledge, M.H.; Aagaard, A.; Györkös, P. Assessment of trace metal toxicity using molecular, physiological and behavioural biomarkers. Mar. Pollut. Bull. 1995, 31, 19–27. [Google Scholar] [CrossRef]
- Lemos, M.F.L.; Soares, A.M.V.M.; Correia, A.C.; Esteves, A.C. Proteins in ecotoxicology—How, why and why not? Proteomics 2010, 10, 873–887. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damasceno, J.M.; Rato, L.D.; Simões, T.; Morão, I.F.C.; Meireles, G.; Novais, S.C.; Lemos, M.F.L. Exposure to the Insecticide Sulfoxaflor Affects Behaviour and Biomarkers Responses of Carcinus maenas (Crustacea: Decapoda). Biology 2021, 10, 1234. https://doi.org/10.3390/biology10121234
Damasceno JM, Rato LD, Simões T, Morão IFC, Meireles G, Novais SC, Lemos MFL. Exposure to the Insecticide Sulfoxaflor Affects Behaviour and Biomarkers Responses of Carcinus maenas (Crustacea: Decapoda). Biology. 2021; 10(12):1234. https://doi.org/10.3390/biology10121234
Chicago/Turabian StyleDamasceno, Jadilson M., Lénia D. Rato, Tiago Simões, Inês F. C. Morão, Gabriela Meireles, Sara C. Novais, and Marco F. L. Lemos. 2021. "Exposure to the Insecticide Sulfoxaflor Affects Behaviour and Biomarkers Responses of Carcinus maenas (Crustacea: Decapoda)" Biology 10, no. 12: 1234. https://doi.org/10.3390/biology10121234
APA StyleDamasceno, J. M., Rato, L. D., Simões, T., Morão, I. F. C., Meireles, G., Novais, S. C., & Lemos, M. F. L. (2021). Exposure to the Insecticide Sulfoxaflor Affects Behaviour and Biomarkers Responses of Carcinus maenas (Crustacea: Decapoda). Biology, 10(12), 1234. https://doi.org/10.3390/biology10121234