Standard Non-Personalized Electric Field Modeling of Twenty Typical tDCS Electrode Configurations via the Computational Finite Element Method: Contributions and Limitations of Two Different Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
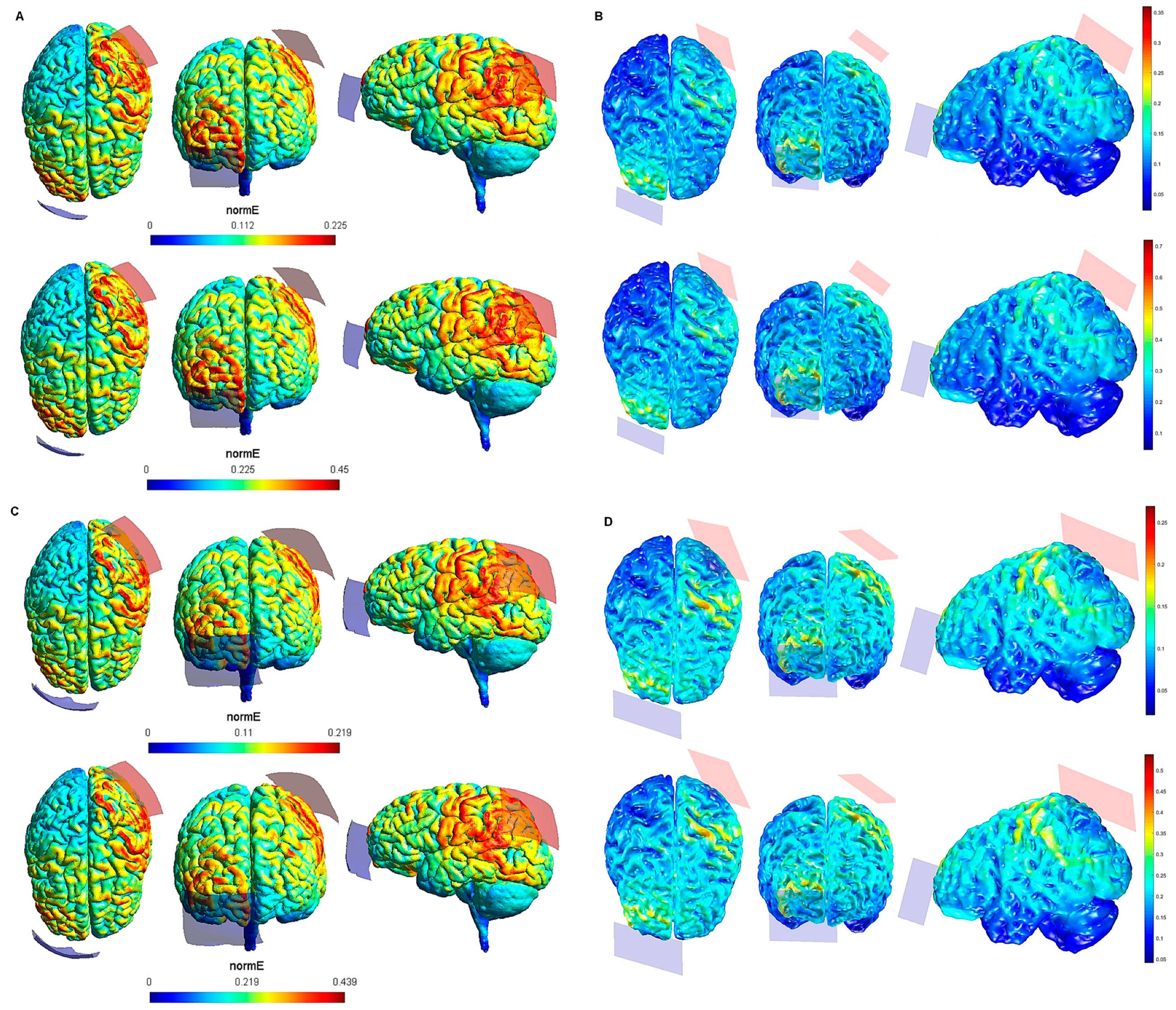
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fertonani, A.; Miniussi, C. Transcranial electrical stimulation: What we know and do not know about mechanisms. Neuroscientist 2017, 23, 109–123. [Google Scholar] [CrossRef]
- Bestmann, S.; Walsh, V. Transcranial electrical stimulation. Curr. Biol. 2017, 27, R1258–R1262. [Google Scholar] [CrossRef] [Green Version]
- Giordano, J.; Bikson, M.; Kappenman, E.S.; Clark, V.P.; Coslett, H.B.; Hamblin, M.R.; Hamilton, R.; Jankord, R.; Kozumbo, W.J.; McKinley, R.A.; et al. Mechanisms and effects of transcranial direct current stimulation. Dose-Response 2017, 15, 1559325816685467. [Google Scholar] [CrossRef] [Green Version]
- Kuo, M.F.; Nitsche, M.A. Effects of transcranial electrical stimulation on cognition. Clin. EEG Neurosci. 2012, 43, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Transcranial direct current stimulation—Update 2011. Restor. Neurol. Neurosci. 2011, 29, 463–492. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Nitsche, M.A. Physiological basis of transcranial direct current stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Nitsche, M.; Bermpohl, F.; Antal, A.; Feredoes, E.; Marcolin, M.A.; Rigonatti, S.P.; Silva, M.T.A.; Paulus, W.; et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 2005, 166, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef] [Green Version]
- Berryhill, M.E.; Martin, D. Cognitive effects of transcranial direct current stimulation in healthy and clinical populations: An overview. J. ECT 2018, 34, e25–e35. [Google Scholar] [CrossRef]
- Dong, L.; Ke, Y.; Liu, S.; Song, X.; Ming, D. Effects of HD-tDCS combined with working memory training on event-related potentials. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Montreal, QC, Canada, 20–24 July 2020; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2020; pp. 3553–3556. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Boggio, P.S.; Fregni, F.; Pascual-Leone, A. Treatment of depression with transcranial direct current stimulation (tDCS): A Review. Exp. Neurol. 2009, 219, 14–19. [Google Scholar] [CrossRef]
- Rivera-Urbina, G.N.; Nitsche, M.A.; Vicario, C.M.; Molero-Chamizo, A. Applications of transcranial direct current stimulation in children and pediatrics. Rev. Neurosci. 2017, 28, 173–184. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef] [Green Version]
- Poreisz, C.; Boros, K.; Antal, A.; Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007, 72, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Molero-Chamizo, A.; Sánchez, M.Á.S.; Riquel, R.M.; Gutiérrez Lérida, C.; Rivera-Urbina, G.N. Regulation and Ethics of Transcranial Electrical Stimulation: A General View. Neurophysiology 2020, 52, 234–238. [Google Scholar] [CrossRef]
- Shin, Y.I.; Foerster, Á.; Nitsche, M.A. Transcranial direct current stimulation (tDCS)—Application in neuropsychology. Neuropsychologia 2015, 69, 154–175. [Google Scholar] [CrossRef] [PubMed]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, M.; Laakso, I.; Tanaka, S.; Hirata, A. Cost of focality in TDCS: Interindividual variability in electric fields. Brain Stimul. 2020, 13, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridding, M.C.; Ziemann, U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010, 588, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, D.; Grittner, U.; Puonti, O.; Flöel, A.; Thielscher, A. Estimation of individually induced e-field strength during transcranial electric stimulation using the head circumference. Brain Stimul. 2021, 14, 1055–1058. [Google Scholar] [CrossRef]
- Ciechanski, P.; Carlson, H.L.; Yu, S.S.; Kirton, A. Modeling transcranial direct-current stimulation-induced electric fields in children and adults. Front. Hum. Neurosci. 2018, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Dmochowski, J.P.; Datta, A.; Bikson, M.; Su, Y.; Parra, L.C. Optimized multi-electrode stimulation increases focality and intensity at target. J. Neural Eng. 2011, 8, 046011. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Truong, D.Q.; Esmaeilpour, Z.; Huang, Y.; Badran, B.W.; Bikson, M. Enhanced tES and tDCS computational models by meninges emulation. J. Neural Eng. 2020, 17, 016027. [Google Scholar] [CrossRef] [PubMed]
- Morales-Quezada, L.; El-Hagrassy, M.M.; Costa, B.; McKinley, R.A.; Lv, P.; Fregni, F. Transcranial direct current stimulation optimization—From physics-based computer simulations to high-fidelity head phantom fabrication and measurements. Front. Hum. Neurosci. 2019, 13, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosayebi-Samani, M.; Jamil, A.; Salvador, R.; Ruffini, G.; Haueisen, J.; Nitsche, M.A. The impact of individual electrical fields and anatomical factors on the neurophysiological outcomes of tDCS: A TMS-MEP and MRI study. Brain Stimul. 2021, 14, 316–326. [Google Scholar] [CrossRef]
