Sex Determination and Differentiation in Teleost: Roles of Genetics, Environment, and Brain
Abstract
Simple Summary
Abstract
1. Introduction
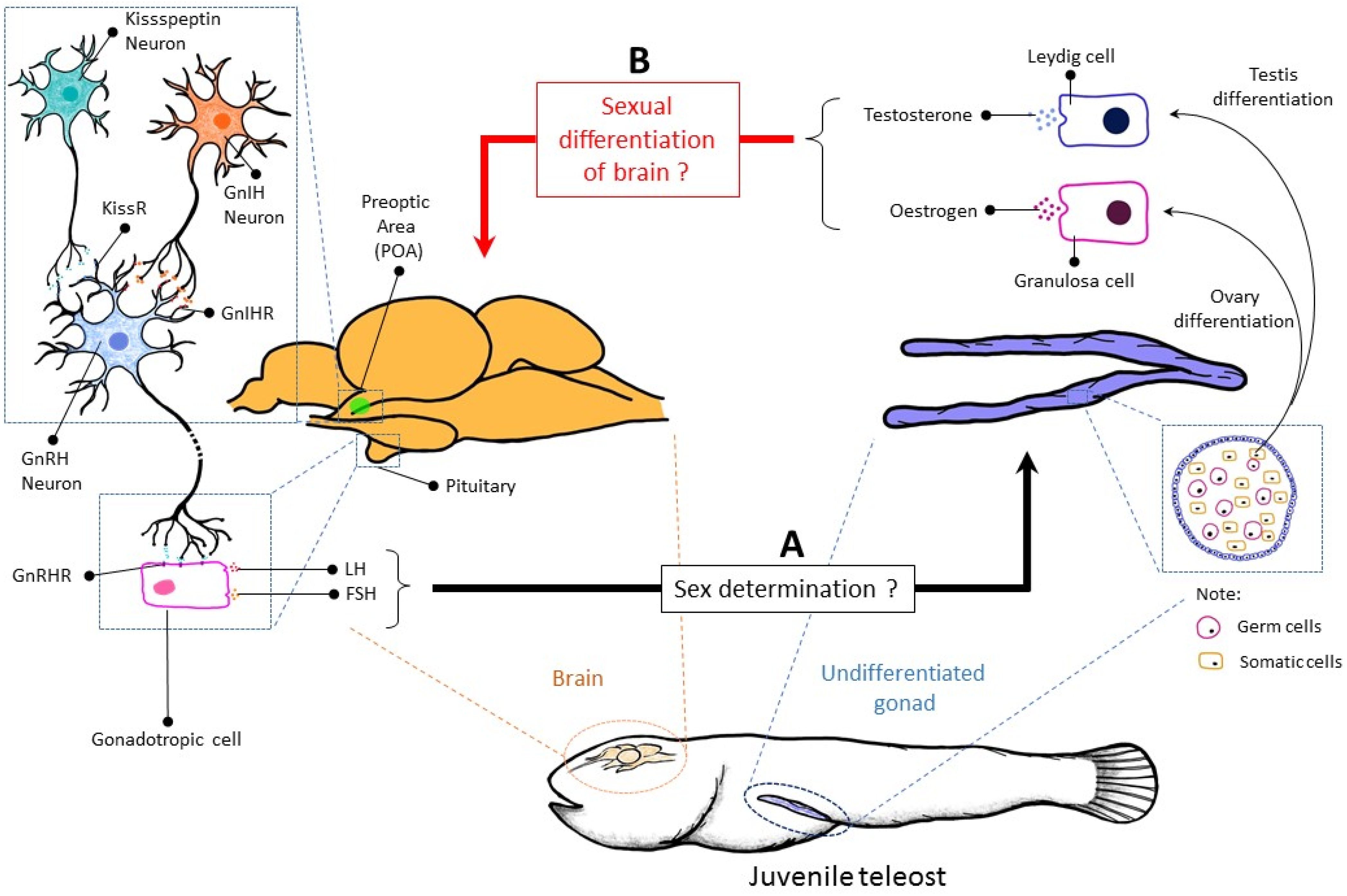
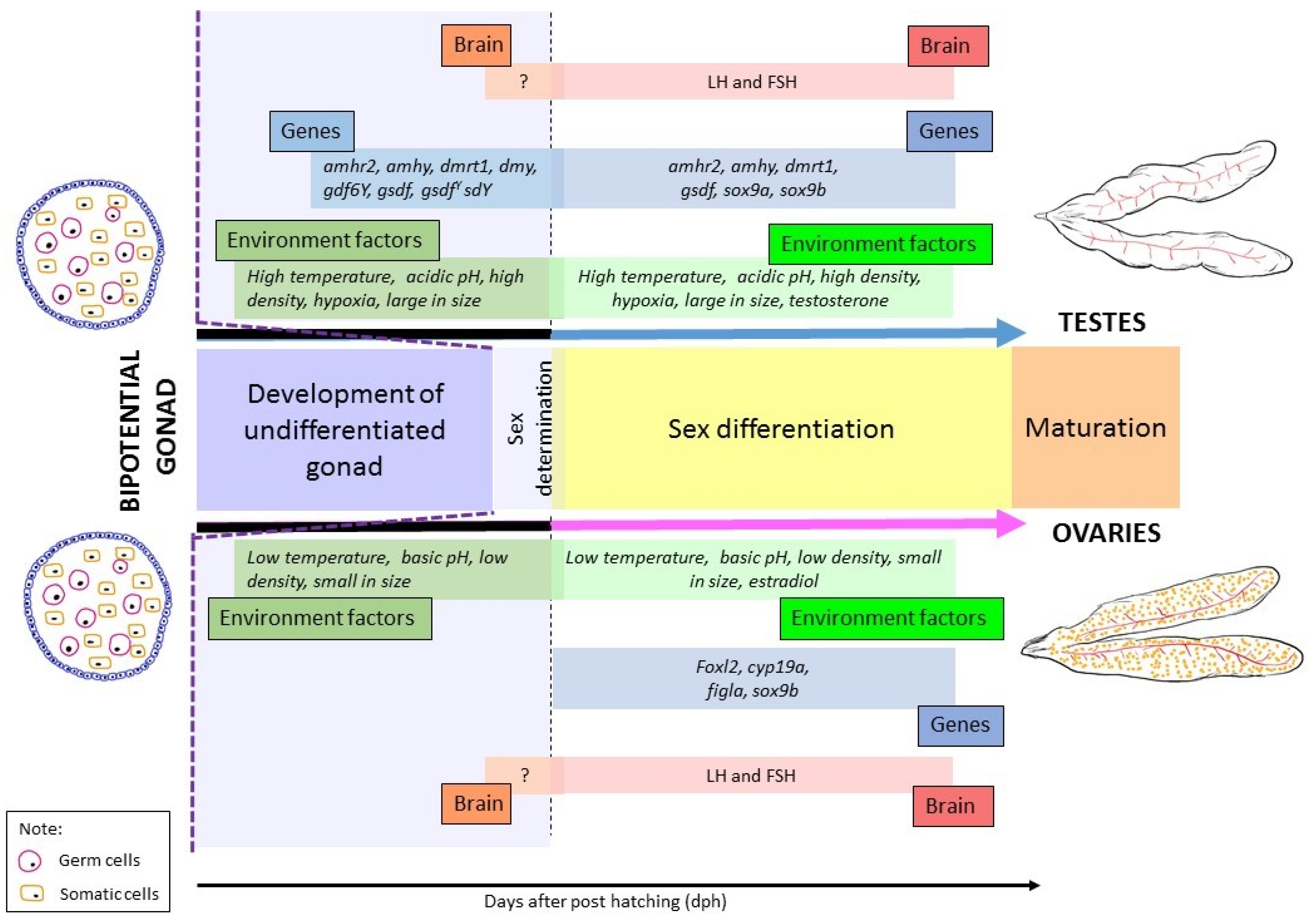
2. Regulation of Sex Determination
2.1. Genetics
2.1.1. The Anti-Müllerian Hormone Receptor Type 2 (amhr2)
2.1.2. The Y-Linked Anti-Müllerian Hormone (amhy)
2.1.3. The Doublesex and Mab-3 Related Transcription Factor (dmrt1)
2.1.4. The DM-domain on the Y-Chromosome (dmy/dmrt1by)
2.1.5. Growth Differentiation Factor 6 on the Y-Chromosome (gdf6Y)
2.1.6. The Gonadal Soma Derived Factor (gsdf) and the Gonadal Soma Derived Factor on the Y-Chromosome (gsdfY)
2.1.7. Sexually Dimorphic on the Y-Chromosome (sdY)
2.1.8. SRY-Related HMG Box 3 on the Y-Chromosome (sox3Y)
2.2. Environment
2.2.1. Temperature
2.2.2. pH
2.2.3. Density and Hypoxia
2.2.4. Social Interactions
2.3. Brain
3. Regulation of Sex Differentiation
3.1. Genetics
3.1.1. The Anti-Mullerian Hormone (amh) and Amh Receptor 2 (amhr2)
3.1.2. The Doublesex and Mab-3 Related Transcription Factor (dmrt1)
3.1.3. Aromatase (cyp19)
3.1.4. The Forkhead Box L2 (foxl2)
3.1.5. Factor in the Germline Alpha (figla)
3.1.6. The Gonadal Soma Derived Factor (gsdf)
3.1.7. SRY-Related HMG Box 9 (sox9)
3.2. Environment
3.2.1. Sex Hormones
3.2.2. Temperature
3.2.3. pH
3.2.4. Social Factors
3.2.5. Density
3.2.6. Hypoxia
3.3. Brain
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Nishimura, T.; Tanaka, M. Gonadal development in fish. Sex. Dev. 2014, 8, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Chakraborty, T.; Paul-Prasanth, B.; Ohta, K.; Nakamura, M. Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiol. Rev. 2021, 101, 1237–1308. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Hamaguchi, S. Novel sex-determining genes in fish and sex chromosome evolution. Dev. Dyn. 2013, 242, 339–353. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Zhao, J.; Shi, H.; Zeng, S.; Ye, K.; Jiang, D.; Zhou, L.; Sun, L.; Tao, W. A tandem duplicate of anti-Müllerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile tilapia, Oreochromis niloticus. PLoS Genet. 2015, 11, e1005678. [Google Scholar] [CrossRef]
- Mustapha, U.F.; Jiang, D.-N.; Liang, Z.-H.; Gu, H.-T.; Yang, W.; Chen, H.-P.; Deng, S.-P.; Wu, T.-L.; Tian, C.-X.; Zhu, C.-H. Male-specific Dmrt1 is a candidate sex determination gene in spotted scat (Scatophagus argus). Aquaculture 2018, 495, 351–358. [Google Scholar] [CrossRef]
- Matsuda, M.; Nagahama, Y.; Shinomiya, A.; Sato, T.; Matsuda, C.; Kobayashi, T.; Morrey, C.E.; Shibata, N.; Asakawa, S.; Shimizu, N. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 2002, 417, 559–563. [Google Scholar] [CrossRef]
- Reichwald, K.; Petzold, A.; Koch, P.; Downie, B.R.; Hartmann, N.; Pietsch, S.; Baumgart, M.; Chalopin, D.; Felder, M.; Bens, M. Insights into sex chromosome evolution and aging from the genome of a short-lived fish. Cell 2015, 163, 1527–1538. [Google Scholar] [CrossRef]
- Sawatari, E.; Shikina, S.; Takeuchi, T.; Yoshizaki, G. A novel transforming growth factor-β superfamily member expressed in gonadal somatic cells enhances primordial germ cell and spermatogonial proliferation in rainbow trout (Oncorhynchus mykiss). Dev. Biol. 2007, 301, 266–275. [Google Scholar] [CrossRef]
- Myosho, T.; Otake, H.; Masuyama, H.; Matsuda, M.; Kuroki, Y.; Fujiyama, A.; Naruse, K.; Hamaguchi, S.; Sakaizumi, M. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics 2012, 191, 163–170. [Google Scholar] [CrossRef]
