Sucrose Is Not the Whole Story: Risk Factors and Oral Health at the Contact (Yakutia, Siberia-16th/19th)
Abstract
:Simple Summary
Abstract
1. Introduction
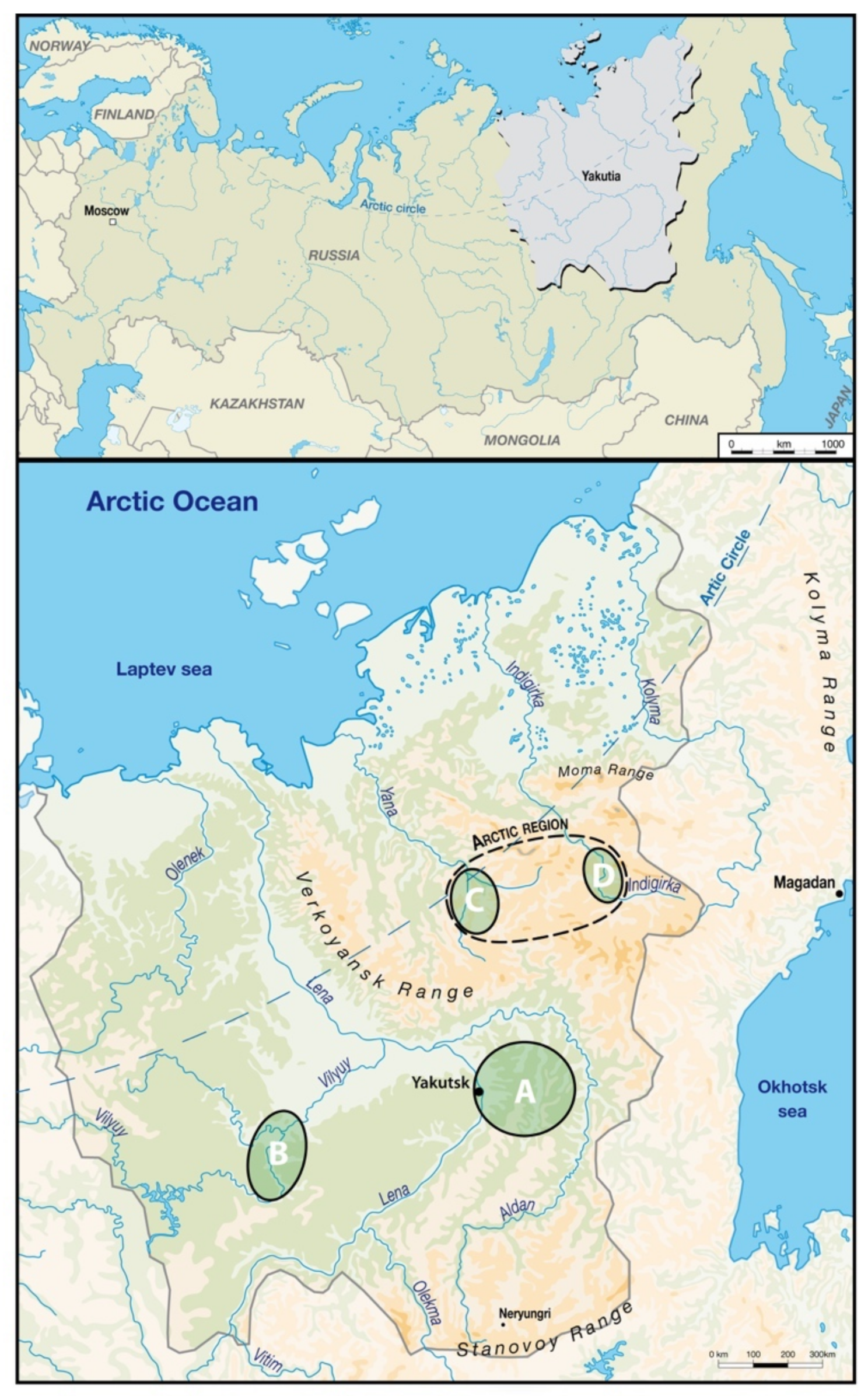
2. Materials and Methods
2.1. Analysis of the Variables
2.2. Statistical Analyses
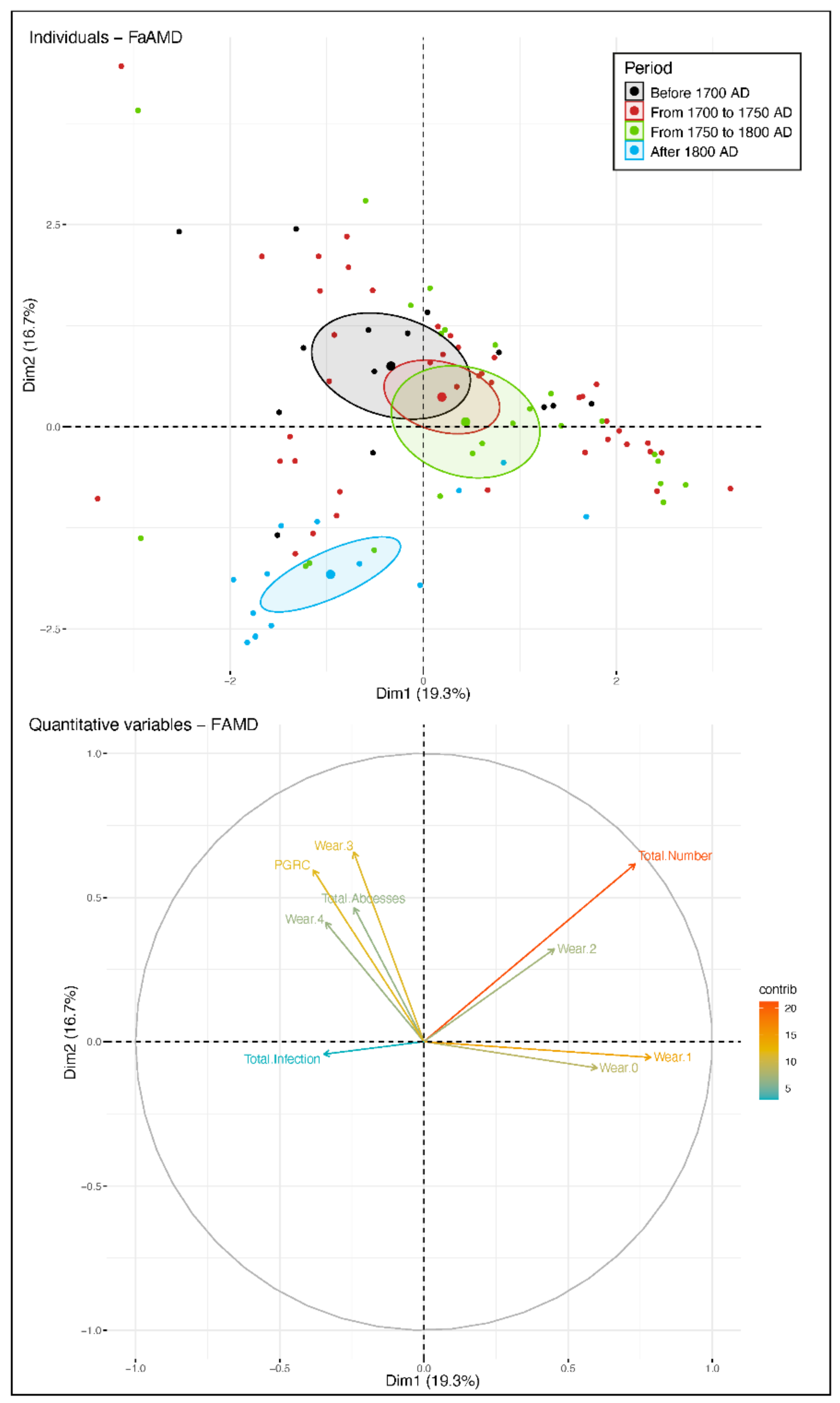
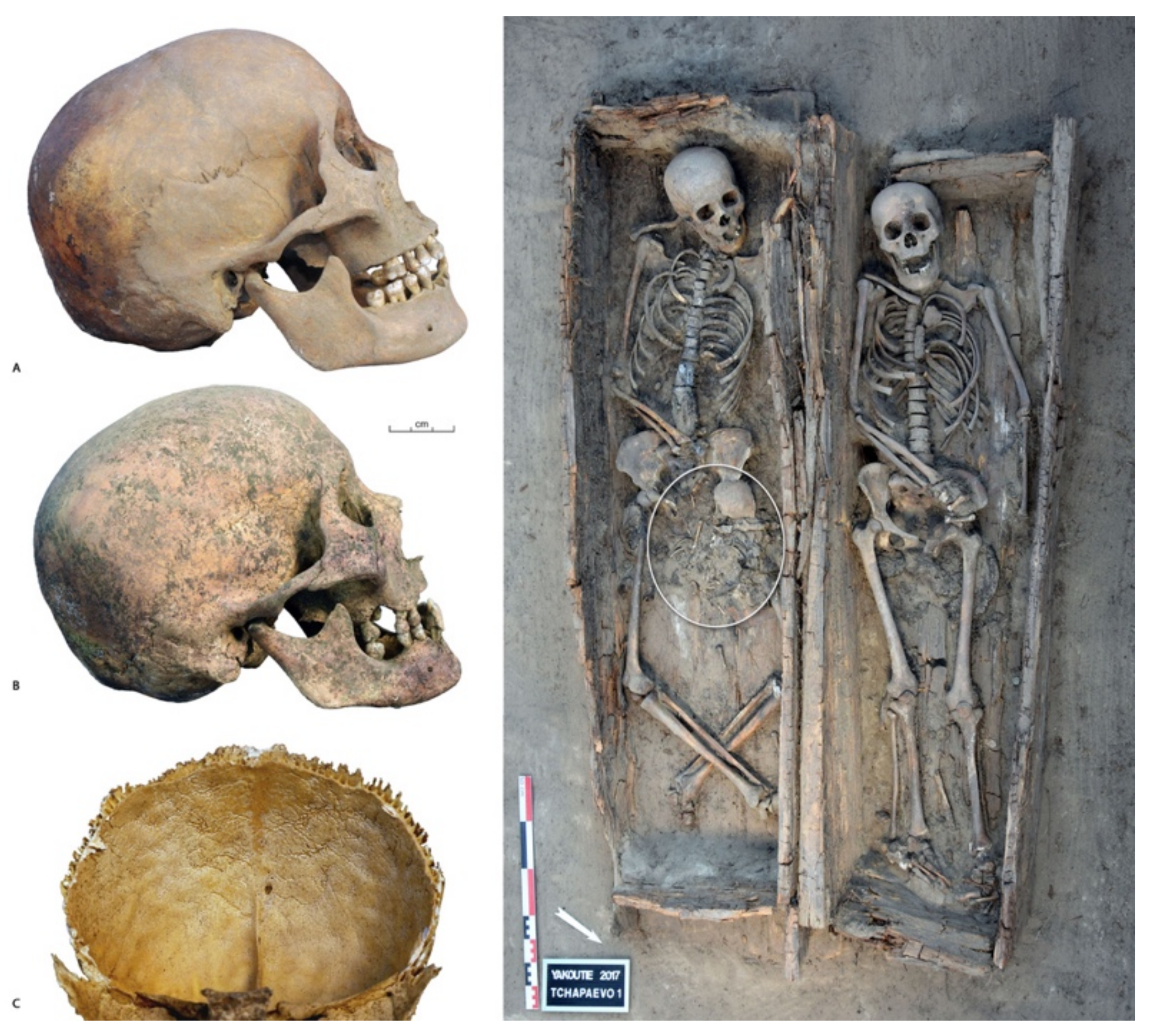
3. Results
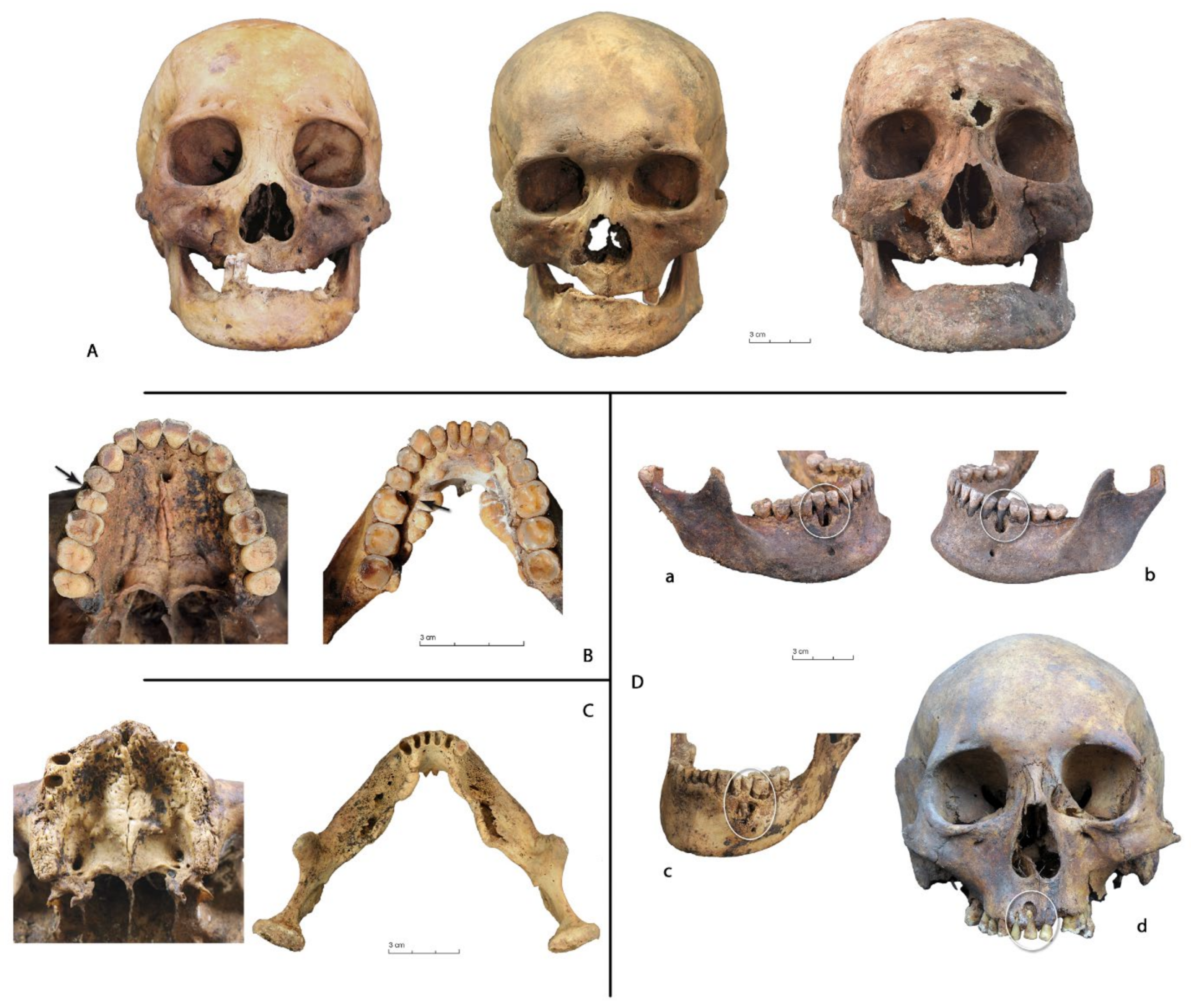
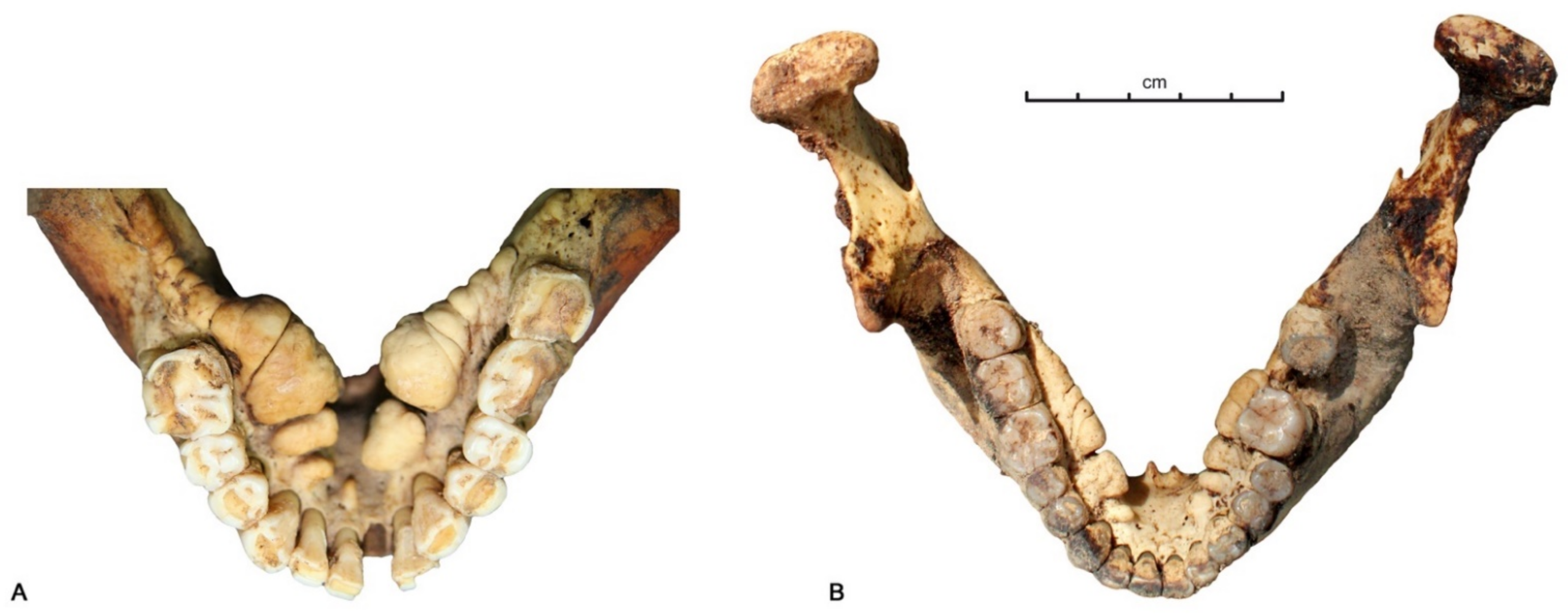
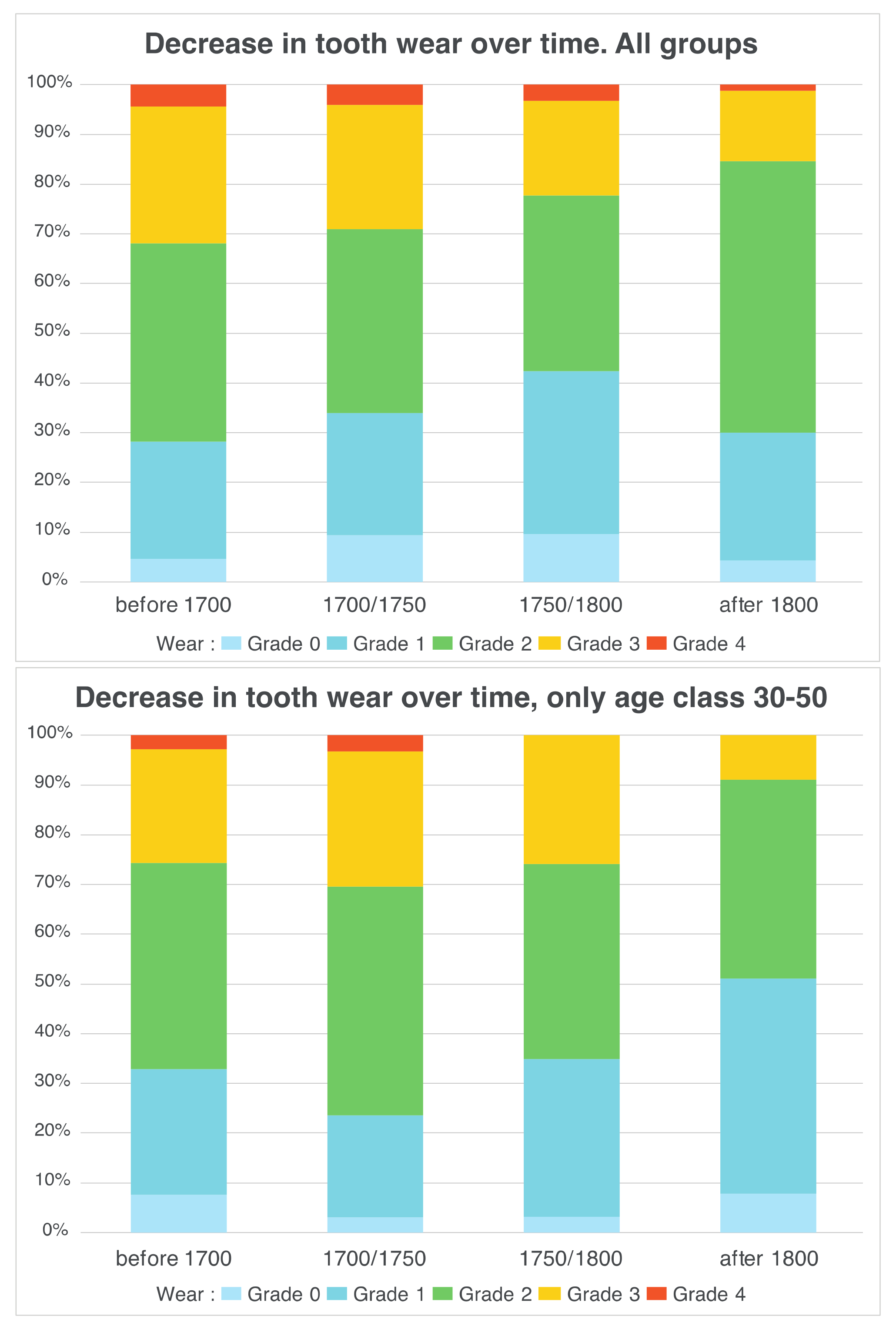
3.1. Data Analysis per Tooth (Data Description)
3.2. Between-Subject Comparisons for Periods and Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gherlone, E.; Polizzi, E.; Tetè, G.; Capparè, P. Dentistry and Covid-19 Pandemic: Operative Indications Post-Lockdown. New Microbiol. 2021, 44, 1–11. [Google Scholar]
- Larsen, C.S. Bioarchaeology of Spanish Florida: The Impact of Colonialism; University Press of Florida: Gainesville, FL, USA, 2001. [Google Scholar]
- Graver, S.M. Dental Health Decline in the Chesapeake Bay, Virginia: The Role of European Contact and Multiple Stressors. Dent. Anthropol. J. 2018, 18, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Botha, D.; Steyn, M. Dental Health of the Late 19th and Early 20th Century Khoesan. HOMO 2015, 66, 187–202. [Google Scholar] [CrossRef] [Green Version]
- Klaus, H.D.; Tam, M.E. Oral Health and the Postcontact Adaptive Transition: A Contextual Reconstruction of Diet in Morrope, Peru. Am. J. Phys. Anthropol. 2010, 609, 594–609. [Google Scholar] [CrossRef]
- Gagnon, C.M. Exploring Oral Paleopathology in the Central Andes: A Review. Int. J. Paleopathol. 2020, 29, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.C. 1493: Uncovering the New World Columbus Created; Knopf: New York, NY, USA, 2011. [Google Scholar]
- Müller, A.; Hussein, K. Meta-Analysis of Teeth from European Populations before and after the 18th Century Reveals a Shift towards Increased Prevalence of Caries and Tooth Loss. Arch. Oral Biol. 2017, 73, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zvénigorosky, V.; Duchesne, S.; Romanova, L.; Gérard, P.; Solovyev, A.; Romanov, G.; Barashkov, N.; Fedorova, S.; Ludes, B.; Crubézy, E.; et al. The Genetic Legacy of Legendary and Historical Siberian Chieftains. Commun. Biol. 2020, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Crubézy, E.; Nikolaeva, D. Vainqueurs Ou Vaincus? Odile Jaco: Paris, France, 2017. [Google Scholar]
