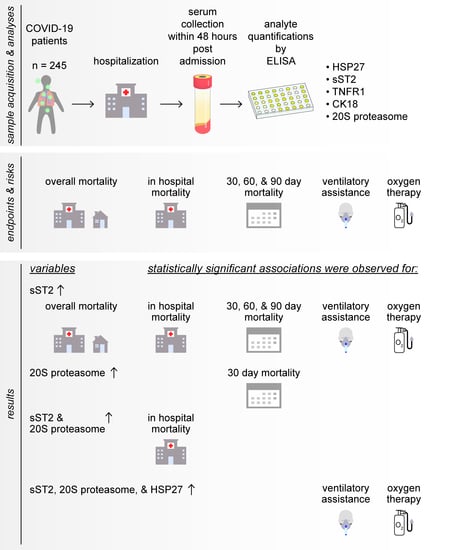
Clinical Relevance of Elevated Soluble ST2, HSP27 and 20S Proteasome at Hospital Admission in Patients with COVID-19
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Serum Samples
2.3. Quantification of Serum HSP27, TNFR1, sST2, cCK18, and 20S Proteasome
2.4. Statistical Analysis
3. Results
3.1. Study Population Characteristics
3.2. Soluble ST2 Serum Content Is Significantly Associated with Worse Overall Survival
3.3. Elevated sST2 Acts as an Potential Prognostic Factor for 30-, 60- and 90-Days Mortality, Elevated 20S Proteasome Levels Were Associated with Worse 30-Days Mortality
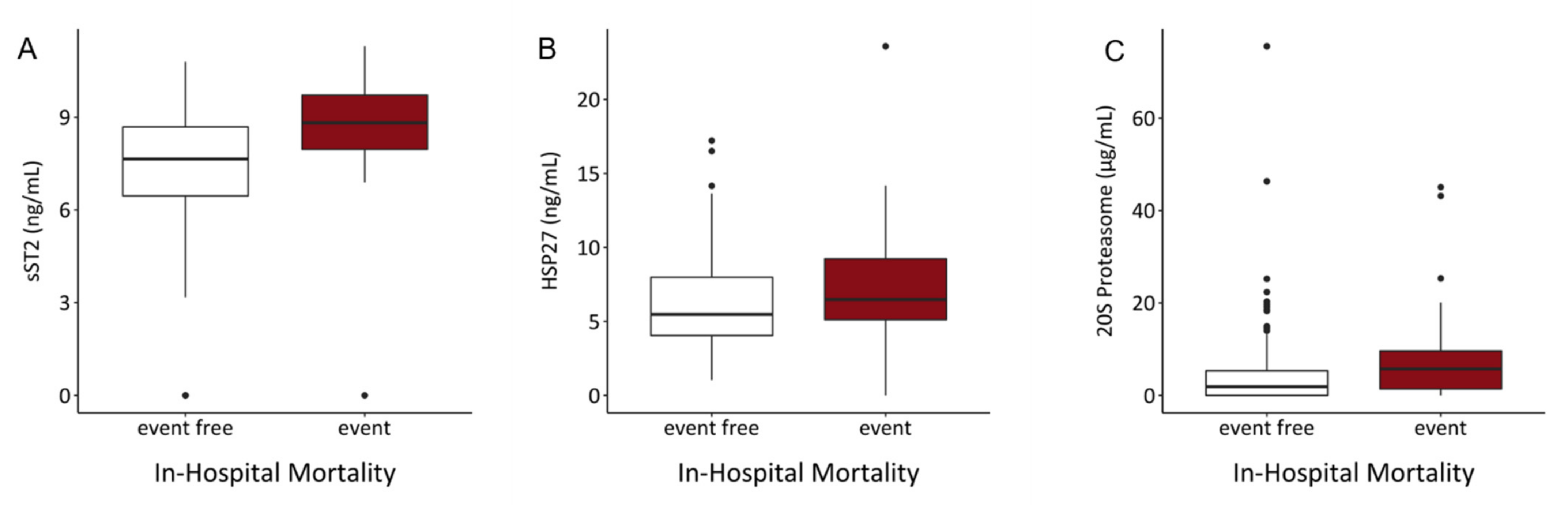
3.4. sST2 and 20S Proteasome Serum Levels Are Significant Predictors for In-Hospital Mortality
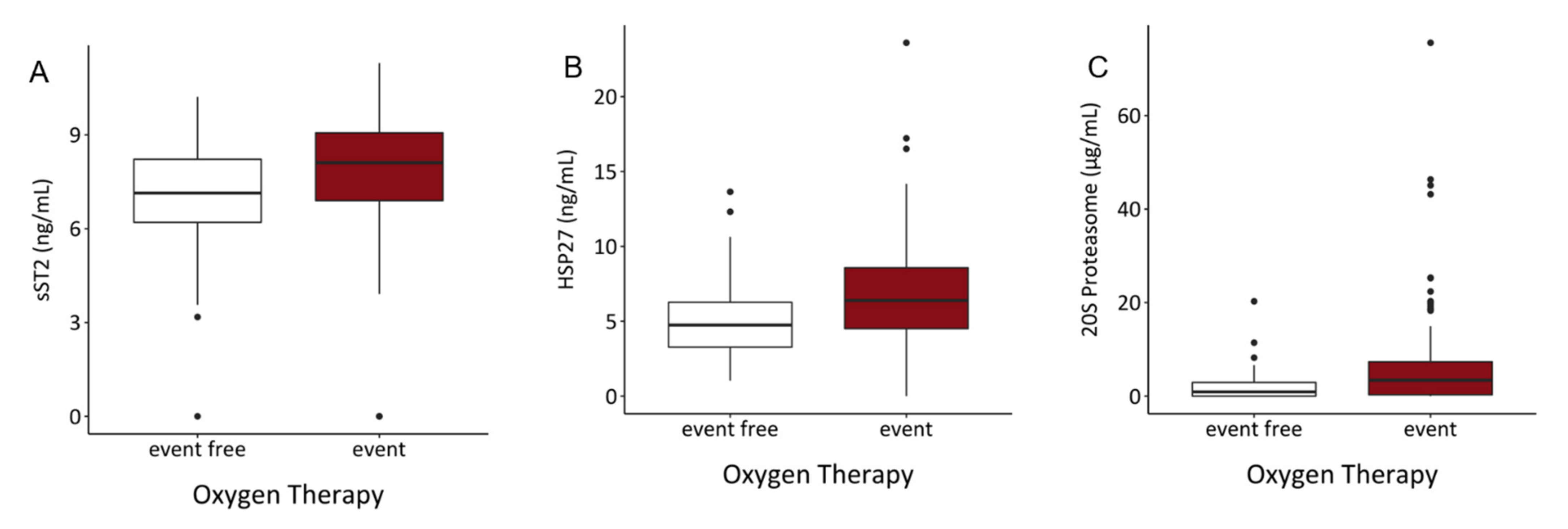