- Opitz, A.; Paulus, W.; Will, S.; Antunes, A.; Thielscher, A. Determinants of the electric field during transcranial direct current stimulation. Neuroimage 2015, 109, 140–150. [Google Scholar] [CrossRef]
- Shahid, S.S.; Song, B.; Salman, H.; de Oliveira, M.M.; Wen, P. Use of electric field orientation as an index for estimating the contribution of model complexity in transcranial direct current stimulation forward head model development. IET Sci. Meas. Technol. 2015, 9, 596–605. [Google Scholar] [CrossRef]
- Guerra, A.; López-Alonso, V.; Cheeran, B.; Suppa, A. Variability in non-invasive brain stimulation studies: Reasons and results. Neurosci. Lett. 2020, 719, 133330. [Google Scholar] [CrossRef] [Green Version]
- Datta, A.; Zhou, X.; Su, Y.; Parra, L.C.; Bikson, M. Validation of finite element model of transcranial electrical stimulation using scalp potentials: Implications for clinical dose. J. Neural Eng. 2013, 10, 036018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuling, T.; Wagner, S.; Wolters, C.H.; Zaehle, T.; Herrmann, C.S. Finite-element model predicts current density distribution for clinical applications of tDCS and tACS. Front. Psychiatry 2012, 3, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibt, O.; Truong, D.; Khadka, N.; Huang, Y.; Bikson, M. Computational finite element method (FEM) forward modeling workflow for transcranial direct current stimulation (tDCS) current flow on MRI-derived head: Simpleware and COMSOL Multiphysics tutorial. bioRxiv 2019. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Kashyap, R.; Rapp, B.; Oishi, K.; Desmond, J.E.; Chen, S.H.A. Simulation analyses of tDCS montages for the investigation of dorsal and ventral pathways. Sci. Rep. 2019, 9, 12178. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, K.A.; Badran, B.W.; Li, X.; Bikson, M.; George, M.S. Can transcranial electrical stimulation motor threshold estimate individualized tDCS doses over the prefrontal cortex? Evidence from reverse-calculation electric field modeling. Brain Stimul. 2020, 13, 1150–1152. [Google Scholar] [CrossRef] [PubMed]
- Rawji, V.; Ciocca, M.; Zacharia, A.; Soares, D.; Truong, D.; Bikson, M.; Rothwell, J.; Bestmann, S. tDCS changes in motor excitability are specific to orientation of current flow. Brain Stimul. 2018, 11, 289–298. [Google Scholar] [CrossRef]
- Evans, C.; Bachmann, C.; Lee, J.S.A.; Gregoriou, E.; Ward, N.; Bestmann, S. Dose-controlled tDCS reduces electric field intensity variability at a cortical target site. Brain Stimul. 2020, 13, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Albizu, A.; Fang, R.; Indahlastari, A.; O’Shea, A.; Stolte, S.E.; See, K.B.; Boutzoukas, E.M.; Kraft, J.N.; Nissim, N.R.; Woods, A.J. Machine learning and individual variability in electric field characteristics predict tDCS treatment response. Brain Stimul. 2020, 13, 1753–1764. [Google Scholar] [CrossRef]
- Kasten, F.H.; Duecker, K.; Maack, M.C.; Meiser, A.; Herrmann, C.S. Integrating electric field modeling and neuroimaging to explain inter-individual variability of tACS effects. Nat. Commun. 2019, 10, 5427. [Google Scholar] [CrossRef] [Green Version]
- Laakso, I.; Tanaka, S.; Koyama, S.; De Santis, V.; Hirata, A. Inter-subject variability in electric fields of motor cortical tDCS. Brain Stimul. 2015, 8, 906–913. [Google Scholar] [CrossRef]
- Laakso, I.; Mikkonen, M.; Koyama, S.; Hirata, A.; Tanaka, S. Can electric fields explain inter-individual variability in transcranial direct current stimulation of the motor cortex? Sci. Rep. 2019, 9, 626. [Google Scholar] [CrossRef] [PubMed]
- Salvador, R.; Biagi, M.C.; Puonti, O.; Splittgerber, M.; Moliadze, V.; Siniatchkin, M.; Thielscher, A.; Ruffini, G. Personalization of multi-electrode setups in tCS/tES: Methods and advantages. In Brain and Human Body Modeling 2020; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 119–135. [Google Scholar]
- Huang, Y.; Liu, A.A.; Lafon, B.; Friedman, D.; Dayan, M.; Wang, X.; Bikson, M.; Doyle, W.K.; Devinsky, O.; Parra, L.C. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. eLife 2017, 6, e18834. [Google Scholar] [CrossRef]
- Liu, A.; Vöröslakos, M.; Kronberg, G.; Henin, S.; Krause, M.R.; Huang, Y.; Opitz, A.; Mehta, A.; Pack, C.C.; Krekelberg, B.; et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat. Commun. 2018, 9, 5092. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, G.B.; Siebner, H.R.; Thielscher, A.; Madsen, K.H. Accessibility of cortical regions to focal TES: Dependence on spatial position, safety, and practical constraints. Neuroimage 2019, 203, 116183. [Google Scholar] [CrossRef] [PubMed]