- Romanova, L.; Balter, V.; Simon, L.; Gerard, P.; Pokatilova, N.; Crubezy, E. Diet of Autochthonous Populations in Yakutia Using Isotopic, Ethnographic, Historical and Archaeological Data. J. Archaeol. Sci. Rep. 2019, 28, 102022. [Google Scholar] [CrossRef]
- Crubézy, E.; Amory, S.; Keyser, C.; Bouakaze, C.; Bodner, M.; Gibert, M.; Röck, A.; Parson, W.; Alexeev, A.; Ludes, B. Human Evolution in Siberia: From Frozen Bodies to Ancient DNA. BMC Evol. Biol. 2010, 10, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyser, C.; Hollard, C.; Gonzalez, A.; Fausser, J.L.; Rivals, E.; Alexeev, A.N.; Riberon, A.; Crubézy, E.; Ludes, B. The Ancient Yakuts: A Population Genetic Enigma. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyser, C.; Zvénigorosky, V.; Gonzalez, A.; Fausser, J.L.; Jagorel, F.; Gérard, P.; Tsagaan, T.; Duchesne, S.; Crubézy, E.; Ludes, B. Genetic Evidence Suggests a Sense of Family, Parity and Conquest in the Xiongnu Iron Age Nomads of Mongolia. Hum. Genet. 2021, 140, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Crubézy, E.; Melnichuk, O.; Alexeev, A. Archaelogy, Genetics and History 15 Years Of Research in Yakutia (2002–2017). Vestnik Arheologii, Antropologii I Etnografii 2020, 4, 110–119. [Google Scholar] [CrossRef]
- Forsyth, J. A History of the Peoples of Siberia. Russia’s North Asian Colony 1581–1990; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Biagini, P.; Thèves, C.; Balaresque, P.; Géraut, A.; Cannet, C.; Keyser, C.; Nikolaeva, D.; Gérard, P.; Duchesne, S.; Orlando, L.; et al. Variola Virus in a 300-Year-Old Siberian Mummy. N. Engl. J. Med. 2012, 367, 2057–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnasheva, N.I. Ekonomika Iakutii v XIX Veke: Pervye Shagi k Rynochnym Ot- Nosheniiam (The Economy of Yakutia in the 19th Century: The First Steps to Market Relations). Cevero-Vostochnyi Gumanit. Vestn. 2015, 2, 15–19. [Google Scholar]
- Crubézy, E. Les Tombes Gelées de Sibérie Orientale. In L’archéologie au Laboratoire; Thiébault, S., Depaepe, P., Eds.; La Découverte: Paris, France, 2013; pp. 49–60. [Google Scholar]