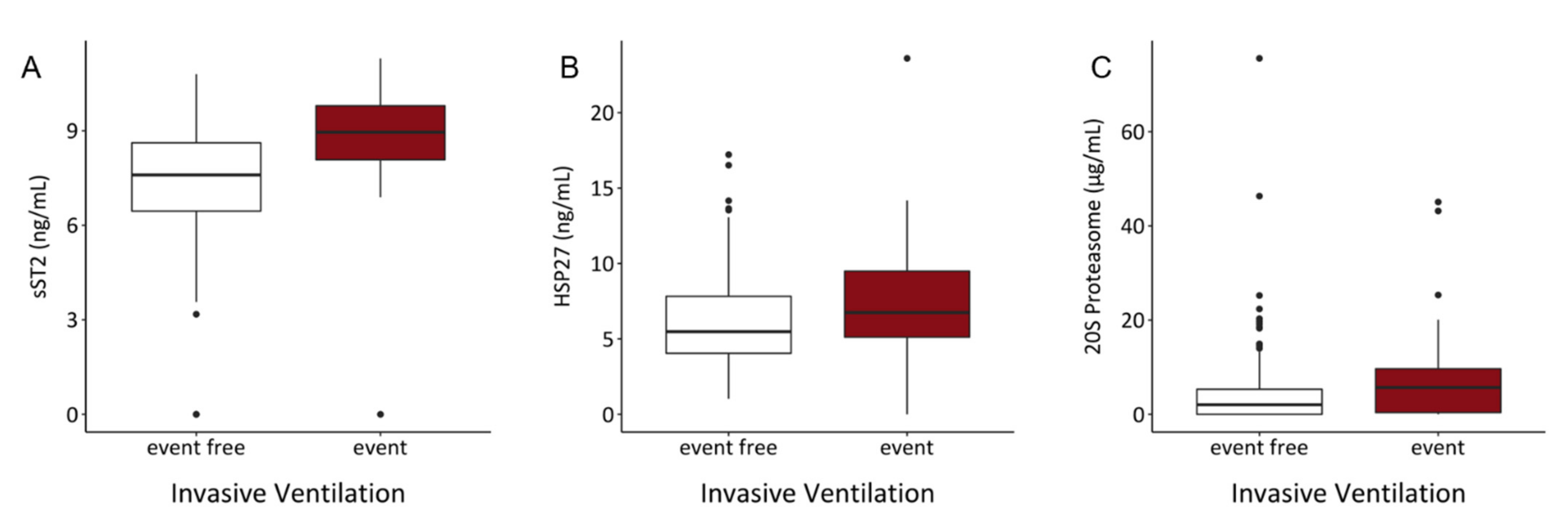
3.5. Serum Content of sST2, HSP27, and 20S Proteasome Might Predict Risk of Intubation and Requirement of Oxygen Therapy
3.6. Correlations between sST2 with 20S Proteasome, HSP27, and TNFR1
4. Discussion
4.1. sST2
4.2. HSP27
4.3. 20S Proteasome
4.4. Survival
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salzberger, B.; Buder, F.; Lampl, B.; Ehrenstein, B.; Hitzenbichler, F.; Hanses, F. Epidemiology of SARS-CoV-2 Infection and COVID-19. Der Internist 2020, 61, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Koch-Institut, R. Epidemiologisches Bulletin Höhere Letalität, Längere Beatmungsdauer Bei COVID-19 Im Vergleich Zu Schwerer Influenza. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/epid_bull_node.html (accessed on 13 July 2021).