- Salvador, R.; Wenger, C.; Nitsche, M.A.; Miranda, P.C. How electrode montage affects transcranial direct current stimulation of the human motor cortex. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Milan, Italy, 25–29 August 2015. [Google Scholar]
- Santos, L.; Martinho, M.; Salvador, R.; Wenger, C.; Fernandes, S.R.; Ripolles, O.; Ruffini, G.; Miranda, P.C. Evaluation of the electric field in the brain during transcranial direct current stimulation: A sensitivity analysis. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Orlando, FA, USA, 16–20 August 2016. [Google Scholar]
- Mezger, E.; Rauchmann, B.S.; Brunoni, A.R.; Bulubas, L.; Thielscher, A.; Werle, J.; Mortazavi, M.; Karali, T.; Stöcklein, S.; Ertl-Wagner, B.; et al. Effects of bifrontal transcranial direct current stimulation on brain glutamate levels and resting state connectivity: Multimodal MRI data for the cathodal stimulation site. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 111–122. [Google Scholar] [CrossRef]
- Saturnino, G.B.; Antunes, A.; Thielscher, A. On the importance of electrode parameters for shaping electric field patterns generated by tDCS. Neuroimage 2015, 120, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Attene, M. A lightweight approach to repairing digitized polygon meshes. Vis. Comput. 2010, 26, 1393–1406. [Google Scholar] [CrossRef]
- Antonenko, D.; Grittner, U.; Saturnino, G.; Nierhaus, T.; Thielscher, A.; Flöel, A. Inter-individual and age-dependent variability in simulated electric fields induced by conventional transcranial electrical stimulation. Neuroimage 2021, 224, 117413. [Google Scholar] [CrossRef]
- Lee, C.; Jung, Y.J.; Lee, S.J.; Im, C.H. COMETS2: An advanced MATLAB toolbox for the numerical analysis of electric fields generated by transcranial direct current stimulation. J. Neurosci. Methods 2017, 277, 56–62. [Google Scholar] [CrossRef]
- Fang, Q.; Boas, D.A. Tetrahedral mesh generation from volumetric binary and grayscale images. In Proceedings of the 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, ISBI 2009, Boston, MA, USA, 28 June–1 July 2009; pp. 1142–1145. [Google Scholar]
- Im, C.H.; Park, J.H.; Shim, M.; Chang, W.H.; Kim, Y.H. Evaluation of local electric fields generated by transcranial direct current stimulation with an extracephalic reference electrode based on realistic 3D body modeling. Phys. Med. Biol. 2012, 57, 2137–2150. [Google Scholar] [CrossRef]
- Ramaraju, S.; Roula, M.A.; McCarthy, P.W. Modelling the effect of electrode displacement on transcranial direct current stimulation (tDCS). J. Neural Eng. 2018, 15, 016019. [Google Scholar] [CrossRef] [PubMed]
- Opitz, A.; Yeagle, E.; Thielscher, A.; Schroeder, C.; Mehta, A.D.; Milham, M.P. On the importance of precise electrode placement for targeted transcranial electric stimulation. Neuroimage 2018, 181, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Chhatbar, P.Y.; Kautz, S.A.; Takacs, I.; Rowland, N.C.; Revuelta, G.J.; George, M.S.; Bikson, M.; Feng, W. Evidence of transcranial direct current stimulation-generated electric fields at subthalamic level in human brain in vivo. Brain Stimul. 2018, 11, 727–733. [Google Scholar] [CrossRef]
- Jog, M.V.; Smith, R.X.; Jann, K.; Dunn, W.; Lafon, B.; Truong, D.; Wu, A.; Parra, L.; Bikson, M.; Wang, D.J.J. In-vivo imaging of magnetic fields induced by Transcranial Direct Current Stimulation (tDCS) in human brain using MRI. Sci. Rep. 2016, 6, 34385. [Google Scholar] [CrossRef] [PubMed]
- Puonti, O.; Saturnino, G.B.; Madsen, K.H.; Thielscher, A. Value and limitations of intracranial recordings for validating electric field modeling for transcranial brain stimulation. Neuroimage 2020, 208, 116431. [Google Scholar] [CrossRef]
- Antonenko, D.; Thielscher, A.; Saturnino, G.B.; Aydin, S.; Ittermann, B.; Grittner, U.; Flöel, A. Towards precise brain stimulation: Is electric field simulation related to neuromodulation? Brain Stimul. 2019, 12, 1159–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedoncker, J.; Brunoni, A.R.; Baeken, C.; Vanderhasselt, M.A. A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: Influence of stimulation parameters. Brain Stimul. 2016, 9, 501–517. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Urbina, G.N.; Batsikadze, G.; Molero-Chamizo, A.; Paulus, W.; Kuo, M.-F.; Nitsche, M.A. Parietal transcranial direct current stimulation modulates primary motor cortex excitability. Eur. J. Neurosci. 2015, 41, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Bawin, S.M.; Sheppard, A.R.; Mahoney, M.D.; Abu-Assal, M.; Adey, W.R. Comparison between the effects of extracellular direct and sinusoidal currents on excitability in hippocampal slices. Brain Res. 1986, 362, 350–354. [Google Scholar] [CrossRef]
- Bawin, S.M.; Sheppard, A.R.; Mahoney, M.D.; Ross Adey, W. Influences of sinusoidal electric fields on excitability in the rat hippocampal slice. Brain Res. 1984, 323, 227–237. [Google Scholar] [CrossRef]