- Moorrees, C.F.A.; Fanning, E.A.; Hunt, E.E., Jr. Formation and Resorption of Three Deciduous Teeth in Children. Am. J. Phys. Anthropol. 1963, 21, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Braga, J.; Heuze, Y.; Chabadel, O.; Sonan, N.K.; Gueramy, A. Non-Adult Dental Age Assessment: Correspondence Analysis and Linear Regression versus Bayesian Predictions. Int. J. Legal Med. 2005, 119, 260–274. [Google Scholar] [CrossRef]
- Stloukal, M.; Hanáková, H. The Length of Long Bones in Ancient Slavonic Populations with Particular Consideration to the Questions of Growth. Homo 1978, 29, 53–69. [Google Scholar]
- Lovejoy, C.O.; Meindl, R.S.; Pryzbeck, T.R.; Mensforth, R.P. Chronological Metamorphosis of the Auricular Surface of the Ilium: A New Method for the Determination of Adult Skeletal Age at Death. Am. J. Phys. Anthropol. 1985, 68, 15–28. [Google Scholar] [CrossRef]
- Schmitt, A. Une Nouvelle Méthode Pour Estimer l’âge Au Décès Des Adultes à Partir de La Surface Sacro-Pelvienne Iliaque. Bull. Mem. Soc. Anthropol. Paris 2005, 17, 89–110. [Google Scholar] [CrossRef]
- Irish, J.; Scott, G. A Companion to Dental Anthropology; Wiley Blackwell: Chichester, UK, 2016. [Google Scholar]
- Khazaei, S.H.; Keshteli, A.; Feizi, A.; Savabi, O.; Adibi, P. Epidemiology and Risk Factors of Tooth Loss among Iranian Adults: Findings from a Large Community-Based Study. BioMed Res. Int. 2013, 2013, 786462. [Google Scholar] [CrossRef]
- Burnett, S. Crown Wear: Identification and Categorization. In A Companion to Dental Anthropology; Irish, J., Scott, D., Richard, G., Eds.; Wiley Blackwell: Oxford, UK, 2016. [Google Scholar]
- Molnar, S. Human Tooth Wear, Tooth Function and Cultural Variability. Am. J. Phys. Anthropol. 1971, 34, 175–189. [Google Scholar] [CrossRef]
- Crubézy, E.; Causse, L.; Delmas, J.; Ludes, B. Le Paysan Médiéval En Rouergue. Cimetière et Église de Canac (Campagnac, Aveyron); Musée Archéologique de Montrozier: Rodez, France, 1998. [Google Scholar]
- Ganss, C.; Klimek, J.; Lussi, A. Accuracy and Consistency of the Visual Diagnosis of Exposed Dentine on Worn Occlusal/Incisal Surfaces. Caries Res. 2006, 40, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Brabant, H.; Sahly, A. La Paléostomatologie En Belgique et En France. Acta Stomatol. Belg. 1962, 355–385. [Google Scholar]