- de Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; lo Tartaro, D.; Mattioli, M.; et al. Marked T Cell Activation, Senescence, Exhaustion and Skewing towards TH17 in Patients with COVID-19 Pneumonia. Nat. Commun. 2020, 11, 3434. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Ho, Y.C. SARS-CoV-2: A Storm Is Raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 Puzzle: Deciphering Pathophysiology and Phenotypes of a New Disease Entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Meng, Z. Immunomodulation for Severe COVID-19 Pneumonia: The State of the Art. Front. Immunol. 2020, 11, e2782. [Google Scholar] [CrossRef]
- Jose, R.J.; Manuel, A. COVID-19 Cytokine Storm: The Interplay between Inflammation and Coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil Extracellular Traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal Coagulation Parameters Are Associated with Poor Prognosis in Patients with Novel Coronavirus Pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 Pneumonia: Different Respiratory Treatments for Different Phenotypes? Intensive Care Med. 2020, 46, e1099. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Chiumello, D.; Rossi, S. COVID-19 Pneumonia: ARDS or Not? Crit. Care 2020, 24, 154. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Salvati, L.; Maggi, L.; Capone, M.; Vanni, A.; Spinicci, M.; Mencarini, J.; Caporale, R.; Peruzzi, B.; Antonelli, A.; et al. Impaired Immune Cell Cytotoxicity in Severe COVID-19 Is IL-6 Dependent. J. Clin. Investig. 2020, 130, 4694–4703. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-Induced Immunosuppression: From Cellular Dysfunctions to Immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Wang, H.; Artigas, A.; Sanitària Parc Taulí, C.; Randy Cron, S.Q.; Polidoro, R.B.; Hagan, R.S.; de Santis Santiago, R.; Schmidt, N.W. Overview: Systemic Inflammatory Response Derived From Lung Injury Caused by SARS-CoV-2 Infection Explains Severe Outcomes in COVID-19. Front. Immunol. 2020, 1, e1626. [Google Scholar] [CrossRef]
- Roth, G.; Moser, B.; Krenn, C.; Brunner, M.; Haisjackl, M.; Almer, G.; Gerlitz, S.; Wolner, E.; Boltz-Nitulescu, G.; Ankersmit, H.J. Susceptibility to Programmed Cell Death in T-Lymphocytes from Septic Patients: A Mechanism for Lymphopenia and Th2 Predominance. Biochem. Biophys. Res. Commun. 2003, 308, 840–846. [Google Scholar] [CrossRef]
- Roth, G.A.; Moser, B.; Krenn, C.; Roth-Walter, F.; Hetz, H.; Richter, S.; Brunner, M.; Jensen-Jarolim, E.; Wolner, E.; Hoetzenecker, K.; et al. Heightened Levels of Circulating 20S Proteasome in Critically Ill Patients. Eur. J. Clin. Investig. 2005, 35, 399–403. [Google Scholar] [CrossRef]
- Roth, G.A.; Krenn, C.; Brunner, M.; Moser, B.; Ploder, M.; Spittler, A.; Pelinka, L.; Sautner, T.; Wolner, E.; Boltz-Nitulescu, G.; et al. Elevated Serum Levels of Epithelial Cell Apoptosis-Specific Cytokeratin 18 Neoepitope M30 in Critically Ill Patients. Shock 2004, 22, 218–220. [Google Scholar] [CrossRef]
- Brunner, M.; Krenn, C.; Roth, G.; Moser, B.; Dworschak, M.; Jensen-Jarolim, E.; Spittler, A.; Sautner, T.; Bonaros, N.; Wolner, E.; et al. Increased Levels of Soluble ST2 Protein and IgG1production in Patients with Sepsis and Trauma. Intensive Care Med. 2004, 30, 1468–1473. [Google Scholar] [CrossRef]
- Mildner, M.; Storka, A.; Lichtenauer, M.; Mlitz, V.; Ghannadan, M.; Hoetzenecker, K.; Nickl, S.; Dome, B.; Tschachler, E.; Ankersmit, H.J. Primary Sources and Immunological Prerequisites for SST2 Secretion in Humans. Cardiovasc. Res. 2010, 87, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Hacker, S.; Lambers, C.; Hoetzenecker, K.; Pollreisz, A.; Aigner, C.; Lichtenauer, M.; Mangold, A.; Niederpold, T.; Zimmermann, M.; Taghavi, S.; et al. Elevated HSP27, HSP70 and HSP90α in Chronic Obstructive Pulmonary Disease: Markers for Immune Activation and Tissue Destruction. Clin. Lab. 2009, 55, 31–40. [Google Scholar] [PubMed]
- Ankersmit, H.J.; Nickl, S.; Hoeltl, E.; Toepker, M.; Lambers, C.; Mitterbauer, A.; Kortuem, B.; Zimmermann, M.; Moser, B.; Bekos, C.; et al. Increased Serum Levels of HSP27 as a Marker for Incipient Chronic Obstructive Pulmonary Disease in Young Smokers. Respiration 2012, 83, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Abers, M.S.; Delmonte, O.M.; Ricotta, E.E.; Fintzi, J.; Fink, D.L.; Almeida de Jesus, A.A.; Zarember, K.A.; Alehashemi, S.; Oikonomou, V.; Desai, J.v.; et al. An Immune-Based Biomarker Signature Is Associated with Mortality in COVID-19 Patients. JCI Insight 2021, 6, e144455. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Rapid Review Cytokine Elevation in Severe and Critical COVID-19: A Rapid Systematic Review, Meta-Analysis, and Comparison with Other Inflammatory Syndromes. Lancet Respir. Med. 2020, 1233, S2213–S2600. [Google Scholar] [CrossRef]
- WHO R&D Blueprint Novel Coronavirus COVID-19 Therapeutic Trial Synopsis. Available online: https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf (accessed on 12 August 2021).