- Quéméré, E.; Perrier, C.; Besnard, A.-L.; Evanno, G.; Bagliniere, J.-L.; Guiguen, Y.; Launey, S. An improved PCR-based method for faster sex determination in brown trout (Salmo trutta) and Atlantic salmon (Salmo salar). Conserv. Genet. Resour. 2014, 6, 825–827. [Google Scholar] [CrossRef]
- Lau, E.S.-W.; Zhang, Z.; Qin, M.; Ge, W. Knockout of Zebrafish Ovarian Aromatase Gene (cyp19a1a) by TALEN and CRISPR/Cas9 Leads to All-male Offspring Due to Failed Ovarian Differentiation. Sci. Rep. 2016, 6, 37357. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, F.; Standke, A.; Gutzeit, H.O. The role of Amh signaling in teleost fish–Multiple functions not restricted to the gonads. Gen. Comp. Endocrinol. 2015, 223, 87–107. [Google Scholar] [CrossRef]
- Wu, G.-C.; Chiu, P.-C.; Lyu, Y.-S.; Chang, C.-F. The expression of amh and amhr2 is associated with the development of gonadal tissue and sex change in the protandrous black porgy, Acanthopagrus schlegeli. Biol. Reprod. 2010, 83, 443–453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Hattori, R.S.; Sarida, M.; García, E.L.; Strüssmann, C.A.; Yamamoto, Y. Expression profiles of amhy and major sex-related genes during gonadal sex differentiation and their relation with genotypic and temperature-dependent sex determination in pejerrey Odontesthes bonariensis. Gen. Comp. Endocrinol. 2018, 265, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-H.; Yang, H.-H.; Li, M.-R.; Sun, Y.-L.; Jiang, X.-L.; Xie, Q.-P.; Wang, T.-R.; Shi, H.-J.; Sun, L.-N.; Zhou, L.-Y. Antagonistic roles of Dmrt1 and Foxl2 in sex differentiation via estrogen production in tilapia as demonstrated by TALENs. Endocrinology 2013, 154, 4814–4825. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Tsai, Y.-J.; Chang, C.-F. The roles of Cyp19ala and DMRT1 during gonadal sex differentiation and sex change in Orange-spotted grouper, Epinephelus coioides. J. Mar. Sci. Technol. 2019, 27, 11. [Google Scholar]
- Rashid, H.; Kitano, H.; Lee, K.H.; Nii, S.; Shigematsu, T.; Kadomura, K.; Yamaguchi, A.; Matsuyama, M. Fugu (Takifugu rubripes) sexual differentiation: CYP19 regulation and aromatase inhibitor induced testicular development. Sex. Dev. 2007, 1, 311–322. [Google Scholar] [CrossRef]
- Fajkowska, M.; Ostaszewska, T.; Rzepkowska, M. Molecular mechanisms of sex differentiation in sturgeons. Rev. Aquac. 2020, 12, 1003–1027. [Google Scholar] [CrossRef]
- Liang, S.; Wang, W.; Wang, L.; Wu, Z.; Zou, Y.; Tan, X.; Liu, Y.; Peng, Z.; You, F. Figla gene roles in the proliferation of oocytes in the olive flounder Paralichthys olivaceus. Aquaculture 2020, 528, 735493. [Google Scholar] [CrossRef]
- Inaba, H.; Hara, S.; Horiuchi, M.; Ijiri, S.; Kitano, T. Gonadal expression profiles of sex-specific genes during early sexual differentiation in Japanese eel Anguilla japonica. Fish. Sci. 2021, 87, 203–209. [Google Scholar] [CrossRef]
- Martinez-Bengochea, A.; Doretto, L.; Rosa, I.; Oliveira, M.; Silva, C.; Silva, D.; Santos, G.; Santos, J.; Avelar, M.; Silva, L. Effects of 17β-estradiol on early gonadal development and expression of genes implicated in sexual differentiation of a South American teleost, Astyanax altiparanae. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 248, 110467. [Google Scholar] [CrossRef]
- Graves, J.A.M.; Peichel, C.L. Are homologies in vertebrate sex determination due to shared ancestry or to limited options? Genome Biol. 2010, 11, 1–12. [Google Scholar]
- McClelland, K.; Bowles, J.; Koopman, P. Male sex determination: Insights into molecular mechanisms. Asian J. Androl. 2012, 14, 164. [Google Scholar] [CrossRef]