- Esclassan, R.; Grimoud, A.M.; Ruas, M.P.; Donat, R.; Sevin, A.; Astie, F.; Lucas, S.; Crubezy, E. Dental Caries, Tooth Wear and Diet in an Adult Medieval (12th–14th Century) Population from Mediterranean France. Arch. Oral Biol. 2009, 54, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, S.; Crubézy, E. Les Cimetières Du Haut Moyen Âge En Languedoc; Presses Universitaires de Perpignan: Perpignan, France, 2015. [Google Scholar]
- Hillson, S. Dental Anthropology; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Bender, I.B.; Seltzer, S. Roentgenographic and Direct Observation of Experimental Lesions in Bone: II. J. Endod. 2003, 29, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Brothwell, D. Digging Up Bones; Cornell University Press: Itaca, NY, USA, 1981. [Google Scholar]
- Dias, G.; Tayles, N. Abscess Cavity”—A Misnomer. Int. J. Osteoarchaeol. 1997, 7, 458–554. [Google Scholar] [CrossRef]
- Ogden, A. Advances in the Paleopathology of Teeth and Jaws. In Advances in Human Paleopathology; Pinhasi, R., Mays, S., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 283–307. [Google Scholar]
- Romanova, L.; Stépanoff, C.; Telmon, N.; Crubézy, E. Health Access Inequities and Magic Medicine: The First Ancient Evidence? Lancet 2020, 395, 1343–1344. [Google Scholar] [CrossRef]
- Cameron, C.E. The Cracked Tooth Syndrome: Additional Findings. J. Am. Dent. Assoc. 1976, 93, 971–977. [Google Scholar] [CrossRef]
- Banerji, S.; Mehta, S.B.; Millar, B.J. Cracked Tooth Syndrome. Part 1: Aetiology and Diagnosis. Br. Dent. J. 2010, 208, 459–463. [Google Scholar] [CrossRef] [Green Version]
- Léonard, A.; Bayle, P.; Murail, P.; Bruzek, J. Oral Exostoses: An Assessment of two Hundred Years of Research. BMSAP 2014, 26, 1–22. [Google Scholar] [CrossRef]
- Movsesjan, A.; Mamonova, N.N.; Rychkov, J.C. Programma in Metodolika Issledovanija Anomalij Cerepa. Vopr Anthropol. 1975, 51, 127–150. [Google Scholar]
- Reichart, P.A.; Neuhaus, F.; Sookasem, M. Prevalence of Torus Palatinus and torus mandibularis in Germans and Thai. Community Dent. Oral Epidemiol. 1988, 16, 61–64. [Google Scholar] [CrossRef]