- Miftode, R.-S.; Petriș, A.O.; Onofrei Aursulesei, V.; Cianga, C.; Costache, I.-I.; Mitu, O.; Miftode, I.-L.; Șerban, I.-L. The Novel Perspectives Opened by ST2 in the Pandemic: A Review of Its Role in the Diagnosis and Prognosis of Patients with Heart Failure and COVID-19. Diagnostics 2021, 11, 175. [Google Scholar] [CrossRef]
- Zeng, Z.; Hong, X.Y.; Li, Y.; Chen, W.; Ye, G.; Li, Y.; Luo, Y. Serum-Soluble ST2 as a Novel Biomarker Reflecting Inflammatory Status and Illness Severity in Patients with COVID-19. Biomark. Med. 2020, 14, 1619–1629. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Januzzi, J.L. The Biology of ST2: The International ST2 Consensus Panel. Am. J. Cardiol. 2015, 115, 3B–7B. [Google Scholar] [CrossRef]
- Traxler, D.; Zimmermann, M.; Simader, E.; Veraar, C.M.; Moser, B.; Mueller, T.; Mildner, M.; Dannenberg, V.; Lainscak, M.; Jug, B.; et al. The Inflammatory Markers SST2, HSP27 and HsCRP as a Prognostic Biomarker Panel in Chronic Heart Failure Patients. Clin. Chim. Acta 2020, 510, 507–514. [Google Scholar] [CrossRef]
- Hacker, S.; Dieplinger, B.; Werba, G.; Nickl, S.; Roth, G.A.; Krenn, C.G.; Mueller, T.; Ankersmit, H.J.; Haider, T. Increased Serum Concentrations of Soluble ST2 Predict Mortality after Burn Injury. Clin. Chem. Lab. Med. 2018, 56, 2079–2087. [Google Scholar] [CrossRef]
- Kumar, S.; Tzimas, M.N.; Griswold, D.E.; Young, P.R. Expression of ST2, an Interleukin-1 Receptor Homologue, Is Induced by Proinflammatory Stimuli. Biochem. Biophys. Res. Commun. 1997, 235, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Opfermann, P.; Simader, E.; Felli, A.; Bevilacqua, M.; Holaubek, C.; Dworschak, M.; Mouhieddine, M.; Zimpfer, D.; Ankersmit, J.H.; Steinlechner, B. Early SST2 Liberation after Implantation of a Left Ventricular Assist Device in Patients with Advanced Heart Failure. J. Immunol. Res. 2020, 2020, 5826176. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Gong, J.; Murshid, A. Extracellular HSPs: The Complicated Roles of Extracellular HSPs in Immunity. Front. Immunol. 2016, 7, e159. [Google Scholar] [CrossRef]
- Salari, S.; Seibert, T.; Chen, Y.-X.; Hu, T.; Shi, C.; Zhao, X.; Cuerrier, C.M.; Raizman, J.E.; O’Brien, E.R. Extracellular HSP27 Acts as a Signaling Molecule to Activate NF-ΚB in Macrophages. Cell Stress Chaperones 2013, 18, 53–63. [Google Scholar] [CrossRef]
- Haider, T.; Simader, E.; Glück, O.; Ankersmit, H.J.; Heinz, T.; Hajdu, S.; Negrin, L.L. Systemic Release of Heat-Shock Protein 27 and 70 Following Severe Trauma. Sci. Rep. 2019, 9, 9595. [Google Scholar] [CrossRef] [PubMed]
- Polanowska-Grabowska, R.; Gear, A.R.L. Heat-Shock Proteins and Platelet Function. Platelets 2000, 11, 6–22. [Google Scholar] [CrossRef]
- Tian, M.; Zhu, L.; Lin, H.; Lin, Q.; Huang, P.; Yu, X.; Jing, Y. Hsp-27 Levels and Thrombus Burden Relate to Clinical Outcomes in Patients with ST-Segment Elevation Myocardial Infarction. Oncotarget 2017, 8, 73733–73744. [Google Scholar] [CrossRef]
- Zimmermann, M.; Traxler, D.; Bekos, C.; Simader, E.; Mueller, T.; Graf, A.; Lainscak, M.; Marčun, R.; Košnik, M.; Fležar, M.; et al. Heat Shock Protein 27 as a Predictor of Prognosis in Patients Admitted to Hospital with Acute COPD Exacerbation. Cell Stress Chaperones 2020, 25, 141–149. [Google Scholar] [CrossRef]
- Traxler, D.; Zimmermann, M.; Simader, E.; Einwallner, E.; Copic, D.; Graf, A.; Mueller, T.; Veraar, C.; Lainscak, M.; Marčun, R.; et al. Fractional Heat Shock Protein 27 Urine Excretion as a Short-Term Predictor in Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Ann. Transl. Med. 2021, 9, e117. [Google Scholar] [CrossRef]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and Endotheliopathy: Crucial Contributors to COVID-19 Thromboinflammation. Nat. Rev. Cardiol. 2021, 18, e1. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Nickl, S.; Lambers, C.; Hacker, S.; Mitterbauer, A.; Hoetzenecker, K.; Rozsas, A.; Ostoros, G.; Laszlo, V.; Hofbauer, H.; et al. Discrimination of Clinical Stages in Non-Small Cell Lung Cancer Patients by Serum HSP27 and HSP70: A Multi-Institutional Case-Control Study. Clin. Chim. Acta 2012, 413, 1115–1120. [Google Scholar] [CrossRef]
- Circulating 20S Proteasome in Patients with Non-Metastasized Breast Cancer | Anticancer Research. Available online: https://ar.iiarjournals.org/content/31/6/2197.long (accessed on 12 August 2021).