- Jefferys, J.G.R.; Deans, J.; Bikson, M.; Fox, J. Effects of weak electric fields on the activity of neurons and neuronal networks. Radiat. Prot. Dosimetry 2003, 106, 321–323. [Google Scholar] [CrossRef]
- Bikson, M.; Inoue, M.; Akiyama, H.; Deans, J.K.; Fox, J.E.; Miyakawa, H.; Jefferys, J.G.R. Effect of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J. Physiol. 2004, 557, 175–190. [Google Scholar] [CrossRef]
- Faria, P.; Hallett, M.; Miranda, P.C. A finite element analysis of the effect of electrode area and inter-electrode distance on the spatial distribution of the current density in tDCS. J. Neural Eng. 2011, 8, 066017. [Google Scholar] [CrossRef] [Green Version]
- Metwally, M.K.; Cho, Y.S.; Park, H.J.; Kim, T.S. Investigation of the electric field components of tDCS via anisotropically conductive gyri-specific finite element head models. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, San Diego, CA, USA, 28 August–1 September 2012; pp. 5514–5517. [Google Scholar]
- Suh, H.S.; Kim, S.H.; Lee, W.H.; Kim, T.S. Realistic simulation of transcranial direct current stimulation via 3-D high-resolution finite element analysis: Effect of tissue anisotropy. In Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine, EMBC, Minneapolis, MN, USA, 3–6 September 2009; IEEE Computer Society: Washington, DC, USA, 2009; pp. 638–641. [Google Scholar]
- Caulfield, K.A.; Badran, B.W.; DeVries, W.H.; Summers, P.M.; Kofmehl, E.; Li, X.; Borckardt, J.J.; Bikson, M.; George, M.S. Transcranial electrical stimulation motor threshold can estimate individualized tDCS dosage from reverse-calculation electric-field modeling. Brain Stimul. 2020, 13, 961–969. [Google Scholar] [CrossRef]
- Agboada, D.; Mosayebi Samani, M.; Jamil, A.; Kuo, M.F.; Nitsche, M.A. Expanding the parameter space of anodal transcranial direct current stimulation of the primary motor cortex. Sci. Rep. 2019, 9, 18185. [Google Scholar] [CrossRef]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Biabani, M.; Aminitehrani, M.; Zoghi, M.; Farrell, M.; Egan, G.; Jaberzadeh, S. The effects of transcranial direct current stimulation on short-interval intracortical inhibition and intracortical facilitation: A systematic review and meta-analysis. Rev. Neurosci. 2017, 29, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Parkin, B.L.; Bhandari, M.; Glen, J.C.; Walsh, V. The physiological effects of transcranial electrical stimulation do not apply to parameters commonly used in studies of cognitive neuromodulation. Neuropsychologia 2019, 128, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strube, W.; Bunse, T.; Nitsche, M.A.; Nikolaeva, A.; Palm, U.; Padberg, F.; Falkai, P.; Hasan, A. Bidirectional variability in motor cortex excitability modulation following 1 mA transcranial direct current stimulation in healthy participants. Physiol. Rep. 2016, 4, e12884. [Google Scholar] [CrossRef]
- Antal, A.; Polania, R.; Schmidt-Samoa, C.; Dechent, P.; Paulus, W. Transcranial direct current stimulation over the primary motor cortex during fMRI. Neuroimage 2011, 55, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Batsikadze, G.; Kuo, H.I.; Meesen, R.L.J.; Dechent, P.; Paulus, W.; Nitsche, M.A. Current intensity- and polarity-specific online and aftereffects of transcranial direct current stimulation: An fMRI study. Hum. Brain Mapp. 2020, 41, 1644–1666. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.H.; Ko, M.H.; Ahn, S.H.; Kim, Y.H.; Song, J.C.; Lee, C.H.; Chang, M.C.; Jang, S.H. Primary motor cortex activation by transcranial direct current stimulation in the human brain. Neurosci. Lett. 2008, 435, 56–59. [Google Scholar] [CrossRef]
- Amadi, U.; Ilie, A.; Johansen-Berg, H.; Stagg, C.J. Polarity-specific effects of motor transcranial direct current stimulation on fMRI resting state networks. Neuroimage 2014, 88, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Lang, N.; Nitsche, M.A.; Dileone, M.; Mazzone, P.; de Andrés-Arés, J.; Diaz-Jara, L.; Paulus, W.; di Lazzaro, V.; Oliviero, A. Transcranial direct current stimulation effects on I-wave activity in humans. J. Neurophysiol. 2011, 105, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Callejon-Leblic, M.A.; Miranda, P.C. A computational analysis of the electric field components in transcranial direct current stimulation. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Berlin, Germany, 23–27 July 2019; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2019; pp. 5913–5917. [Google Scholar]
- Seo, H.; Jun, S.C. Relation between the electric field and activation of cortical neurons in transcranial electrical stimulation. Brain Stimul. 2019, 12, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.; Wen, P.; Ahfock, T. Assessment of electric field distribution in anisotropic cortical and subcortical regions under the influence of tDCS. Bioelectromagnetics 2014, 35, 41–57. [Google Scholar] [CrossRef] [Green Version]