- Jainkittivong, A.; Langlais, R.P. Buccal and Palatal Exostoses: Prevalence and Concurrence with Tori. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2000, 90, 48–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huberty, C. Discriminant Analysis. Rev. Educ. Res. 1975, 45, 543–598. [Google Scholar] [CrossRef]
- Todorov, V. Robust Selection of Variables in Linear Discriminant. Stat. Meth. Appl. 2007, 15, 395–407. [Google Scholar] [CrossRef]
- Lanfranco, L.; Eggers, S. Caries through Time: An Anthropological Overview. In Contemporary Approach to Dental Caries; Li, M.-Y., Ed.; Intech: Rijeka, Croatia, 2012; pp. 3–34. [Google Scholar]
- Afonin, M. Ojmâkono-Borogonskij Nasleg [Village Oïmiakon-Borogon]. Izv. Âkutskogo Otd. IRGO 1929, V3, 50–60. [Google Scholar]
- Savvin, A.A. Piŝa Âkutov Do Razvitiâ Zemledeliâ ([Food of the Yakuts before the Development of Agriculture]); Opyt istor.; IGI AN RS (Ya): Yakutsk, Russia, 1947. [Google Scholar]
- Middendorf, A. Putešestvie Na Sever i Vostok Sibiri: Sever i Vostok Sibiri v Estestvenno-Istoričeskom Otnošenii. In Korennye žiteli Sibiri [The Indigenous Inhabitants of Siberia]; Tipografiia Imperatorskoi Akademii Nauk: Saint-Pétersbourg, Russia, 1878; pp. 619–833. [Google Scholar]
- Berg, L. Otkrytie Kamčatki i Èkspediciâ Beringa, 1725–1742 [The Discovery of Katchatka and the Bering Expedition, 1725–1742]; Leningrad, Russia, 1946. [Google Scholar]
- Zackrisson, O.; Ostlund, L.; Korhonen, O.; Bergman, I. Vegetation History and Archaeobotany the Ancient Use of Pinus Sylvestris L. (Scots Pine) Inner Bark by Sami People in Northern Sweden, Related to Cultural and Ecological Factors. Veg. Hist. Archaeobot. 2000, 9, 99–109. [Google Scholar] [CrossRef]
- Nikolaev-Somogotto, S.I. Piŝa Âkutov (v Svete Sosednih Kul’tur) [The Food of Yakuts (Comparing to Neighboring Cultures)]; Yakutskii: Yakutsk, Russia, 2009. [Google Scholar]
- Basharin, G.P. Istoriâ Agrarnyh Otnošenij v 2 Tomah [The History of Agrarian Relations in 2 Volumes]; Art-Fleks: Moskva, Russia, 2003. [Google Scholar]
- Seroshevskij, V. Iakuty: Opyt Etnograficheskogo Issledovaniia [The Yakuts: An Ethnographic Research Experiment], V.1. Spb.