- Majetschak, M.; Zedler, S.; Romero, J.; Albright, J.M.; Kraft, R.; Kovacs, E.J.; Faist, E.; Gamelli, R.L. Circulating Proteasomes After Burn Injury. J. Burn Care Res. 2010, 31, e243. [Google Scholar] [CrossRef]
- Egerer, K.; Kuckelkorn, U.; Rudolph, P.E.; Rückert, J.C.; Dörner, T.; Burmester, G.-R.; Kloetzel, P.-M.; Feist, E. Circulating Proteasomes Are Markers of Cell Damage and Immunologic Activity in Autoimmune Diseases. J. Rheumatol. 2002, 29, 2045–2052. [Google Scholar]
- DeBruin, G.; Xin, B.T.; Kraus, M.; van der Stelt, M.; van der Marel, G.A.; Kisselev, A.F.; Driessen, C.; Florea, B.I.; Overkleeft, H.S. A Set of Activity-Based Probes to Visualize Human (Immuno)Proteasome Activities. Angew. Chem. 2016, 128, 4271–4275. [Google Scholar] [CrossRef]
- Sixt, S.U.; Alami, R.; Hakenbeck, J.; Adamzik, M.; Kloß, A.; Costabel, U.; Jungblut, P.R.; Dahlmann, B.; Peters, J. Distinct Proteasome Subpopulations in the Alveolar Space of Patients with the Acute Respiratory Distress Syndrome. Mediat. Inflamm. 2012, 2012, 204250. [Google Scholar] [CrossRef][Green Version]
- Sixt, S.U.; Adamzik, M.; Spyrka, D.; Saul, B.; Hakenbeck, J.; Wohlschlaeger, J.; Costabel, U.; Kloß, A.; Giesebrecht, J.; Dahlmann, B.; et al. Alveolar Extracellular 20S Proteasome in Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2009, 179, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Tundo, G.R.; Sbardella, D.; Santoro, A.M.; Coletta, A.; Oddone, F.; Grasso, G.; Milardi, D.; Lacal, P.M.; Marini, S.; Purrello, R.; et al. The Proteasome as a Druggable Target with Multiple Therapeutic Potentialities: Cutting and Non-Cutting Edges. Pharmacol. Ther. 2020, 213, e107579. [Google Scholar] [CrossRef] [PubMed]
- Hetz, H.; Hoetzenecker, K.; Hacker, S.; Faybik, P.; Pollreisz, A.; Moser, B.; Roth, G.; Hoetzenecker, W.; Lichtenauer, M.; Klinger, M.; et al. Caspase-cleaved Cytokeratin 18 and 20 S Proteasome in Liver Degeneration. J. Clin. Lab. Anal. 2007, 21, e277. [Google Scholar] [CrossRef]
- Dwivedi, V.; Yaniv, K.; Sharon, M. Beyond Cells: The Extracellular Circulating 20S Proteasomes. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2021, 1867, e166041. [Google Scholar] [CrossRef] [PubMed]
- Janik, S.; Raunegger, T.; Hacker, P.; Ghanim, B.; Einwallner, E.; Müllauer, L.; Schiefer, A.-I.; Moser, J.; Klepetko, W.; Ankersmit, H.J.; et al. Prognostic and Diagnostic Impact of Fibrinogen, Neutrophil-to-Lymphocyte Ratio, and Platelet-to-Lymphocyte Ratio on Thymic Epithelial Tumors Outcome. Oncotarget 2018, 9, 21861–21875. [Google Scholar] [CrossRef]





| 30-Day Mortality | 60-Day Mortality | 90-Day Mortality | In-Hospital Mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | All Patients (n = 245) | Event (n = 44) | Event-Free (n = 177) | p-Value | Event (n = 52) | Event-Free (n = 168) | p-Value | Event (n = 52) | Event-Free (n = 153) | p-Value | Event (n = 39) | Event-Free (n = 206) | p-Value |
| Age (years) | 76 (64–83) | 80.5 (74–86) | 74 (62–83) | 80 (74–86) | 74 (62–83) | 80 (74–86) | 74 (62–83) | 81 (74–86) | 74 (62.25–82) | ||||
| Sex | |||||||||||||
| Female | 113 (46.12%) | 16 (36.36%) | 83 (46.89%) | ns | 19 (36.54%) | 80 (47.62%) | ns | 19 (36.54%) | 69 (45.1%) | ns | 16 (41.03%) | 97 (47.09%) | ns |
| Male | 132 (53.88%) | 28 (63.64%) | 94 (53.11%) | ns | 33 (63.46%) | 88 (52.38%) | ns | 33 (63.46%) | 84 (54.9%) | ns | 23 (58.97%) | 109 (52.91%) | ns |
| Comorbidities | |||||||||||||
| Diabetes | 98 (40.2%) | 19 (43.2%) | 72 (40.9%) | ns | 25 (48.1%) | 66 (39.5%) | ns | 25 (48.1%) | 60 (39.5%) | ns | 18 (46.2%) | 67 (40.6%) | ns |