- Hara, S.; Furukawa, F.; Mukai, K.; Yazawa, T.; Kitano, T. Peroxisome proliferator-activated receptor alpha is involved in the temperature-induced sex differentiation of a vertebrate. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Römer, U.; Beisenherz, W. Environmental determination of sex in Apistogramma (Cichlidae) and two other freshwater fishes (Teleost). J. Fish Biol. 1996, 48, 714–725. [Google Scholar]
- Ramee, S.W.; Lipscomb, T.N.; DiMaggio, M.A. Evaluation of the effect of larval stocking density, salinity, and temperature on stress response and sex differentiation in the Dwarf Gourami and Rosy Barb. Aquac. Rep. 2020, 16, 100287. [Google Scholar] [CrossRef]
- Robertson, C.E.; Wright, P.A.; Köblitz, L.; Bernier, N.J. Hypoxia-inducible factor-1 mediates adaptive developmental plasticity of hypoxia tolerance in zebrafish, Danio rerio. Proc. R. Soc. B: Biol. Sci. 2014, 281, 20140637. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, F.; Kurth, T.; Meißner, S.; Standke, A.; Hoppe, M.; Zieschang, F.; Reitmayer, C.; Göbel, A.; Kretzschmar, G.; Gutzeit, H.O. The social status of the male Nile tilapia (Oreochromis niloticus) influences testis structure and gene expression. Reproduction 2012, 143, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Miyazoe, D.; Nishiike, Y. A conceptual framework for understanding sexual differentiation of the teleost brain. Gen. Comp. Endocrinol. 2019, 284, 113129. [Google Scholar] [CrossRef]
- Feng, K.; Cui, X.; Song, Y.; Tao, B.; Chen, J.; Wang, J.; Liu, S.; Sun, Y.; Zhu, Z.; Trudeau, V.L.; et al. Gnrh3 Regulates PGC Proliferation and Sex Differentiation in Developing Zebrafish. Endocrinology 2019, 161, bqz024. [Google Scholar] [CrossRef]
- Li, J.; Ge, W. Zebrafish as a model for studying ovarian development: Recent advances from targeted gene knockout studies. Mol. Cell. Endocrinol. 2020, 507, 110778. [Google Scholar] [CrossRef]
- Bej, D.K.; Miyoshi, K.; Hattori, R.S.; Strüssmann, C.A.; Yamamoto, Y. A duplicated, truncated amh gene is involved in male sex determination in an old world silverside. G3 Genes Genomes Genet. 2017, 7, 2489–2495. [Google Scholar] [CrossRef]
- Pan, Q.; Feron, R.; Yano, A.; Guyomard, R.; Jouanno, E.; Vigouroux, E.; Wen, M.; Busnel, J.-M.; Bobe, J.; Concordet, J.-P. Identification of the master sex determining gene in Northern pike (Esox lucius) reveals restricted sex chromosome differentiation. PLoS Genet. 2019, 15, e1008013. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Xie, Y.; Sun, M.; Li, X.; Fitzpatrick, C.K.; Vaux, F.; O’Malley, K.G.; Zhang, Q.; Qi, J.; He, Y. A duplicated amh is the master sex-determining gene for Sebastes rockfish in the Northwest Pacific. Open Biol. 2021, 11, 210063. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, Y.; Wang, W.; Wang, Q.; Zhang, N.; Lin, F.; Wang, N.; Shao, C.; Dong, Z.; Li, Y. Genome editing reveals dmrt1 as an essential male sex-determining gene in Chinese tongue sole (Cynoglossus semilaevis). Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Yano, A.; Nicol, B.; Jouanno, E.; Guiguen, Y. Heritable targeted inactivation of the rainbow trout (Oncorhynchus mykiss) master sex-determining gene using zinc-finger nucleases. Mar. Biotechnol. 2014, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yano, A.; Nicol, B.; Jouanno, E.; Quillet, E.; Fostier, A.; Guyomard, R.; Guiguen, Y. The sexually dimorphic on the Y-chromosome gene (sdY) is a conserved male-specific Y-chromosome sequence in many salmonids. Evol. Appl. 2013, 6, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Mazen, I.; El-Gammal, M.; McElreavey, K.; Elaidy, A.; Abdel-Hamid, M.S. Novel AMH and AMHR2 mutations in two Egyptian families with persistent müllerian duct syndrome. Sex. Dev. 2017, 11, 29–33. [Google Scholar] [CrossRef]