- Popov, G.A. Sočineniâ. Tom 3. Istoriâ Goroda Âkutska 1632–1917 Gg. (Kratkie Očerki) [Compositions. Volume 3. History of the City of Yakutsk 1632–1917 (Short Essays)]; Yakuts, Russia, 2005. [Google Scholar]
- Lee, H.; Honga, J.H.; Hong, Y.; Shina, D.H.; Slepchenkod, S. Caries, Antemortem Tooth Loss and Tooth Wear Observed in Indigenous Peoples and Russian Settlers of 16th to 19th Century West Siberia. Arch. Oral Biol. 2019, 98, 176–181. [Google Scholar] [CrossRef]
- Gessain, R. La Dentition Des Eskimo d’Angmassalik; Génétique, Croissance et Pathologie. Bull. Mem. Soc. Anthropol. Paris 1959, 10, 364–396. [Google Scholar] [CrossRef]
- Anderson, C.A.; Curzon, M.E.J.; Van Loveren, C.; Tatsi, C.; Duggal, M.S. Sucrose and Dental Caries: A Review of the Evidence. Obes. Rev. 2009, 10, 41–54. [Google Scholar] [CrossRef]
- Sheiham, A. Sugar and Dental Decay. Lancet 1983, 321, 282–284. [Google Scholar] [CrossRef]
- Popov, G.A. Sochinenija. Istorija Goroda Jakutska: 1632–1917 [ History of the City of Yakuts: 1632–1917] T3; aGU: Yakutsk, Russia, 2007. [Google Scholar]
- Ushnitskii, I.D. Actual Problems and Prospects of Stomatology Development in the North Conditions: Collection of Articles of the Interregional Scientific-Practical Conference Dedicated to the 100th Anniversary of Stomatological Service of the Republic of Sakha (Yakutia); Publishing House SVFU: Yakutsk, Russia, 2020; p. 352. [Google Scholar]
- Müller, F.; Naharro, M.; Carlsson, G. What Are the Prevalence and Incidence of Tooth Loss in the Adult and Elderly Population in Europe? Clin. Oral Implants Res. 2007, 18, 2–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, R.M. The Dental Health of Elderly People in Britain, 1968 to 1988. Int. Dent. J. 1992, 42, 399–402. [Google Scholar] [PubMed]
- Crespi, R.; Capparè, P.; Gherlone, E. Osteotome Sinus Floor Elevation and Simultaneous Implant Placement in Grafted Biomaterial Sockets: 3 Years of Follow-Up. J. Periodontol. 2010, 81, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparè, P.; Polizzi, E.M.; Gherlone, E.F. Tissue Remodeling after Bone Expansion in Grafted and Ungrafted Sockets. Int. J. Oral Maxillofac. Implants 2014, 29, 699–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespi, R.; Capparè, P.; Polizzi, E.; Gherlone, E. Fresh-Socket Implants of Different Collar Length: Clinical Evaluation in the Aesthetic Zone. Clin. Implant Dent. Relat. Res. 2015, 17, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Vinci, R.; Teté, G.; Lucchetti, F.R.; Capparé, P.; Gherlone, E.F. Implant Survival Rate in Calvarial Bone Grafts: A Retrospective Clinical Study with 10 Year Follow-Up. Clin. Implant Dent. Relat. Res. 2019, 21, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Capparé, P.; Vinci, R.; Di Stefano, D.A.; Traini, T.; Pantaleo, G.; Gherlone, E.F.; Gastaldi, G. Correlation between Initial BIC and the Insertion Torque/Depth Integral Recorded with an Instantaneous Torque-Measuring Implant Motor: An in Vivo Study. Clin. Implant Dent. Relat. Res. 2015, 17 (Suppl. 2), e613–e620. [Google Scholar] [CrossRef] [PubMed]
- Burt, B.A.; Ismail, A.I.; Morrison, E.C.; Beltran, E.D. Risk Factors for Tooth Loss over a 28-Year Period. J. Dent. Res. 1990, 69, 1126–1130. [Google Scholar] [CrossRef]
- Haugejorden, O.; Klock, K.S.; Trovik, T.A. Incidence and Predictors of Self-Reported Tooth Loss in a Representative Sample of Norwegian Adults. Community Dent. Oral Epidemiol. 2003, 31, 28. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.C.; Paolone, G.; Gherlone, E.; Ferracane, J.L.; Brambilla, E. In Vitro Biofilm Formation on Resin-Based Composites after Different Finishing and Polishing Procedures. J. Dent. 2017, 67, 43–52. [Google Scholar] [CrossRef]
- Polizzi, E.; Tetè, G.; Targa, C.; Salviato, B.; Ferrini, F.; Gastaldi, G. Evaluation of the Effectiveness of the Use of the Diode Laser in the Reduction of the Volume of the Edematous Gingival Tissue after Causal Therapy. Int. J. Environ. Res. Public Health 2020, 17, 6192. [Google Scholar] [CrossRef]
- Tetè, G.; D’Orto, B.; Nagni, M.; Agostinacchio, M.; Polizzi, E.; Agliardi, E. Role of Induced Pluripotent Stem Cells (IPSCS) in Bone Tissue Regeneration in Dentistry: A Narrative Review. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. 3), 1–10. [Google Scholar]
- Capparè, P.; Tetè, G.; Sberna, M.T.; Panina-Bordignon, P. The Emerging Role of Stem Cells in Regenerative Dentistry. Curr. Gene Ther. 2020, 20, 259–268. [Google Scholar] [CrossRef]
- Mace, M.; Richeval, C.; Romanova, L.; Gerard, P.; Duchesne, S.; Cannet, C.; Boyarskikh, I.; Gerault, A.; Zvenigorosky, V.; Nikolaeva, D.; et al. Impact of Socio-Economic Traditions on Current Tobacco and Tea Addictions (Siberia 17th to 20th Century). medRxiv 2021. [Google Scholar] [CrossRef]
- Olyksandrovič. Ugolok Dalekogo Severa [A Little Corner in the Far North]. Sib. Gazeta. Tomsk 1884, 185–188. [Google Scholar]