| Hypertension | 183 (74.4%) | 35 (79.5%) | 131 (74.4%) | ns | 43 (82.7%) | 122 (73.1%) | ns | 43 (82.7%) | 111 (73.0%) | ns | 33 (84.6%) | 121 (73.3%) | ns |
| Transplantation | 2 (0.8%) | 0 (0%) | 1 (0.6%) | ns | 0 (0%) | 1 (0.6%) | ns | 0 (0.0%) | 1 (0.7%) | ns | 0 (0.0%) | 1 (0.6%) | ns |
| Liver cirrhosis | 2 (0.8%) | 1 (2.4%) | 1 (0.6%) | ns | 1 (2.0%) | 1 (0.6%) | ns | 1 (2.0%) | 1 (0.7%) | ns | 1 (2.7%) | 1 (0.6%) | ns |
| Heart failure | 64 (26.1%) | 21 (48.8%) | 38 (21.5%) | <0.01 | 22 (43.1%) | 37 (22.0%) | <0.01 | 22 (43.1%) | 36 (23.5%) | 0.01 | 18 (47.4%) | 40 (24.1%) | <0.01 |
| Medication at admission | |||||||||||||
| ACE Inhibitors | 75 (30.6%) | 15 (34.1%) | 52 (29.5%) | ns | 18 (34.6%) | 49 (29.3%) | ns | 18 (34.6%) | 44 (28.9%) | ns | 12 (30.8%) | 50 (30.3%) | ns |
| Angiotensin-Receptor Blocker | 87 (35.5%) | 11 (25.0%) | 69 (39.2%) | ns | 15 (28.8%) | 64 (38.3%) | ns | 15 (28.8%) | 60 (39.5%) | ns | 10 (25.6%) | 65 (39.4%) | ns |
| Calcium Inhibitors | 32 (13.1%) | 6 (13.6%) | 20 (11.4%) | ns | 6 (11.5%) | 20 (12.0%) | ns | 6 (11.5%) | 20 (13.2%) | ns | 6 (15.4%) | 20 (12.2%) | ns |
| Glucocorticoids | 14 (5.7%) | 5 (11.4%) | 7 (4.0%) | ns | 5 (9.6%) | 7 (4.2%) | ns | 5 (9.6%) | 7 (4.6%) | ns | 3 (7.7%) | 9 (5.5%) | ns |
| Aspirin | 52 (21.2%) | 8 (18.2%) | 41 (23.3%) | ns | 12 (23.1%) | 38 (22.2%) | ns | 12 (23.1%) | 35 (23.0%) | ns | 7 (17.9%) | 40 (24.2%) | ns |
| Heparin | 5 (2.0%) | 2 (4.5%) | 3 (1.7%) | ns | 2 (3.8%) | 3 (1.8%) | ns | 2 (3.8%) | 3 (2.0%) | ns | 1 (2.6%) | 4 (2.4%) | ns |
| NOACs | 56 (22.9%) | 15 (34.1%) | 36 (20.5%) | ns | 15 (28.8%) | 36 (21.6%) | ns | 15 (28.8%) | 34 (22.4%) | ns | 11 (28.2%) | 38 (23.0%) | ns |
| Hospitalization (days) | 10 (6–16) | ||||||||||||
| 30-Day Mortality | 60-Day Mortality | 90-Day Mortality | In-Hospital Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | All Patients (n = 245) | Event (n = 44) | Event-Free (n = 177) | Event (n = 52) | Event-Free (n = 168) | Event (n = 52) | Event-Free (n = 153) | Event (n = 39) | Event-Free (n = 206) |
| WHO Outcome Classification at admission | |||||||||
| 2 | 3 (1.22%) | 0 (0%) | 3 (1.69%) | 0 (0%) | 3 (1.79%) | 0 (0%) | 2 (1.31%) | 0 (0%) | 3 (1.46%) |
| 3 | 63 (25.71%) | 8 (18.18%) | 50 (28.25%) | 11 (21.15%) | 47 (27.98%) | 11 (21.15%) | 43 (28.1%) | 7 (17.95%) | 56 (27.18%) |
| 4 | 137 (55.92%) | 22 (50%) | 100 (56.5%) | 26 (50%) | 96 (57.14%) | 26 (50%) | 86 (56.21%) | 18 (46.15%) | 119 (57.77%) |
| 5 | 41 (16.73%) | 13 (29.55%) | 24 (13.56%) | 14 (26.92%) | 22 (13.1%) | 14 (26.92%) | 22 (14.38%) | 13 (33.33%) | 28 (13.59%) |
| 6 | 1 (0.41%) | 1 (2.27%) | 0 (0%) | 1 (1.92%) | 0 (0%) | 1 (1.92%) | 0 (0%) | 1 (2.56%) | 0 (0%) |
| at discharge | |||||||||
| 0 | 26 (10.61%) | 0 (0%) | 19 (10.73%) | 0 (0%) | 19 (11.31%) | 0 (0%) | 19 (12.42%) | 0 (0%) | 26 (12.62%) |
| 1 | 14 (5.71%) | 2 (4.55%) | 11 (6.21%) | 3 (5.77%) | 10 (5.95%) | 3 (5.77%) | 9 (5.88%) | 1 (2.56%) | 13 (6.31%) |
| 2 | 131 (53.47%) | 2 (4.55%) | 115 (64.97%) | 5 (9.62%) | 111 (66.07%) | 5 (9.62%) | 105 (68.63%) | 0 (0%) | 131 (63.59%) |
| 3 | 18 (7.35%) | 0 (0%) | 17 (9.6%) | 0 (0%) | 17 (10.12%) | 0 (0%) | 13 (8.5%) | 0 (0%) | 18 (8.74%) |
| 4 | 12 (4.9%) | 1 (2.27%) | 10 (5.65%) | 1 (1.92%) | 10 (5.95%) | 1 (1.92%) | 7 (4.58%) | 1 (2.56%) | 11 (5.34%) |
| 5 | 1 (0.41%) | 0 (0%) | 1 (0.56%) | 1 (1.92%) | 0 (0%) | 1 (1.92%) | 0 (0%) | 0 (0%) | 1 (0.49%) |