- Kamiya, T.; Kai, W.; Tasumi, S.; Oka, A.; Matsunaga, T.; Mizuno, N.; Fujita, M.; Suetake, H.; Suzuki, S.; Hosoya, S. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genet. 2012, 8, e1002798. [Google Scholar] [CrossRef]
- Zheng, S.; Long, J.; Liu, Z.; Tao, W.; Wang, D. Identification and evolution of TGF-β signaling pathway members in twenty-four animal species and expression in Tilapia. Int. J. Mol. Sci. 2018, 19, 1154. [Google Scholar] [CrossRef]
- Curzon, A.; Shirak, A.; Dor, L.; Zak, T.; Perelberg, A.; Seroussi, E.; Ron, M. A duplication of the Anti-Müllerian hormone gene is associated with genetic sex determination of different Oreochromis niloticus strains. Heredity 2020, 125, 317–327. [Google Scholar] [CrossRef]
- Carlsson, I.B.; Scott, J.E.; Visser, J.; Ritvos, O.; Themmen, A.; Hovatta, O. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum. Reprod. 2006, 21, 2223–2227. [Google Scholar] [CrossRef] [PubMed]
- Adolfi, M.C.; Nakajima, R.T.; Nobrega, R.H.; Schartl, M. Intersex, Hermaphroditism, and Gonadal Plasticity in Vertebrates: Evolution of the Mullerian Duct and Amh/Amhr2 Signaling. Annu. Rev. Anim. Biosci 2019, 7, 149–172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.N.; Yang, H.H.; Li, M.H.; Shi, H.J.; Zhang, X.B.; Wang, D.S. gsdf is a downstream gene of dmrt1 that functions in the male sex determination pathway of the Nile tilapia. Mol. Reprod. Dev. 2016, 83, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Hattori, R.S.; Murai, Y.; Oura, M.; Masuda, S.; Majhi, S.K.; Sakamoto, T.; Fernandino, J.I.; Somoza, G.M.; Yokota, M.; Strüssmann, C.A. A Y-linked anti-Müllerian hormone duplication takes over a critical role in sex determination. Proc. Natl. Acad. Sci. USA 2012, 109, 2955–2959. [Google Scholar] [CrossRef] [PubMed]
- Mawaribuchi, S.; Ito, Y.; Ito, M. Independent evolution for sex determination and differentiation in the DMRT family in animals. Biol. Open 2019, 8, bio041962. [Google Scholar] [CrossRef]
- Nanda, I.; Kondo, M.; Hornung, U.; Asakawa, S.; Winkler, C.; Shimizu, A.; Shan, Z.; Haaf, T.; Shimizu, N.; Shima, A. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc. Natl. Acad. Sci. USA 2002, 99, 11778–11783. [Google Scholar] [CrossRef]
- Forconi, M.; Canapa, A.; Barucca, M.; Biscotti, M.A.; Capriglione, T.; Buonocore, F.; Fausto, A.M.; Makapedua, D.M.; Pallavicini, A.; Gerdol, M. Characterization of sex determination and sex differentiation genes in Latimeria. PLoS ONE 2013, 8, e56006. [Google Scholar]
- Crespo, B.; Gómez, A.; Mazón, M.J.; Carrillo, M.; Zanuy, S. Isolation and characterization of Ff1 and Gsdf family genes in European sea bass and identification of early gonadal markers of precocious puberty in males. Gen. Comp. Endocrinol. 2013, 191, 155–167. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chiba, A.; Sato, T.; Myosho, T.; Yamamoto, J.; Okamura, T.; Onishi, Y.; Sakaizumi, M.; Hamaguchi, S.; Iguchi, T. Estrogen alters gonadal soma-derived factor (Gsdf)/Foxl2 expression levels in the testes associated with testis-ova differentiation in adult medaka, Oryzias latipes. Aquat. Toxicol. 2017, 191, 209–218. [Google Scholar] [CrossRef]
- Shibata, Y.; Paul-Prasanth, B.; Suzuki, A.; Usami, T.; Nakamoto, M.; Matsuda, M.; Nagahama, Y. Expression of gonadal soma derived factor (GSDF) is spatially and temporally correlated with early testicular differentiation in medaka. Gene Expr. Patterns 2010, 10, 283–289. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, G.; Li, M.; Zhu, F.; Liu, Q.; Naruse, K.; Herpin, A.; Nagahama, Y.; Li, J.; Hong, Y. Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Bertho, S.; Herpin, A.; Branthonne, A.; Jouanno, E.; Yano, A.; Nicol, B.; Muller, T.; Pannetier, M.; Pailhoux, E.; Miwa, M. The unusual rainbow trout sex determination gene hijacked the canonical vertebrate gonadal differentiation pathway. Proc. Natl. Acad. Sci. USA 2018, 115, 12781–12786. [Google Scholar] [CrossRef]