- Dietrich, T.; Maserejian, N.N.; Joshipura, K.J.; Krall, E.A.; Garcia, R.I. Tobacco Use and Incidence of Tooth Loss among US Male Health Professionals. J. Dent. Res. 2007, 86, 373–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gherlone, E.F.; Capparé, P.; Tecco, S.; Polizzi, E.; Pantaleo, G.; Gastaldi, G.; Grusovin, M.G. A Prospective Longitudinal Study on Implant Prosthetic Rehabilitation in Controlled HIV-Positive Patients with 1-Year Follow-Up: The Role of CD4+ Level, Smoking Habits, and Oral Hygiene. Clin. Implant Dent. Relat. Res. 2016, 18, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, G.; Campus, G.; Strohmenger, L.; Lingström, P. Tobacco and Dental Caries: A Systematic Review. Acta Odontol. Scand. 2013, 71, 363–371. [Google Scholar] [CrossRef]
- Copeland, L.B.; Krall, E.A.; Brown, L.J.; Garcia, R.I.; Streckfus, C.F. Predictors of Tooth Loss in Two US Adult Populations. J. Public Health Dent. 2004, 64, 31–37. [Google Scholar] [CrossRef]
- Koyama, Y.; Kuriyama, S.; Aida, J.; Sone, T.; Nakaya, N.; Ohmori-Matsuda, K.; Hozawa, A.; Tsuji, I. Association between Green Tea Consumption and Tooth Loss: Cross-Sectional Results from the Ohsaki Cohort 2006 Study. Prev. Med. 2010, 50, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Braz-Silva, P.H.; Bergamini, M.L.; Mardegan, A.P.; De Rosa, C.S.; Hasseus, B.; Jonasson, P. Inflammatory Profile of Chronic Apical Periodontitis: A Literature Review. Acta Odontol. Scand. 2019, 77, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.P.; Serpa, M.S.; Sobral, A.P.V.; Arruda, J.A.A.; Silva, L.V.O.; Noronha, M.S.; Kato, C.O.; Mesquita, R.A.; Schuch, L.F.; Gomes, A.P.N.; et al. A Retrospective Multicentre Study of Cystic Lesions and Odontogenic Tumours in Older People. Gerodontology 2018, 35, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Giscard, P.-H.; Turbat, T.; Crubézy, E. Le Premier Empire Des Steppes En Mongolie. Éditions Faton; Edts Faton: Paris, France, 2014; 383p. [Google Scholar]
- Dabernat, H.; Thèves, C.; Bouakaze, C.; Nikolaeva, D.; Keyser, C.; Mokrousov, I.; Géraut, A.; Duchesne, S.; Gérard, P.; Alexeev, A.N.; et al. Tuberculosis Epidemiology and Selection in an Autochthonous Siberian Population from the 16th–19th Century. PLoS ONE 2014, 9, e89877. [Google Scholar] [CrossRef]

| Regions | Central Yakutia | Arctic (Verkhoyansk and Oymyakon) | Vilyuy | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Periods | Below 30 | 30–50 | Above 50 | Below 30 | 30–50 | Above 50 | Below 30 | 30–50 | Above 50 | Below 30 | 30–50 | Above 50 |
| Before 1700 | 0 | 8 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 8 | 6 |
| 1700–1750 | 9 | 13 | 8 | 0 | 6 | 2 | 2 | 1 | 1 | 11 | 20 | 11 |
| 1750–1800 | 2 | 9 | 3 | 4 | 2 | 2 | 0 | 0 | 3 | 6 | 11 | 8 |
| After 1800 | 0 | 3 | 5 | 1 | 3 | 2 | 0 | 0 | 1 | 1 | 6 | 8 |
| Total | 11 | 33 | 19 | 5 | 11 | 6 | 2 | 1 | 8 | 18 | 45 | 33 |
| Tooth | Missing Teeth | Tooth Positions That Can Be Analysed | ||
|---|---|---|---|---|
| N | % | N | % | |
| 18 | 0 | 0.0 | 96 | 100.0 |
| 17 | 1 | 1.0 | 95 | 99.0 |
| 16 | 2 | 2.1 | 94 | 98.0 |
| 15 | 2 | 2.1 | 94 | 98.0 |
| 14 | 2 | 2.1 | 94 | 98.0 |
| 13 | 4 | 4.2 | 92 | 96.0 |
| 12 | 3 | 3.1 | 93 | 96.9 |
| 11 | 3 | 3.1 | 93 | 96.9 |
| 21 | 3 | 3.1 | 93 | 96.9 |
| 22 | 2 | 2.1 | 94 | 97.9 |
| 23 | 3 | 3.1 | 93 | 96.9 |
| 24 | 4 | 4.2 | 92 | 95.8 |
| 25 | 3 | 3.1 | 93 | 96.9 |
| 26 | 2 | 2.1 | 94 | 97.9 |
| 27 | 4 | 4.2 | 92 | 95.8 |
| 28 | 4 | 4.2 | 92 | 95.8 |
| 38 | 2 | 2.1 | 94 | 97.9 |
| 37 | 3 | 3.1 | 93 | 96.9 |
| 36 | 3 | 3.1 | 93 | 96.9 |
| 35 | 3 | 3.1 | 93 | 96.9 |
| 34 | 3 | 3.1 | 93 | 96.9 |
| 33 | 3 | 3.1 | 93 | 96.9 |
| 32 | 3 | 3.1 | 93 | 96.9 |
| 31 | 3 | 3.1 | 93 | 96.9 |
| 41 | 2 | 2.1 | 94 | 97.9 |
| 42 | 2 | 2.1 | 94 | 97.9 |
| 43 | 1 | 1.0 | 95 | 99.0 |
| 44 | 2 | 2.1 | 94 | 97.9 |
| 45 | 2 | 2.1 | 94 | 97.9 |
| 46 | 2 | 2.1 | 94 | 97.9 |
| 47 | 2 | 2.1 | 94 | 97.9 |
| 48 | 2 | 2.1 | 94 | 97.9 |
| Total | 80 | 2992 | ||
| Tooth | Loss a.m | Gran and Cyst | Fracture | Caries | Agenesis | Active.absc. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | |
| 18 | 45 | 46.9 | 3 | 3.1 | 0 | 0.0 | 0 | 0.0 | 14 | 14.6 | 2 | 2.1 |
| 17 | 36 | 37.5 | 5 | 5.2 | 1 | 1.0 | 0 | 0.0 | 1 | 1.0 | 2 | 2.1 |
| 16 | 31 | 32.9 | 6 | 6.2 | 4 | 4.2 | 0 | 0.0 | 0 | 0.0 | 2 | 2.1 |
| 15 | 27 | 28.1 | 2 | 2.1 | 2 | 2.1 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 14 | 27 | 28.1 | 3 | 3.1 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 13 | 29 | 30.2 | 2 | 2.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 12 | 32 | 33.3 | 4 | 4.2 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | 1 | 1.0 |
| 11 | 39 | 40.6 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 21 | 36 | 37.5 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 22 | 36 | 37.5 | 5 | 5.2 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | 1 | 1.0 |