| 7 | 1 (0.41%) | 0 (0%) | 1 (0.56%) | 0 (0%) | 1 (0.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.49%) |
| 8 | 42 (17.14%) | 39 (88.64%) | 3 (1.69%) | 42 (80.77%) | 0 (0%) | 42 (80.77%) | 0 (0%) | 37 (94.87%) | 5 (2.43%) |
| 30-Day Mortality | 60-Day Mortality | 90-Day Mortality | In-Hospital Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Molecules | All Patients (n = 245) | Event (n = 44) | Event-Free(n = 177) | Event (n = 52) | Event-Free (n = 168) | Event (n = 52) | Event-Free (n = 153) | Event (n = 39) | Event-Free (n = 206) |
| sST2 (ng/mL) | 7.9 | 8.8 (7.8–9.7) | 7.7 (6.5–8.7) | 8.7 (7.6–9.6) | 7.7 (6.5–8.7) | 8.7 (7.6–9.7) | 7.7 (6.5–8.7) | 8.8 (7.9–9.8) | 7.7 (6.5–8.7) |
| HSP27 (ng/mL) | 5.8 | 6.6 (5.3–9.5) | 5.5 (4.1–8.0) | 6.7 (5.1–9.0) | 5.5 (4.0–8.2) | 6.7 (5.0–9.0) | 5.4 (4.0–8.1) | 6.5 (5.0–9.5) | 5.5 (4.0–8.1) |
| 20S proteasome (µg/mL) | 2.2 | 5.2 (0.3–9.6) | 1.8 (0–5.4) | 4.4 (0.3–9.2) | 5.3 (0–5.3) | 4.4 (0.3–9.2) | 2.1 (0–5.6) | 5.7 (1.1–9.8) | 2.0 (0–5.4) |
| cCK18 (U/L) | 162 | 151 (127–233) | 173 (130–274) | 151 (127–234) | 170 (130–259) | 151 (127–234) | 170 (130–268) | 148 (126–233) | 171 (131–265) |
| TNFR1 (ng/mL) | 2.4 | 2.7 (2.0–3.9) | 2.3 (1.7–3.9) | 2.7 (1.9–4.0) | 2.4 (1.7–3.9) | 2.7 (1.9–4.0) | 2.3 (1.7–3.7) | 2.6 (2.0–3.6) | 2.3 (1.7–3.9) |
| Prognostic Markers | Hazard Ratio | Lower CL | Upper CL | p-Value |
|---|---|---|---|---|
| Univariable Cox-Regression | ||||
| Age (years) | 1.047 | 1.02 | 1.074 | <0.001 |
| HSP27 (ng/mL) | 1.083 | 0.998 | 1.176 | 0.055 |
| sST2 (ng/mL) | 1.364 | 1.13 | 1.647 | 0.001 |
| TNFR1 (ng/mL) | 1.053 | 0.98 | 1.132 | 0.162 |
| cCK18 (1000 U/L) | 0.595 | 0.13 | 2.735 | 0.505 |
| 20S proteasome (µg/mL) | 1.019 | 0.998 | 1.041 | 0.076 |
| Multivariable Cox-Regression | ||||
| Age (years) | 1.047 | 1.019 | 1.075 | <0.001 |
| sST2 (ng/mL) | 1.353 | 1.116 | 1.639 | 0.002 |
| Prognostic Markers | Odds Ratio | Lower CL | Upper CL | p-Value |
|---|---|---|---|---|
| Univariable Logistic Regression | ||||
| Age (years) | 1.056 | 1.023 | 1.094 | 0.001 |
| HSP27 (ng/mL) | 1.102 | 0.997 | 1.22 | 0.056 |
| sST2 (ng/mL) | 1.536 | 1.216 | 1.991 | <0.001 |
| TNFR1 (ng/mL) | 1.046 | 0.931 | 1.16 | 0.408 |
| cCK18 (1000 U/L) | 0.723 | 0.101 | 3.097 | 0.702 |
| 20S proteasome (µg/mL) | 1.037 | 1.002 | 1.077 | 0.044 |
| Multivariable Logistic Regression | ||||
| Age (years) | 1.059 | 1.024 | 1.1 | 0.002 |
| sST2 (ng/mL) | 1.457 | 1.144 | 1.911 | 0.004 |
| 20S proteasome (µg/mL) | 1.034 | 0.997 | 1.076 | 0.072 |
| Prognostic Markers | Odds Ratio | Lower CL | Upper CL | p-Value |
|---|---|---|---|---|
| Univariable Logistic Regression | ||||
| Age (years) | 1.052 | 1.023 | 1.087 | <0.001 |
| HSP27 (ng/mL) | 1.092 | 0.992 | 1.204 | 0.071 |
| sST2 (ng/mL) | 1.332 | 1.091 | 1.659 | 0.007 |
| TNFR1 (ng/mL) | 1.07 | 0.964 | 1.186 | 0.19 |
| cCK18 (1000 U/L) | 0.566 | 0.081 | 2.42 | 0.502 |
| 20S proteasome (µg/mL) | 1.032 | 0.997 | 1.07 | 0.076 |
| Multivariable Logistic Regression | ||||
| Age (years) | 1.051 | 1.02 | 1.086 | 0.002 |
| sST2 (ng/mL) | 1.298 | 1.061 | 1.624 | 0.016 |
| Prognostic Markers | Odds Ratio | Lower CL | Upper CL | p-Value |
|---|---|---|---|---|
| Univariable Logistic Regression | ||||
| Age (years) | 1.053 | 1.022 | 1.088 | 0.001 |
| HSP27 (ng/mL) | 1.096 | 0.996 | 1.209 | 0.061 |
| sST2 (ng/mL) | 1.335 | 1.095 | 1.663 | 0.007 |
| TNFR1(ng/mL) | 1.098 | 0.984 | 1.236 | 0.098 |
| cCK18 (1000 U/L) | 0.536 | 0.076 | 2.296 | 0.463 |
| 20S proteasome (µg/mL) | 1.027 | 0.993 | 1.065 | 0.122 |
| Multivariable Logistic Regression | ||||