- Gubbay, J.; Collignon, J.; Koopman, P.; Capel, B.; Economou, A.; Münsterberg, A.; Vivian, N.; Goodfellow, P.; Lovell-Badge, R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 1990, 346, 245–250. [Google Scholar] [CrossRef]
- Sinclair, A.H.; Berta, P.; Palmer, M.S.; Hawkins, J.R.; Griffiths, B.L.; Smith, M.J.; Foster, J.W.; Frischauf, A.-M.; Lovell-Badge, R.; Goodfellow, P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990, 346, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.W.; Graves, J.A. An SRY-related sequence on the marsupial X chromosome: Implications for the evolution of the mammalian testis-determining gene. Proc. Natl. Acad. Sci. USA 1994, 91, 1927–1931. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.; Hughes, J.; White, S.; Sekido, R.; Tan, J.; Arboleda, V.; Rogers, N.; Knower, K.; Rowley, L.; Eyre, H.; et al. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J. Clin. Investig. 2011, 121, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Takehana, Y.; Matsuda, M.; Myosho, T.; Suster, M.L.; Kawakami, K.; Shin, T.; Kohara, Y.; Kuroki, Y.; Toyoda, A.; Fujiyama, A. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Senthilkumaran, B. Expression analysis of sox3 during testicular development, recrudescence, and after hCG induction in catfish, Clarias batrachus. Sex. Dev. 2014, 8, 376–386. [Google Scholar] [CrossRef]
- Herpin, A.; Schartl, M. Plasticity of gene-regulatory networks controlling sex determination: Of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Rep. 2015, 16, 1260–1274. [Google Scholar] [CrossRef]
- Hong, Q.; Li, C.; Ying, R.; Lin, H.; Li, J.; Zhao, Y.; Cheng, H.; Zhou, R. Loss-of-function of sox3 causes follicle development retardation and reduces fecundity in zebrafish. Protein Cell 2019, 10, 347–364. [Google Scholar] [CrossRef]
- Navara, K.J. Mechanisms of environmental sex determination in fish, amphibians, and reptiles. In Choosing Sexes; Springer: Cham, Switzerland, 2018; pp. 213–240. [Google Scholar]
- Yamamoto, Y.; Hattori, R.S.; Patiño, R.; Strüssmann, C.A. Environmental regulation of sex determination in fishes: Insights from Atheriniformes. Curr. Top. Dev. Biol. 2019, 134, 49–69. [Google Scholar]
- Bhattacharya, I.; Modi, D. Sex Determination in Teleost Fish. In Recent Updates in Molecular Endocrinology and Reproductive Physiology of Fish; Springer: Singapore, 2021; pp. 121–138. [Google Scholar]
- Santi, S.; Gennotte, V.; Muller, M.; Melard, C.; Toguyeni, A.; Mandiki, S.N.; Rougeot, C. Sex-ratio, early sex steroid profiles and cyp19a1b, dmrt1 and foxl2 gene expressions upon high temperature treatment of undifferentiated African catfish juveniles (Clarias gariepinus). Aquaculture 2019, 499, 140–148. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kajiura-Kobayashi, H.; Guan, G.; Nagahama, Y. Sexual dimorphic expression of DMRT1 and Sox9a during gonadal differentiation and hormone-induced sex reversal in the teleost fish Nile tilapia (Oreochromis niloticus). Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008, 237, 297–306. [Google Scholar]
- Navarro-Martín, L.; Viñas, J.; Ribas, L.; Díaz, N.; Gutiérrez, A.; Di Croce, L.; Piferrer, F. DNA Methylation of the Gonadal Aromatase (cyp19a) Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass. PLOS Genet. 2011, 7, e1002447. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Sun, L.X.; Zhu, J.J.; Zhao, Y.; Wang, H.; Liu, H.J.; Ji, X.S. Epigenetic control of cyp19a1a expression is critical for high temperature induced Nile tilapia masculinization. J. Therm. Biol. 2017, 69, 76–84. [Google Scholar] [CrossRef]