| 23 | 28 | 29.2 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 24 | 28 | 29.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 25 | 25 | 26.0 | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 26 | 36 | 37.5 | 7 | 7.3 | 2 | 2.1 | 0 | 0.0 | 0 | 0.0 | 4 | 4.2 |
| 27 | 35 | 36.5 | 4 | 4.2 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 3 | 3.1 |
| 28 | 41 | 42.7 | 4 | 4.2 | 0 | 0.0 | 0 | 0.0 | 14 | 14.6 | 3 | 3.1 |
| 38 | 28 | 29.2 | 3 | 3.1 | 0 | 0.0 | 0 | 0.0 | 8 | 8.3 | 2 | 2.1 |
| 37 | 33 | 34.4 | 3 | 3.1 | 1 | 1.0 | 1 | 1.0 | 0 | 0.0 | 1 | 1.0 |
| 36 | 27 | 28.1 | 8 | 8.3 | 3 | 3.1 | 1 | 1.0 | 0 | 0.0 | 1 | 1.0 |
| 35 | 21 | 21.9 | 3 | 3.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 34 | 18 | 18.7 | 2 | 2.1 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | 1 | 1.0 |
| 33 | 17 | 17.7 | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 32 | 20 | 20.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 31 | 27 | 28.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 41 | 27 | 28.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 42 | 21 | 21.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 |
| 43 | 17 | 17.7 | 2 | 2.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 44 | 19 | 19.8 | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 45 | 18 | 18.7 | 1 | 1.0 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 46 | 25 | 26.0 | 6 | 6.2 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 47 | 30 | 31.2 | 2 | 2.1 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 48 | 29 | 30.2 | 2 | 2.1 | 0 | 0.0 | 0 | 0.0 | 13 | 13.5 | 1 | 1.0 |
| Total | 908 | 80 | 21 | 2 | 54 | 36 | ||||||
| Tooth | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| 18 | 16 | 16.7 | 9 | 9.4 | 7 | 7.3 | 2 | 2.9 | 0 | 0.0 |
| 17 | 8 | 8.3 | 26 | 27.1 | 19 | 19.8 | 3 | 3.1 | 0 | 0.0 |
| 16 | 1 | 1.0 | 18 | 18.7 | 31 | 32.3 | 10 | 10.4 | 3 | 3.1 |
| 15 | 4 | 4.2 | 31 | 32.3 | 19 | 19.8 | 10 | 10.4 | 3 | 3.1 |
| 14 | 4 | 4.2 | 24 | 25.0 | 18 | 18.7 | 13 | 13.5 | 7 | 7.3 |
| 13 | 2 | 2.1 | 13 | 13.6 | 26 | 27.1 | 18 | 18.7 | 4 | 4.2 |
| 12 | 0 | 0.0 | 6 | 6.2 | 25 | 26.0 | 23 | 24.0 | 4 | 4.2 |
| 11 | 1 | 1.0 | 4 | 4.2 | 21 | 21.9 | 24 | 25.0 | 4 | 4.2 |
| 21 | 1 | 1.0 | 4 | 4.2 | 23 | 24.0 | 25 | 26.0 | 3 | 3.1 |
| 22 | 0 | 0.0 | 5 | 5.2 | 25 | 26.0 | 24 | 25.0 | 3 | 3.1 |
| 23 | 2 | 2.1 | 9 | 9.4 | 29 | 30.2 | 19 | 19.8 | 5 | 5.2 |
| 24 | 2 | 2.1 | 26 | 27.1 | 15 | 15.6 | 16 | 16.7 | 3 | 3.1 |
| 25 | 4 | 4.2 | 27 | 28.1 | 20 | 20.8 | 14 | 14.6 | 2 | 2.1 |
| 26 | 1 | 1.0 | 16 | 16.7 | 29 | 30.2 | 8 | 8.3 | 4 | 4.2 |
| 27 | 7 | 7.3 | 24 | 25.0 | 17 | 17.7 | 7 | 7.3 | 0 | 0.0 |
| 28 | 11 | 11.5 | 11 | 11.5 | 6 | 6.2 | 4 | 4.2 | 0 | 0.0 |
| 38 | 22 | 22.9 | 22 | 22.9 | 10 | 10.4 | 2 | 2.1 | 1 | 1.0 |
| 37 | 8 | 8.3 | 27 | 28.1 | 23 | 24.0 | 1 | 1.0 | 1 | 1.0 |
| 36 | 0 | 0.0 | 18 | 18.7 | 31 | 32.3 | 14 | 14.6 | 3 | 3.1 |
| 35 | 6 | 6.2 | 20 | 20.8 | 33 | 34.4 | 12 | 12.5 | 0 | 0.0 |
| 34 | 3 | 3.1 | 21 | 21.9 | 34 | 35.4 | 14 | 14.6 | 1 | 1.0 |
| 33 | 2 | 2.1 | 11 | 11.4 | 34 | 35.4 | 26 | 27.1 | 2 | 2.1 |
| 32 | 1 | 1.0 | 6 | 6.2 | 36 | 37.5 | 28 | 29.2 | 1 | 1.0 |
| 31 | 0 | 0.0 | 5 | 5.2 | 32 | 33.3 | 24 | 25.0 | 3 | 3.1 |
| 41 | 0 | 0.0 | 6 | 6.2 | 30 | 31.2 | 22 | 22.9 | 4 | 4.2 |
| 42 | 0 | 0.0 | 8 | 8.3 | 34 | 35.4 | 28 | 29.2 | 2 | 2.1 |
| 43 | 2 | 2.1 | 12 | 12.5 | 35 | 36.5 | 26 | 27.1 | 2 | 2.1 |
| 44 | 3 | 3.1 | 27 | 28.1 | 30 | 31.2 | 11 | 11.5 | 3 | 3.1 |
| 45 | 7 | 7.3 | 27 | 28.1 | 30 | 31.2 | 10 | 10.4 | 1 | 1.0 |
| 46 | 1 | 1.0 | 22 | 22.9 | 29 | 30.2 | 13 | 13.5 | 4 | 4.2 |
| 47 | 12 | 12.5 | 26 | 27.1 | 18 | 18.7 | 7 | 7.3 | 0 | 0.0 |
| 48 | 24 | 25.0 | 14 | 14.6 | 7 | 7.3 | 4 | 4.2 | 1 | 1.0 |
| Total | 155 | 525 | 776 | 462 | 74 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crubézy, E.; Duchesne, S.; Razafindrazaka, H.; Romanova, L.; Gérard, P.; Alcouffe, A.; Esclassan, R.; Melnichuk, O.; Ushnitsky, I.; Ludes, B.; et al. Sucrose Is Not the Whole Story: Risk Factors and Oral Health at the Contact (Yakutia, Siberia-16th/19th). Biology 2021, 10, 974. https://doi.org/10.3390/biology10100974
Crubézy E, Duchesne S, Razafindrazaka H, Romanova L, Gérard P, Alcouffe A, Esclassan R, Melnichuk O, Ushnitsky I, Ludes B, et al. Sucrose Is Not the Whole Story: Risk Factors and Oral Health at the Contact (Yakutia, Siberia-16th/19th). Biology. 2021; 10(10):974. https://doi.org/10.3390/biology10100974
Chicago/Turabian StyleCrubézy, Eric, Sylvie Duchesne, Harilanto Razafindrazaka, Liubomira Romanova, Patrice Gérard, Ameline Alcouffe, Rémi Esclassan, Olga Melnichuk, Innokenty Ushnitsky, Bertrand Ludes, and et al. 2021. "Sucrose Is Not the Whole Story: Risk Factors and Oral Health at the Contact (Yakutia, Siberia-16th/19th)" Biology 10, no. 10: 974. https://doi.org/10.3390/biology10100974