- Gordon, P.C.; Zrenner, C.; Desideri, D.; Belardinelli, P.; Zrenner, B.; Brunoni, A.R.; Ziemann, U. Modulation of cortical responses by transcranial direct current stimulation of dorsolateral prefrontal cortex: A resting-state EEG and TMS-EEG study. Brain Stimul. 2018, 11, 1024–1032. [Google Scholar] [CrossRef]
- Caulfield, K.A.; Indahlastari, A.; Nissim, N.R.; Lopez, J.W.; Fleischmann, H.H.; Woods, A.J.; George, M.S. Electric field strength from prefrontal transcranial direct current stimulation determines degree of working memory response: A potential application of reverse-calculation modeling? Neuromodulation 2020. [Google Scholar] [CrossRef]
- Hanenberg, C.; Getzmann, S.; Lewald, J. Transcranial direct current stimulation of posterior temporal cortex modulates electrophysiological correlates of auditory selective spatial attention in posterior parietal cortex. Neuropsychologia 2019, 131, 160–170. [Google Scholar] [CrossRef]
- Grasso, P.A.; Tonolli, E.; Bortoletto, M.; Miniussi, C. tDCS over posterior parietal cortex increases cortical excitability but decreases learning: An ERPs and TMS-EEG study. Brain Res. 2021, 1753, 147227. [Google Scholar] [CrossRef]
- Pollok, B.; Keitel, A.; Foerster, M.; Moshiri, G.; Otto, K.; Krause, V. The posterior parietal cortex mediates early offline-rather than online-motor sequence learning. Neuropsychologia 2020, 146, 107555. [Google Scholar] [CrossRef]
- Tomio, R.; Akiyama, T.; Horikoshi, T.; Ohira, T.; Yoshida, K. Visualization of the electric field evoked by transcranial electric stimulation during a craniotomy using the finite element method. J. Neurosci. Methods 2015, 256, 157–167. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Y.; Guan, H.; Zhang, J.; Zhang, S. Validation of numerical simulation for transcranial direct current stimulation with spherical phantom. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Montreal, QC, Canada, 20–24 July 2020; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2020; pp. 3565–3568. [Google Scholar]
- Saturnino, G.B.; Wartman, W.A.; Makarov, S.N.; Thielscher, A. Accurate TMS head modeling: Interfacing SimNIBS and BEM-FMM in a MATLAB-based module. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Montreal, QC, Canada, 20–24 July 2020; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2020; pp. 5326–5329. [Google Scholar]
- Gomez, L.J.; Dannhauer, M.; Peterchev, A.V. Fast computational optimization of TMS coil placement for individualized electric field targeting. Neuroimage 2021, 228, 117696. [Google Scholar] [CrossRef]
- Mondino, M.; Fonteneau, C.; Simon, L.; Dondé, C.; Haesebaert, F.; Poulet, E.; Brunelin, J. Advancing clinical response characterization to frontotemporal transcranial direct current stimulation with electric field distribution in patients with schizophrenia and auditory hallucinations: A pilot study. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Indahlastari, A.; Chauhan, M.; Schwartz, B.; Sadleir, R.J. Changing head model extent affects finite element predictions of transcranial direct current stimulation distributions. J. Neural Eng. 2016, 13, 066006. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Marquez, C.M.; Zhang, X.; Swinnen, S.P.; Meesen, R.; Wenderoth, N. Task-specific effect of transcranial direct current stimulation on motor learning. Front. Hum. Neurosci. 2013, 7, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karok, S.; Fletcher, D.; Witney, A.G. Task-specificity of unilateral anodal and dual-M1 tDCS effects on motor learning. Neuropsychologia 2017, 94, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, R.; Rehman, A.U.; You, X. Dorsolateral prefrontal cortex and task-switching performance: Effects of anodal transcranial direct current stimulation. Neuroscience 2020, 446, 94–101. [Google Scholar] [CrossRef]
- Pope, P.A.; Brenton, J.W.; Miall, R.C. Task-specific facilitation of cognition by anodal transcranial direct current stimulation of the prefrontal cortex. Cereb. Cortex 2015, 25, 4551–4558. [Google Scholar] [CrossRef] [Green Version]
- Dubreuil-Vall, L.; Chau, P.; Ruffini, G.; Widge, A.S.; Camprodon, J.A. tDCS to the left DLPFC modulates cognitive and physiological correlates of executive function in a state-dependent manner. Brain Stimul. 2019, 12, 1456–1463. [Google Scholar] [CrossRef]
- Dennison, O.; Gao, J.; Lim, L.W.; Stagg, C.J.; Aquili, L. Catecholaminergic modulation of indices of cognitive flexibility: A pharmaco-tDCS study. Brain Stimul. 2019, 12, 290–295. [Google Scholar] [CrossRef]
- Greenwood, P.M.; Blumberg, E.J.; Scheldrup, M.R. Hypothesis for cognitive effects of transcranial direct current stimulation: Externally- and internally-directed cognition. Neurosci. Biobehav. Rev. 2018, 86, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Klaus, J.; Hartwigsen, G. Failure to improve verbal fluency with transcranial direct current stimulation. Neuroscience 2020, 449, 123–133. [Google Scholar] [CrossRef]