- Reddon, A.R.; Hurd, P.L. Water pH during early development influences sex ratio and male morph in a West African cichlid fish, Pelvicachromis pulcher. Zoology 2013, 116, 139–143. [Google Scholar] [CrossRef]
- Baroiller, J.-F.; D’Cotta, H.; Saillant, E. Environmental effects on fish sex determination and differentiation. Sex. Dev. 2009, 3, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.A. Effect of pH on sex ratio in cichlids and a poecilliid (Teleost). Copeia 1985, 1985, 233–235. [Google Scholar] [CrossRef]
- Davey, A.J.; Jellyman, D.J. Sex determination in freshwater eels and management options for manipulation of sex. Rev. Fish Biol. Fish. 2005, 15, 37–52. [Google Scholar] [CrossRef]
- Tzeng, W.N.; Han, Y.S.; He, J.T. The sex ratios and growth strategies of wild and captive Japanese eels Anguilla japonica. In Developments in Understanding Fish Growth. International Congress on the Biology of Fish; Small, B., MacKinlay, D., Eds.; University of British Columbia: Vancouver, BC, Canada, 2002; pp. 25–42. [Google Scholar]
- Abdel-Tawwab, M.; Hagras, A.E.; Elbaghdady, H.A.M.; Monier, M.N. Dissolved oxygen level and stocking density effects on growth, feed utilization, physiology, and innate immunity of Nile Tilapia, Oreochromis niloticus. J. Appl. Aquac. 2014, 26, 340–355. [Google Scholar] [CrossRef]
- Strüssmann, C.A.; Yamamoto, Y.; Hattori, R.S.; Fernandino, J.I.; Somoza, G.M. Where the Ends Meet: An Overview of Sex Determination in Atheriniform Fishes. Sex. Dev. 2021, 15, 1–13. [Google Scholar] [CrossRef]
- Bailey, R.C.; Young, V.H.; Keenleyside, M.H. Variation in the mating system and associated parental behaviour of captive and free-living Cichlasoma nigrofasciatum (Pisces, Cichlidae). Behaviour 1990, 112, 202–220. [Google Scholar] [CrossRef]
- Oldfield, R.G.; McCrary, J.; McKaye, K.R. Habitat use, social behavior, and female and male size distributions of juvenile Midas cichlids, Amphilophus cf. citrinellus, in Lake Apoyo, Nicaragua. Caribb. J. Sci. 2006, 42, 197. [Google Scholar]
- Berejikian, B.A.; Tezak, E.P.; Schroder, S.L.; Flagg, T.A.; Knudsen, C.M. Competitive differences between newly emerged offspring of captive-reared and wild coho salmon. Trans. Am. Fish. Soc. 1999, 128, 832–839. [Google Scholar] [CrossRef]
- Dwyer, A.A.; Quinton, R. Anatomy and Physiology of the Hypothalamic-Pituitary-Gonadal (HPG) Axis. In Advanced Practice in Endocrinology Nursing; Springer: Berlin/Heidelberg, Germany, 2019; pp. 839–852. [Google Scholar]
- Kumar, P.; Behera, P.; Christina, L.; Kailasam, M. Sex Hormones and Their Role in Gonad Development and Reproductive Cycle of Fishes. In Recent Updates in Molecular Endocrinology and Reproductive Physiology of Fish; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–22. [Google Scholar]
- Shivers, B.; Harlan, R.; Morrell, J.; Pfaff, D. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature 1983, 304, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, E. Rapid direct action of estradiol in GnRH neurons: Findings and implications. Front. Endocrinol. 2012, 2, 106. [Google Scholar]
- Ogawa, S.; Parhar, I.S. Single-cell gene profiling reveals social status-dependent modulation of nuclear hormone receptors in GnRH neurons in a male cichlid fish. Int. J. Mol. Sci. 2020, 21, 2724. [Google Scholar] [CrossRef]
- Chang, J.P.; Pemberton, J.G. Comparative aspects of GnRH-Stimulated signal transduction in the vertebrate pituitary–Contributions from teleost model systems. Mol. Cell. Endocrinol. 2018, 463, 142–167. [Google Scholar] [CrossRef]