| Age (years) | 1.051 | 1.02 | 1.087 | 0.002 |
| sST2 (ng/mL) | 1.304 | 1.068 | 1.631 | 0.014 |
| Prognostic Markers | Odds Ratio | Lower CL | Upper CL | p-Value |
|---|---|---|---|---|
| Univariable Logistic Regression | ||||
| Age (years) | 1.062 | 1.027 | 1.104 | <0.001 |
| HSP27 (ng/mL) | 1.101 | 0.993 | 1.22 | 0.063 |
| sST2 (ng/mL) | 1.697 | 1.316 | 2.253 | <0.001 |
| TNFR1 (ng/mL) | 1.079 | 0.963 | 1.2 | 0.16 |
| cCK18 (1000 U/L) | 0.291 | 0.019 | 1.957 | 0.298 |
| 20S proteasome (µg/mL) | 1.044 | 1.008 | 1.085 | 0.019 |
| Multivariable Logistic Regression | ||||
| Age (years) | 1.068 | 1.029 | 1.114 | 0.001 |
| sST2 (ng/mL) | 1.631 | 1.245 | 2.21 | <0.001 |
| 20S proteasome (µg/mL) | 1.041 | 1.003 | 1.085 | 0.033 |
| Prognostic Markers | Odds Ratio | Lower CL | Upper CL | p-Value |
|---|---|---|---|---|
| Univariable Logistic Regression | ||||
| Age (years) | 0.995 | 0.974 | 1.016 | 0.662 |
| HSP27 (ng/mL) | 1.218 | 1.091 | 1.374 | <0.001 |
| sST2 (ng/mL) | 1.258 | 1.082 | 1.475 | 0.003 |
| TNFR1 (ng/mL) | 1.107 | 0.98 | 1.289 | 0.146 |
| cCK18 (1000 U/L) | 0.747 | 0.213 | 2.985 | 0.651 |
| 20S proteasome (µg/mL) | 1.177 | 1.084 | 1.3 | <0.001 |
| Multivariable Logistic Regression | ||||
| HSP27 (ng/mL) | 1.134 | 1.012 | 1.282 | 0.037 |
| sST2 (ng/mL) | 1.175 | 1.003 | 1.38 | 0.046 |
| 20S proteasome (µg/mL) | 1.147 | 1.054 | 1.269 | 0.004 |
| Prognostic Markers | Odds Ratio | Lower CL | Upper CL | p-Value |
|---|---|---|---|---|
| Univariable Logistic Regression | ||||
| Age (years) | 1.053 | 1.021 | 1.09 | 0.002 |
| HSP27 (ng/mL) | 1.108 | 1.003 | 1.225 | 0.041 |
| sST2 (ng/mL) | 1.79 | 1.39 | 2.373 | <0.001 |
| TNFR1 (ng/mL) | 1.065 | 0.951 | 1.182 | 0.24 |
| cCK18 (1000 U/L) | 0.806 | 0.112 | 3.45 | 0.799 |
| 20S proteasome (µg/mL) | 1.039 | 1.004 | 1.079 | 0.031 |
| Multivariable Logistic Regression | ||||
| Age (years) | 1.055 | 1.02 | 1.096 | 0.003 |
| HSP27 (ng/mL) | 1.028 | 0.915 | 1.155 | 0.635 |
| sST2 (ng/mL) | 1.709 | 1.306 | 2.31 | <0.001 |
| 20S proteasome (µg/mL) | 1.032 | 0.993 | 1.074 | 0.094 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wendt, R.; Lingitz, M.-T.; Laggner, M.; Mildner, M.; Traxler, D.; Graf, A.; Krotka, P.; Moser, B.; Hoetzenecker, K.; Kalbitz, S.; et al. Clinical Relevance of Elevated Soluble ST2, HSP27 and 20S Proteasome at Hospital Admission in Patients with COVID-19. Biology 2021, 10, 1186. https://doi.org/10.3390/biology10111186
Wendt R, Lingitz M-T, Laggner M, Mildner M, Traxler D, Graf A, Krotka P, Moser B, Hoetzenecker K, Kalbitz S, et al. Clinical Relevance of Elevated Soluble ST2, HSP27 and 20S Proteasome at Hospital Admission in Patients with COVID-19. Biology. 2021; 10(11):1186. https://doi.org/10.3390/biology10111186
Chicago/Turabian StyleWendt, Ralph, Marie-Therese Lingitz, Maria Laggner, Michael Mildner, Denise Traxler, Alexandra Graf, Pavla Krotka, Bernhard Moser, Konrad Hoetzenecker, Sven Kalbitz, and et al. 2021. "Clinical Relevance of Elevated Soluble ST2, HSP27 and 20S Proteasome at Hospital Admission in Patients with COVID-19" Biology 10, no. 11: 1186. https://doi.org/10.3390/biology10111186
APA StyleWendt, R., Lingitz, M.-T., Laggner, M., Mildner, M., Traxler, D., Graf, A., Krotka, P., Moser, B., Hoetzenecker, K., Kalbitz, S., Lübbert, C., Beige, J., & Ankersmit, H. J. (2021). Clinical Relevance of Elevated Soluble ST2, HSP27 and 20S Proteasome at Hospital Admission in Patients with COVID-19. Biology, 10(11), 1186. https://doi.org/10.3390/biology10111186