- Wischnewski, M.; Mantell, K.E.; Opitz, A. Identifying regions in prefrontal cortex related to working memory improvement: A novel meta-analytic method using electric field modeling. bioRxiv 2021. [Google Scholar] [CrossRef]
- Khorrampanah, M.; Seyedarabi, H.; Daneshvar, S.; Farhoudi, M. Optimization of montages and electric currents in tDCS. Comput. Biol. Med. 2020, 125, 103998. [Google Scholar] [CrossRef] [PubMed]
- Salehinejad, M.A.; Nejati, V.; Mosayebi-Samani, M.; Mohammadi, A.; Wischnewski, M.; Kuo, M.F.; Avenanti, A.; Vicario, C.M.; Nitsche, M.A. Transcranial direct current stimulation in ADHD: A systematic review of efficacy, safety, and protocol-induced electrical field modeling results. Neurosci. Bull. 2020, 36, 1191–1212. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Bikson, M.; Fregni, F. Transcranial direct current stimulation in patients with skull defects and skull plates: High-resolution computational FEM study of factors altering cortical current flow. Neuroimage 2010, 52, 1268–1278. [Google Scholar] [CrossRef] [Green Version]
- Datta, A.; Truong, D.; Minhas, P.; Parra, L.C.; Bikson, M. Inter-individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Front. Psychiatry 2012, 3, 91. [Google Scholar] [CrossRef] [Green Version]
- Puonti, O.; Van Leemput, K.; Saturnino, G.B.; Siebner, H.R.; Madsen, K.H.; Thielscher, A. Accurate and robust whole-head segmentation from magnetic resonance images for individualized head modeling. Neuroimage 2020, 219, 117044. [Google Scholar] [CrossRef]
- Mikkonen, M.; Laakso, I. Effects of posture on electric fields of non-invasive brain stimulation. Phys. Med. Biol. 2019, 64, 065019. [Google Scholar] [CrossRef]
- Thomas, C.; Ghodratitoostani, I.; Delbem, A.C.B.; Ali, A.; Datta, A. Influence of gender-related differences in transcranial direct current stimulation: A Computational Study. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Berlin, Germany, 23–27 July 2019; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2019; pp. 5196–5199. [Google Scholar]
- Aparício, L.V.M.; Guarienti, F.; Razza, L.B.; Carvalho, A.F.; Fregni, F.; Brunoni, A.R. A systematic review on the acceptability and tolerability of transcranial direct current stimulation treatment in neuropsychiatry trials. Brain Stimul. 2016, 9, 671–681. [Google Scholar] [CrossRef] [PubMed]
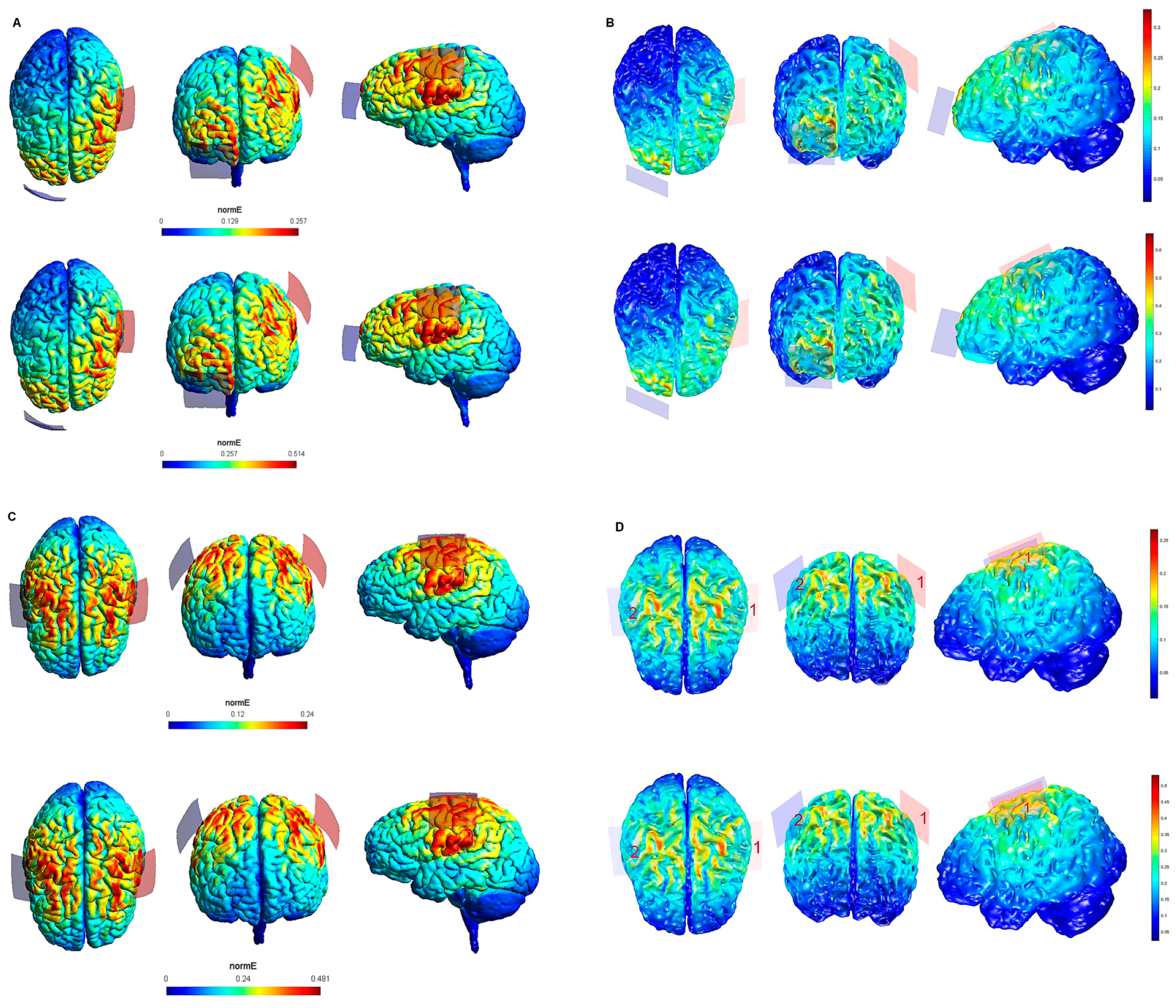
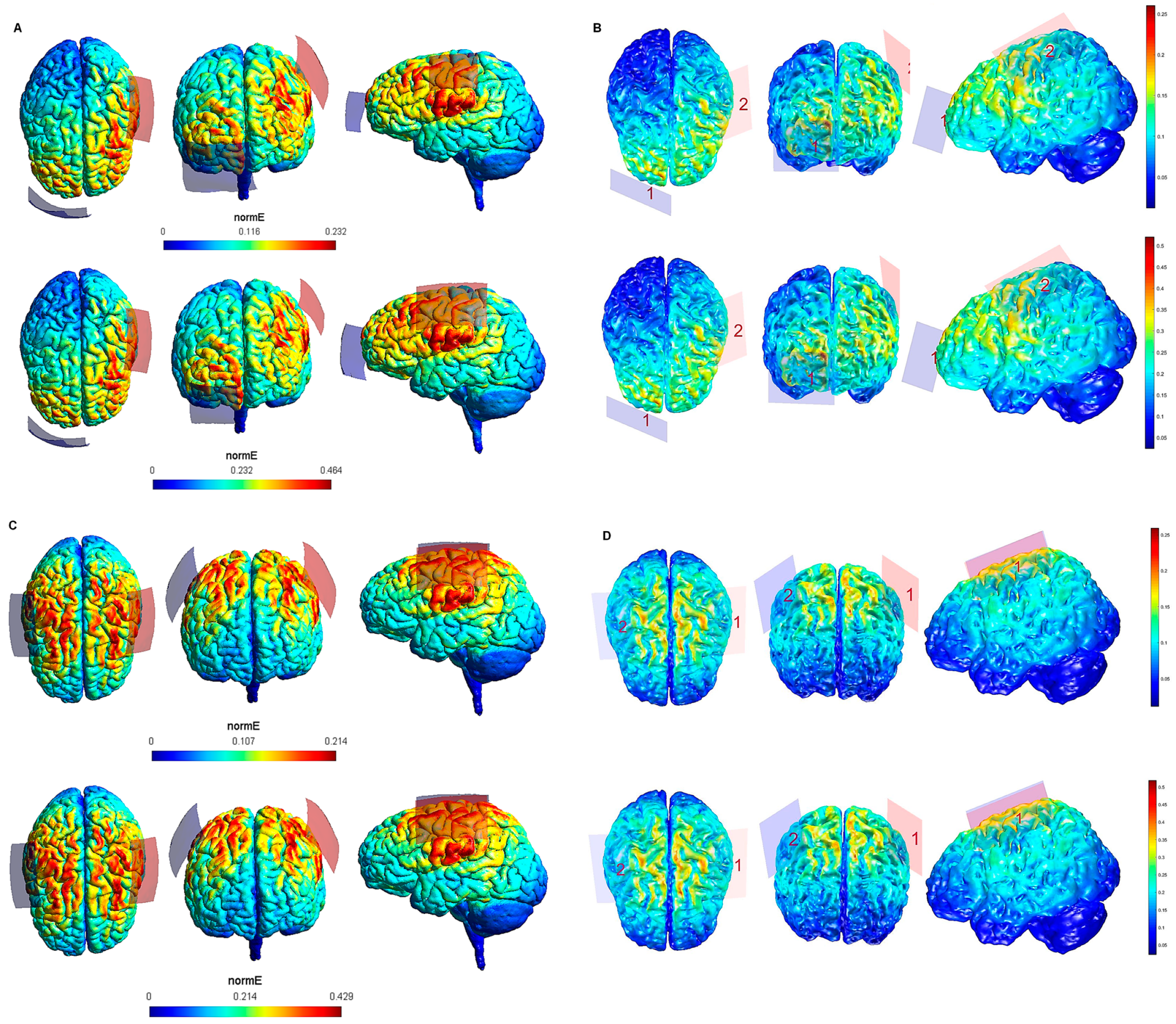
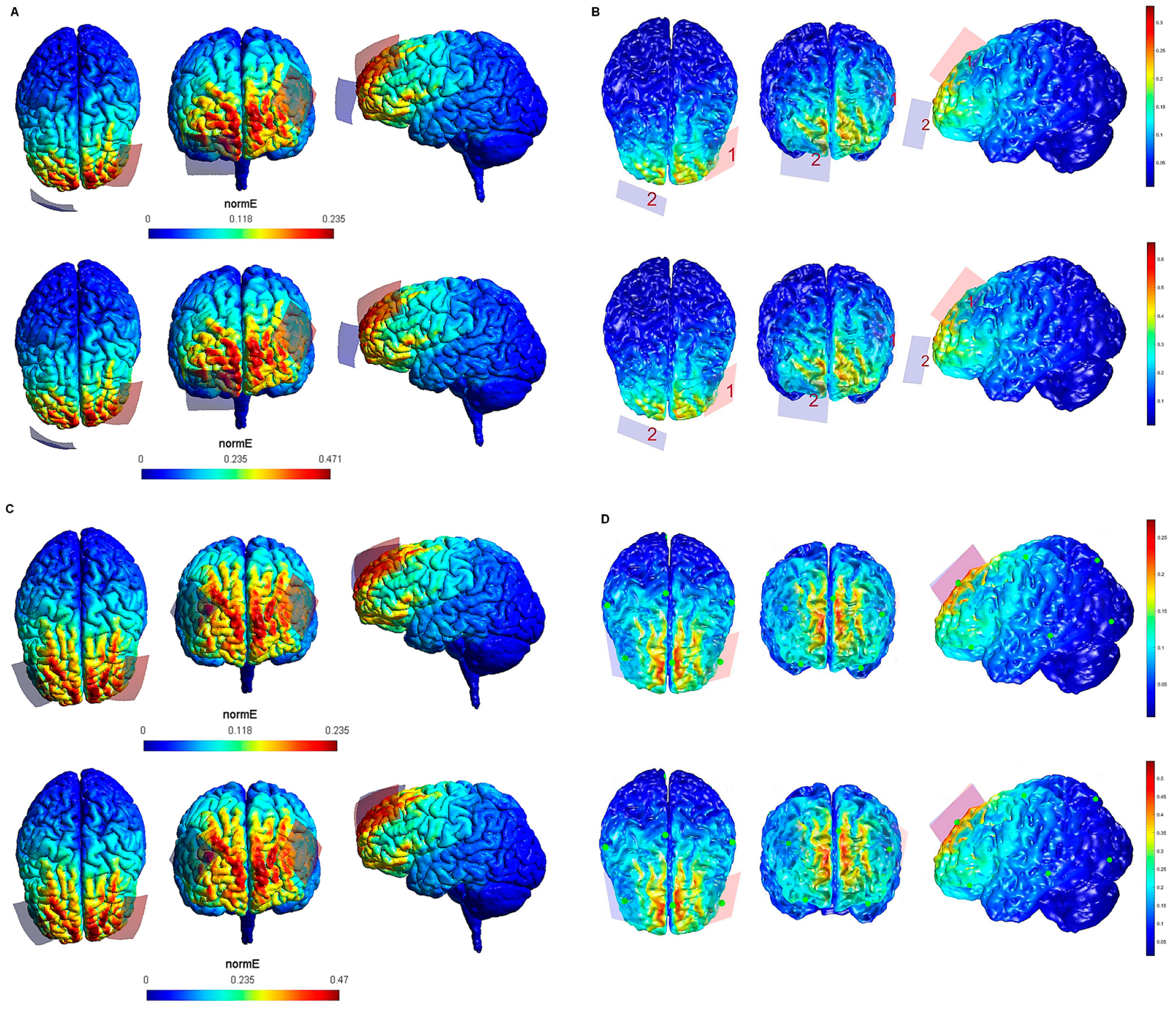
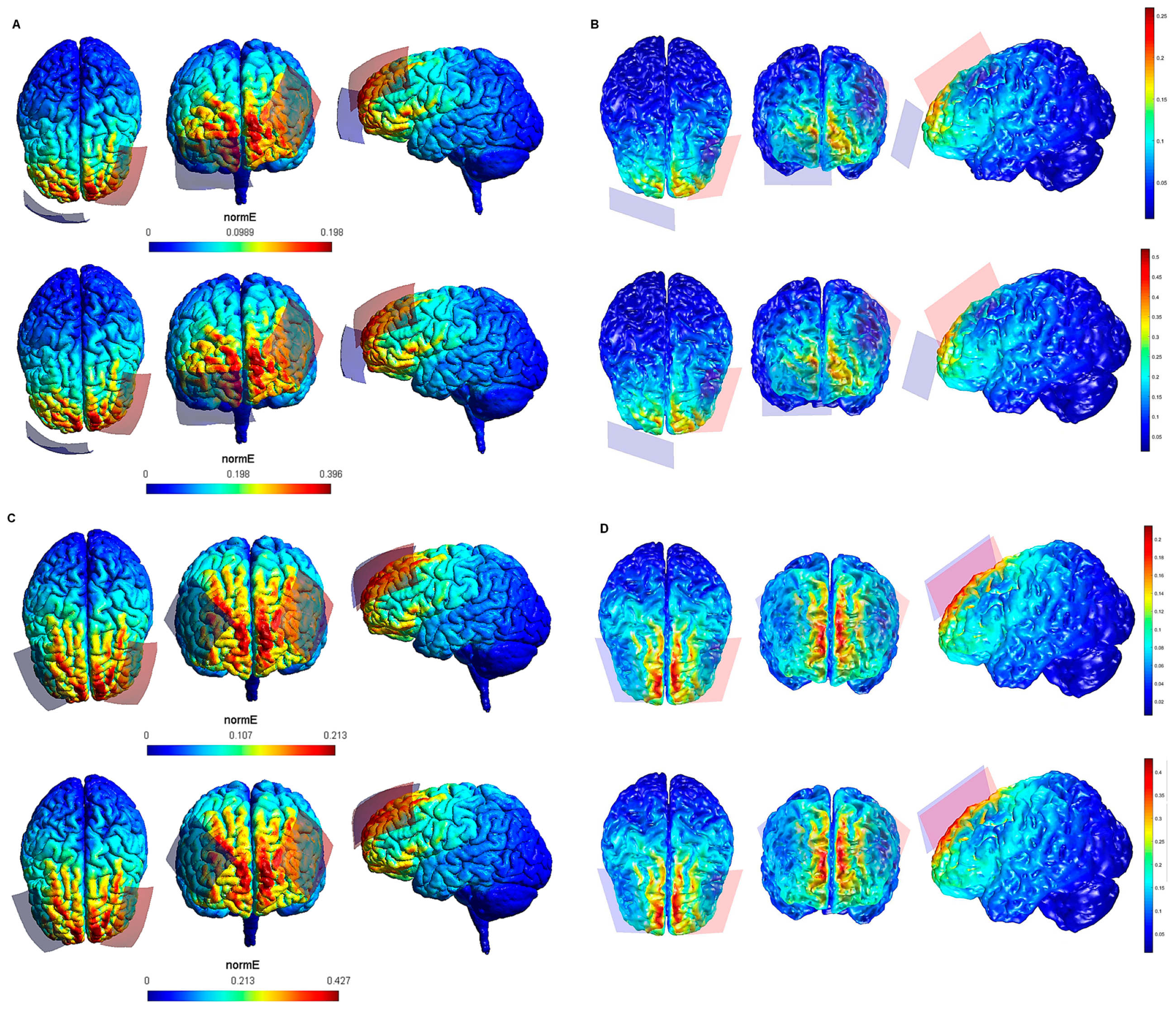
| Electrode Configuration | Anode Location | Cathode Location | Electrode Size | Current Intensity (V/m) | Current Intensity at the Electrode- Skin Interface | Maximum Electric Field in the Brain (SimNIBS—COMETS) |
|---|---|---|---|---|---|---|
| 1 | lM1 | rSOR | 5 cm × 4 cm (20 cm2) | 1 mA | 0.05 mA/cm2 | 0.257—0.3 V/m |
| 2 | lM1 | rSOR | 5 cm × 4 cm (20 cm2) | 2 mA | 0.1 mA/cm2 | 0.514—0.6 V/m |
| 3 | lM1 | rM1 | 5 cm × 4 cm (20 cm2) | 1 mA | 0.05 mA/cm2 | 0.24—0.25 V/m |
| 4 | lM1 | rM1 | 5 cm × 4 cm (20 cm2) | 2 mA | 0.1 mA/cm2 | 0.481—0.5 V/m |
| 5 | lM1 | rSOR | 7 cm × 5 cm (35 cm2) | 1 mA | 0.02 mA/cm2 | 0.232—0.25 V/m |
| 6 | lM1 | rSOR | 7 cm × 5 cm (35 cm2) | 2 mA | 0.05 mA/cm2 | 0.464—0.5 V/m |
| 7 | lM1 | rM1 | 7 cm × 5 cm (35 cm2) | 1 mA | 0.02 mA/cm2 | 0.214—0.25 V/m |
| 8 | lM1 | rM1 | 7 cm × 5 cm (35 cm2) | 2 mA | 0.05 mA/cm2 | 0.429—0.5 V/m |
| 9 | lDLPFC lDLPFC | rSOR | 5 cm × 4 cm (20 cm2) | 1 mA | 0.05 mA/cm2 | 0.235—0.3 V/m |
| 10 | lDLPFC | rSOR | 5 cm × 4 cm (20 cm2) | 2 mA | 0.1 mA/cm2 | 0.471—0.6 V/m |
| 11 | lDLPFC | rDLPFC | 5 cm × 4 cm (20 cm2) | 1 mA | 0.05 mA/cm2 | 0.235—0.25 V/m |
| 12 | lDLPFC | rDLPFC | 5 cm × 4 cm (20 cm2) | 2 mA | 0.1 mA/cm2 | 0.47—0.5 V/m |
| 13 | lDLPFC | rSOR | 7 cm × 5 cm (35 cm2) | 1 mA | 0.02 mA/cm2 | 0.198—0.25 V/m |
| 14 | lDLPFC | rSOR | 7 cm × 5 cm (35 cm2) | 2 mA | 0.05 mA/cm2 | 0.396—0.5 V/m |
| 15 | lDLPFC | rDLPFC | 7 cm × 5 cm (35 cm2) | 1 mA | 0.02 mA/cm2 | 0.213—0.2 V/m |
| 16 | lPPC | rDLPFC | 7 cm × 5 cm (35 cm2) | 2 mA | 0.05 mA/cm2 | 0.427—0.4 V/m |
| 17 | lPPC | rSOR | 5 cm × 4 cm (20 cm2) | 1 mA | 0.05 mA/cm2 | 0.225—0.35 V/m |
| 18 | lPPC | rSOR | 5 cm × 4 cm (20 cm2) | 2 mA | 0.1 mA/cm2 | 0.45—0.7 V/m |
| 19 | lPPC | rSOR | 7 cm × 5 cm (35 cm2) | 1 mA | 0.02 mA/cm2 | 0.219—0.25 V/m |
| 20 | rSOR | 7 cm × 5 cm (35 cm2) | 2 mA | 0.05 mA/cm2 | 0.439—0.5 V/m |
| Electrode Configuration | PMC (S) | PMC (C) | vmPFC (S) | vmPFC (C) | dmPFC (S) | dmPFC (C) |
| 1 (lM1/rSOR-20 cm2-1 mA) | >0.129 <0.257 V/m | >0.1 <0.2 V/m | >0.05 <0.1 V/m | >0.08 <0.14 V/m | >0.1 <0.17 V/m | >0.13 <0.18 V/m |