- Ye, M.; Chen, Y. Zebrafish as an emerging model to study gonad development. Comput. Struct. Biotechnol. J. 2020, 18, 2373. [Google Scholar] [CrossRef]
- Son, Y.L.; Ubuka, T.; Tsutsui, K. Molecular Mechanisms of Gonadotropin-Inhibitory Hormone (GnIH) Actions in Target Cells and Regulation of GnIH Expression. Front. Endocrinol. 2019, 10, 110. [Google Scholar] [CrossRef]
- Filby, A.L.; Aerle, R.v.; Duitman, J.; Tyler, C.R. The Kisspeptin/Gonadotropin-Releasing Hormone Pathway and Molecular Signaling of Puberty in Fish. Biol. Reprod. 2008, 78, 278–289. [Google Scholar] [CrossRef]
- Behringer, R.R.; Finegold, M.J.; Cate, R.L. Müllerian-inhibiting substance function during mammalian sexual development. Cell 1994, 79, 415–425. [Google Scholar] [CrossRef]
- Josso, N.; Racine, C.; di Clemente, N.; Rey, R.; Xavier, F. The role of anti-Müllerian hormone in gonadal development. Mol. Cell. Endocrinol. 1998, 145, 3–7. [Google Scholar] [CrossRef]
- Wang, X.; Orban, L. Anti-Müllerian hormone and 11 β-hydroxylase show reciprocal expression to that of aromatase in the transforming gonad of zebrafish males. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2007, 236, 1329–1338. [Google Scholar] [CrossRef]
- Yan, Y.-L.; Batzel, P.; Titus, T.; Sydes, J.; Desvignes, T.; BreMiller, R.; Draper, B.; Postlethwait, J.H. A Hormone That Lost Its Receptor: Anti-Müllerian Hormone (AMH) in Zebrafish Gonad Development and Sex Determination. Genetics 2019, 213, 529. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Germline stem cells are critical for sexual fate decision of germ cells. Bioessays 2016, 38, 1227–1233. [Google Scholar] [CrossRef]
- Guan, G.; Xu, S.; Guo, A.; Zhao, X.; Zhang, Y.; Sun, K.; Kang, Y.; Chang, Y.; Wu, X.; Chen, L. A compromised gsdf signaling leads to gamatogenesis confusion and subfertility in medaka. bioRxiv 2018, 238436. [Google Scholar] [CrossRef]
- Yan, Y.-L.; Desvignes, T.; Bremiller, R.; Wilson, C.; Dillon, D.; High, S.; Draper, B.; Buck, C.L.; Postlethwait, J. Gonadal soma controls ovarian follicle proliferation through Gsdf in zebrafish. Dev. Dyn. 2017, 246, 925–945. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Froschauer, A.; Kitano, A.; Nanda, I.; Hornung, U.; Volff, J.-N.; Asakawa, S.; Mitani, H.; Naruse, K.; Tanaka, M. Molecular cloning and characterization of DMRT genes from the medaka Oryzias latipes and the platyfish Xiphophorus maculatus. Gene 2002, 295, 213–222. [Google Scholar] [CrossRef]
- Kobayashi, T.; Matsuda, M.; Kajiura-Kobayashi, H.; Suzuki, A.; Saito, N.; Nakamoto, M.; Shibata, N.; Nagahama, Y. Two DM domain genes, DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2004, 231, 518–526. [Google Scholar]
- Winkler, C.; Hornung, U.; Kondo, M.; Neuner, C.; Duschl, J.; Shima, A.; Schartl, M. Developmentally regulated and non-sex-specific expression of autosomal dmrt genes in embryos of the Medaka fish (Oryzias latipes). Mech. Dev. 2004, 121, 997–1005. [Google Scholar] [CrossRef]
- Tao, W.; Chen, J.; Tan, D.; Yang, J.; Sun, L.; Wei, J.; Conte, M.A.; Kocher, T.D.; Wang, D. Transcriptome display during tilapia sex determination and differentiation as revealed by RNA-Seq analysis. BMC Genom. 2018, 19, 363. [Google Scholar] [CrossRef]
- Wei, L.; Li, X.; Li, M.; Tang, Y.; Wei, J.; Wang, D. Dmrt1 directly regulates the transcription of the testis-biased Sox9b gene in Nile tilapia (Oreochromis niloticus). Gene 2019, 687, 109–115. [Google Scholar] [CrossRef]
- Haddad, N.G.; Eugster, E.A. Chapter 121—Precocious Puberty. In Endocrinology: Adult and Pediatric, 7th ed.; Jameson, J.L., De Groot, L.J., de Kretser, D.M., Giudice, L.C., Grossman, A.B., Melmed, S., Potts, J.T., Weir, G.C., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 2130–2141.e2135. [Google Scholar]