| 2 (lM1/rSOR-20 cm2-2 mA) | >0.257 <0.35 V/m | >0.25 <0.35 V/m | >0.2 <0.3 V/m | >0.24 <0.34 V/m | >0.25 <0.36 V/m | >0.24 <0.35 V/m |
| 3 (lM1/rM1-20 cm2-1 mA) | >0.12 <0.17 V/m | >0.12 <0.15 V/m | >0.04 <0.08 V/m | >0.05 <0.1 V/m | >0.06 <0.17 V/m | >0.08 <0.14 V/m |
| 4 (lM1/rM1-20 cm2-2 mA) | >0.15 <0.35 V/m | >0.16 <0.34 V/m | >0.1 <0.2 V/m | >0.12 <0.2 V/m | >0.09 <0.2 V/m | >0.12 <0.19 V/m |
| 5 (lM1/rSOR-35 cm2-1 mA) | >0.07 <0.19 V/m | >0.09 <0.17 V/m | >0.08 <0.16 V/m | >0.09 <0.16 V/m | >0.09 <0.16 V/m | >0.11 <0.17 V/m |
| 6 (lM1/rSOR-35 cm2-2 mA) | >0.17 <0.36 V/m | >0.19 <0.33 V/m | >0.17 <0.29 V/m | >0.19 <0.31 V/m | >0.2 <0.32 V/m | >0.2 <0.31 V/m |
| 7 (lM1/rM1-35 cm2-1 mA) | >0.07 <0.17 V/m | >0.07 <0.17 V/m | >0.06 <0.11 V/m | >0.05 <0.12 V/m | >0.07 <0.13 V/m | >0.07 <0.13 V/m |
| 8 (lM1/rM1-35 cm2-2 mA) | >0.15 <0.36 V/m | >0.15 <0.34 V/m | >0.11 <0.22 V/m | >0.11 <0.23 V/m | >0.15 <0.25 V/m | >0.15 <0.26 V/m |
| 9 (lDLPFC/rSOR-20 cm2-1 mA) | >0.06 <0.15 V/m | >0.07 <0.14 V/m | >0.12 <0.2 V/m | >0.15 <0.24 V/m | >0.1 <0.21 V/m | >0.14 <0.22 V/m |
| 10 (lDLPFC/rSOR-20 cm2-2 mA) | >0.12 <0.29 V/m | >0.12 <0.3 V/m | >0.25 <0.4 V/m | >0.29 <0.49 V/m | >0.18 <0.41 V/m | >0.25 <0.43 V/m |
| 11 (lDLPFC/rDLPF-20 cm2-1 mA) | >0.07 <0.17 V/m | >0.07 <0.17 V/m | >0.14 <0.22 V/m | >0.14 <0.22 V/m | >0.1 <0.21 V/m | >0.15 <0.24 V/m |
| 12 (lDLPFC/rDLPF-20 cm2-2 mA) | >0.14 <0.35 V/m | >0.12 <0.33 V/m | >0.29 <0.41 V/m | >0.27 <0.41 V/m | >0.23 <0.39 V/m | >0.29 <0.48 V/m |
| 13 (lDLPFC/rSOR-35 cm2-1 mA) | >0.07 <0.13 V/m | >0.05 <0.11 V/m | >0.13 <0.19 V/m | >0.15 <0.19 V/m | >0.07 <0.17 V/m | >0.09 <0.19 V/m |
| 14 (lDLPFC/rSOR-35 cm2-2 mA) | >0.13 <0.27 V/m | >0.1 <0.22 V/m | >0.25 <0.35 V/m | >0.32 <0.39 V/m | >0.14 <0.36 V/m | >0.19 <0.37 V/m |
| 15 (lDLPFC/rDLPF-35 cm2-1 mA) | >0.07 <0.19 V/m | >0.04 <0.15 V/m | >0.17 <0.2 V/m | >0.13 <0.19 V/m | >0.12 <0.18 V/m | >0.13 <0.19 V/m |
| 16 (lDLPFC/rDLPF-35 cm2-2 mA) | >0.14 <0.33 V/m | >0.09 <0.32 V/m | >0.32 <0.39 V/m | >0.28 <0.39 V/m | >0.24 <0.39 V/m | >0.27 <0.38 V/m |
| 17 (lPPC/rSOR-20 cm2-1 mA) | >0.07 <0.17 V/m | >0.08 <0.15 V/m | >0.05 <0.14 V/m | >0.1 <0.17 V/m | >0.07 <0.17 V/m | >0.1 <0.16 V/m |
| 18 (lPPC/rSOR-20 cm2-2 mA) | >0.16 <0.34 V/m | >0.18 <0.3 V/m | >0.12 <0.26 V/m | >0.19 <0.35 V/m | >0.18 <0.29 V/m | >0.18 <0.34 V/m |
| 19 (lPPC/rSOR-35 cm2-1 mA) | >0.08 <0.17 V/m | >0.08 <0.13 V/m | >0.06 <0.15 V/m | >0.1 <0.18 V/m | >0.06 <0.16 V/m | >0.08 <0.17 V/m |
| 20 (lPPC/rSOR-35 cm2-2 mA) | >0.16 <0.34 V/m | >0.14 <0.25 V/m | >0.17 <0.26 V/m | >0.19 <0.29 V/m | >0.16 <0.27 V/m | >0.18 <0.29 V/m |
| Electrode Configuration | OFC (S) | OFC (C) | APC (S) | APC (C) | STL (S) | STL (C) |
| 1 (lM1/rSOR-20 cm2-1 mA) | >0.05 <0.13 V/m | >0.07 <0.16 V/m | >0.08 <0.129 V/m | >0.08 <0.13 V/m | >0.08 <0.16 V/m | >0.1 <0.17 V/m |
| 2 (lM1/rSOR-20 cm2-2 mA) | >0.12 <0.27 V/m | >0.17 <0.3 V/m | >0.13 <0.26 V/m | >0.18 <0.27 V/m | >0.18 <0.29 V/m | >0.18 <0.31 V/m |
| 3 (lM1/rM1-20 cm2-1 mA) | >0.05 <0.1 V/m | >0.04 <0.08 V/m | >0.12 <0.15 V/m | >0.1 <0.17 V/m | >0.08 <0.17 V/m | >0.08 <0.12 V/m |
| 4 (lM1/rM1-20 cm2-2 mA) | >0.1 <0.2 V/m | >0.1 <0.15 V/m | >0.24 <0.36 V/m | >0.2 <0.35 V/m | >0.18 <0.29 V/m | >0.15 <0.25 V/m |
| 5 (lM1/rSOR-35 cm2-1 mA) | >0.07 <0.14 V/m | >0.08 <0.15 V/m | >0.09 <0.14 V/m | >0.07 <0.15 V/m | >0.08 <0.15 V/m | >0.07 <0.17 V/m |
| 6 (lM1/rSOR-35 cm2-2 mA) | >0.16 <0.29 V/m | >0.16 <0.29 V/m | >0.16 <0.29 V/m | >0.15 <0.29 V/m | >0.16 <0.29 V/m | >0.16 <0.32 V/m |
| 7 (lM1/rM1-35 cm2-1 mA) | >0.06 <0.1 V/m | >0.06 <0.09 V/m | >0.08 <0.15 V/m | >0.09 <0.17 V/m | >0.07 <0.13 V/m | >0.06 <0.13 V/m |
| 8 (lM1/rM1-35 cm2-2 mA) | >0.12 <0.19 V/m | >0.12 <0.15 V/m | >0.15 <0.31 V/m | >0.17 <0.32 V/m | >0.13 <0.27 V/m | >0.12 <0.25 V/m |
| 9 (lDLPFC/rSOR-20 cm2-1 mA) | >0.11 <0.2 V/m | >0.09 <0.26 V/m | >0.03 <0.09 V/m | >0.06 <0.12 V/m | >0.03 <0.08 V/m | >0.04 <0.13 V/m |
| 10 (lDLPFC/rSOR-20 cm2-2 mA) | >0.19 <0.39 V/m | >0.18 <0.5 V/m | >0.07 <0.17 V/m | >0.1 <0.21 V/m | >0.08 <0.18 V/m | >0.09 <0.25 V/m |
| 11 (lDLPFC/rDLPF-20 cm2-1 mA) | >0.09 <0.2 V/m | >0.07 <0.21 V/m | >0.03 <0.08 V/m | >0.04 <0.11 V/m | >0.05 <0.08 V/m | >0.05 <0.11 V/m |
| 12 (lDLPFC/rDLPF-20 cm2-2 mA) | >0.18 <0.39 V/m | >0.13 <0.4 V/m | >0.06 <0.16 V/m | >0.07 <0.19 V/m | >0.09 <0.16 V/m | >0.1 <0.2 V/m |
| 13 (lDLPFC/rSOR-35 cm2-1 mA) | >0.12 <0.18 V/m | >0.1 <0.19 V/m | >0.02 <0.08 V/m | >0.05 <0.11 V/m | >0.04 <0.08 V/m | >0.05 <0.1 V/m |
| 14 (lDLPFC/rSOR-35 cm2-2 mA) | >0.21 <0.36 V/m | >0.21 <0.39 V/m | >0.09 <0.15 V/m | >0.1 <0.2 V/m | >0.08 <0.16 V/m | >0.09 <0.19 V/m |
| 15 (lDLPFC/rDLPF-35 cm2-1 mA) | >0.12 <0.18 V/m | >0.09 <0.17 V/m | >0.05 <0.1 V/m | >0.04 <0.09 V/m | >0.04 <0.07 V/m | >0.04 <0.08 V/m |
| 16 (lDLPFC/rDLPF-35 cm2-2 mA) | >0.24 <0.38 V/m | >0.18 <0.34 V/m | >0.1 <0.18 V/m | >0.09 <0.18 V/m | >0.08 <0.15 V/m | >0.09 <0.16 V/m |
| 17 (lPPC/rSOR-20 cm2-1 mA) | >0.05 <0.11 V/m | >0.08 <0.18 V/m | >0.1 <0.19 V/m | >0.09 <0.21 V/m | >0.1 <0.19 V/m | >0.08 <0.17 V/m |
| 18 (lPPC/rSOR-20 cm2-2 mA) | >0.09 <0.23 V/m | >0.12 <0.31 V/m | >0.19 <0.34 V/m | >0.18 <0.41 V/m | >0.19 <0.35 V/m | >0.13 <0.32 V/m |
| 19 (lPPC/rSOR-35 cm2-1 mA) | >0.05 <0.12 V/m | >0.07 <0.13 V/m | >0.09 <0.19 V/m | >0.08 <0.17 V/m | >0.1 <0.18 V/m | >0.08 <0.13 V/m |
| 20 (lPPC/rSOR-35 cm2-2 mA) | >0.11 <0.23 V/m | >0.13 <0.29 V/m | >0.18 <0.34 V/m | >0.14 <0.34 V/m | >0.19 <0.35 V/m | >0.13 <0.27 V/m |
| Electrode Configuration | M1 (S) | M1 (C) | DLPFC (S) | DLPFC (C) | PPC (S) | PPC (C) |
| 1 (lM1/rSOR-20 cm2-1 mA) | ROI | ROI | >0.13 <0.21 V/m | >0.11 <0.19 V/m | >0.07 <0.16 V/m | >0.07 <0.17 V/m |
| 2 (lM1/rSOR-20 cm2-2 mA) | ROI | ROI | >0.26 <0.39 V/m | >0.21 <0.38 V/m | >0.17 <0.32 V/m | >0.15 <0.36 V/m |
| 3 (lM1/rM1-20 cm2-1 mA) | ROI | ROI | >0.08 <0.2 V/m | >0.08 <0.17 V/m | >0.08 <0.17 V/m | >0.07 <0.14 V/m |
| 4 (lM1/rM1-20 cm2-2 mA) | ROI | ROI | >0.17 <0.38 V/m | >0.14 <0.31 V/m | >0.14 <0.31 V/m | >0.14 <0.29 V/m |
| 5 (lM1/rSOR-35 cm2-1 mA) | ROI | ROI | >0.1 <0.17 V/m | >0.09 <0.16 V/m | >0.08 <0.15 V/m | >0.07 <0.13 V/m |
| 6 (lM1/rSOR-35 cm2-2 mA) | ROI | ROI | >0.15 <0.37 V/m | >0.19 <0.33 V/m | >0.16 <0.29 V/m | >0.14 <0.28 V/m |
| 7 (lM1/rM1-35 cm2-1 mA) | ROI | ROI | >0.07 <0.17 V/m | >0.07<0.17 V/m | >0.07<0.13 V/m | >0.07 <0.14 V/m |
| 8 (lM1/rM1-35 cm2-2 mA) | ROI | ROI | >0.16 <0.36 V/m | >0.14 <0.31 V/m | >0.13 <0.29 V/m | >0.14 <0.29 V/m |
| 9 (lDLPFC/rSOR-20 cm2-1 mA) | >0.05 <0.15 V/m | >0.05 <0.14 V/m | ROI | ROI | >0.02 <0.06 V/m | >0.05 <0.07 V/m |
| 10 (lDLPFC/rSOR-20 cm2-2 mA) | >0.1 <0.27 V/m | >0.1 <0.27 V/m | ROI | ROI | >0.04 <0.11 V/m | >0.09 <0.16 V/m |
| 11 (lDLPFC/rDLPF-20 cm2-1 mA) | >0.06 <0.15 V/m | >0.06 <0.14 V/m | ROI | ROI | >0.02 <0.05 V/m | >0.04 <0.07 V/m |
| 12 (lDLPFC/rDLPF-20 cm2-2 mA) | >0.11 <0.27 V/m | >0.11 <0.29 V/m | ROI | ROI | >0.04 <0.13 V/m | >0.06 <0.13 V/m |
| 13 (lDLPFC/rSOR-35 cm2-1 mA) | >0.05 <0.14 V/m | >0.05 <0.12 V/m | ROI | ROI | >0.02 <0.05 V/m | >0.03 <0.07 V/m |
| 14 (lDLPFC/rSOR-35 cm2-2 mA) | >0.09 <0.25 V/m | >0.09 <0.23 V/m | ROI | ROI | >0.05 <0.1 V/m | >0.05 <0.12 V/m |
| 15 (lDLPFC/rDLPF-35 cm2-1 mA) | >0.05 <0.14 V/m | >0.05 <0.13 V/m | ROI | ROI | >0.03 <0.06 V/m | >0.03 <0.07 V/m |
| 16 (lDLPFC/rDLPF-35 cm2-2 mA) | >0.1 <0.26 V/m | >0.07 <0.25 V/m | ROI | ROI | >0.05 <0.12 V/m | >0.05 <0.15 V/m |
| 17 (lPPC/rSOR-20 cm2-1 mA) | >0.08 <0.18 V/m | >0.08 <0.17 V/m | >0.08 <0.19 V/m | >0.08 <0.18 V/m | ROI | ROI |
| 18 (lPPC/rSOR-20 cm2-2 mA) | >0.18 <0.35 V/m | >0.13 <0.32 V/m | >0.18 <0.35 V/m | >0.18 <0.36 V/m | ROI | ROI |
| 19 (lPPC/rSOR-35 cm2-1 mA) | >0.08 <0.19 V/m | >0.07 <0.17 V/m | >0.08 <0.19 V/m | >0.07 <0.17 V/m | ROI | ROI |
| 20 (lPPC/rSOR-35 cm2-2 mA) | >0.18 <0.35 V/m | >0.14 <0.32 V/m | >0.17 <0.35 V/m | >0.14 <0.32 V/m | ROI | ROI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molero-Chamizo, A.; Nitsche, M.A.; Gutiérrez Lérida, C.; Salas Sánchez, Á.; Martín Riquel, R.; Andújar Barroso, R.T.; Alameda Bailén, J.R.; García Palomeque, J.C.; Rivera-Urbina, G.N. Standard Non-Personalized Electric Field Modeling of Twenty Typical tDCS Electrode Configurations via the Computational Finite Element Method: Contributions and Limitations of Two Different Approaches. Biology 2021, 10, 1230. https://doi.org/10.3390/biology10121230
Molero-Chamizo A, Nitsche MA, Gutiérrez Lérida C, Salas Sánchez Á, Martín Riquel R, Andújar Barroso RT, Alameda Bailén JR, García Palomeque JC, Rivera-Urbina GN. Standard Non-Personalized Electric Field Modeling of Twenty Typical tDCS Electrode Configurations via the Computational Finite Element Method: Contributions and Limitations of Two Different Approaches. Biology. 2021; 10(12):1230. https://doi.org/10.3390/biology10121230
Chicago/Turabian StyleMolero-Chamizo, Andrés, Michael A. Nitsche, Carolina Gutiérrez Lérida, Ángeles Salas Sánchez, Raquel Martín Riquel, Rafael Tomás Andújar Barroso, José Ramón Alameda Bailén, Jesús Carlos García Palomeque, and Guadalupe Nathzidy Rivera-Urbina. 2021. "Standard Non-Personalized Electric Field Modeling of Twenty Typical tDCS Electrode Configurations via the Computational Finite Element Method: Contributions and Limitations of Two Different Approaches" Biology 10, no. 12: 1230. https://doi.org/10.3390/biology10121230
APA StyleMolero-Chamizo, A., Nitsche, M. A., Gutiérrez Lérida, C., Salas Sánchez, Á., Martín Riquel, R., Andújar Barroso, R. T., Alameda Bailén, J. R., García Palomeque, J. C., & Rivera-Urbina, G. N. (2021). Standard Non-Personalized Electric Field Modeling of Twenty Typical tDCS Electrode Configurations via the Computational Finite Element Method: Contributions and Limitations of Two Different Approaches. Biology, 10(12), 1230. https://doi.org/10.3390/biology10121230