- Tchoudakova, A.; Kishida, M.; Wood, E.; Callard, G.V. Promoter characteristics of two cyp19 genes differentially expressed in the brain and ovary of teleost fish. J. Steroid Biochem. Mol. Biol. 2001, 78, 427–439. [Google Scholar] [CrossRef]
- Belgorosky, A.; Guercio, G.; Pepe, C.; Saraco, N.; Rivarola, M.A. Genetic and clinical spectrum of aromatase deficiency in infancy, childhood and adolescence. Horm. Res. 2009, 72, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Sebastian, S.; Takayama, K.; Suzuki, T.; Sasano, H.; Shozu, M. The human CYP19 (aromatase P450) gene: Update on physiologic roles and genomic organization of promoters. J. Steroid Biochem. Mol. Biol. 2003, 86, 219–224. [Google Scholar] [CrossRef]
- Piferrer, F.; Blázquez, M. Aromatase distribution and regulation in fish. Fish Physiol. Biochem. 2005, 31, 215. [Google Scholar] [CrossRef] [PubMed]
- The UniProt, C. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.F.-L.; Yan, Y.-L.; Guiguen, Y.; Postlethwait, J.; Chung, B.-C. Two Cyp19 (P450 aromatase) genes on duplicated zebrafish chromosomes are expressed in ovary or brain. Mol. Biol. Evol. 2001, 18, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Peng, C.; Ye, Z.; Tang, Z.; Li, S.; Xiao, L.; Liu, S.; Yang, Y.; Zhao, M.; Zhang, Y.; et al. An estradiol-17β/miRNA-26a/cyp19a1a regulatory feedback loop in the protogynous hermaphroditic fish, Epinephelus coioides. Mol. Cell. Endocrinol. 2020, 504, 110689. [Google Scholar] [CrossRef] [PubMed]
- Caulier, M.; Brion, F.; Chadili, E.; Turies, C.; Piccini, B.; Porcher, J.-M.; Guiguen, Y.; Hinfray, N. Localization of steroidogenic enzymes and Foxl2a in the gonads of mature zebrafish (Danio rerio). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 188, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Timmers, R.J.M.; Lambert, J.G.D.; Peute, J.; Van Oordt, P.G.W.J.; Vullings, H.G.B. Localization of aromatase in the brain of the male African catfish, Clarias gariepinus (Burchell), by microdissection and biochemical identification. J. Comp. Neurol. 1987, 258, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Strobl-Mazzulla, P.H.; Moncaut, N.P.; López, G.C.; Miranda, L.A.; Canario, A.V.M.; Somoza, G.M. Brain aromatase from pejerrey fish (Odontesthes bonariensis): cDNA cloning, tissue expression, and immunohistochemical localization. Gen. Comp. Endocrinol. 2005, 143, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Yamashita, M.; Kitano, T.; Iguchi, T. An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 137, 11–20. [Google Scholar] [CrossRef]
- Wu, K.; Song, W.; Zhang, Z.; Ge, W. Disruption of dmrt1 rescues the all-male phenotype of the cyp19a1a mutant in zebrafish a novel insight into the roles of aromatase/estrogens in gonadal differentiation and early folliculogenesis. Development 2020, 147, dev182758. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Nagahama, Y.; Nakamura, M. Diversity and plasticity of sex determination and differentiation in fishes. Sex. Dev. 2013, 7, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Kobayashi, Y.; Horiguchi, R.; Hirai, T.; Nakamura, M. Molecular cloning and quantitative expression of sexually dimorphic markers Dmrt1 and Foxl2 during female-to-male sex change in Epinephelus merra. Gen. Comp. Endocrinol. 2008, 157, 75–85. [Google Scholar] [CrossRef]
- Göppert, C.; Harris, R.M.; Theis, A.; Boila, A.; Hohl, S.; Rüegg, A.; Hofmann, H.A.; Salzburger, W.; Böhne, A. Inhibition of Aromatase Induces Partial Sex Change in a Cichlid Fish: Distinct Functions for Sex Steroids in Brains and Gonads. Sex. Dev. 2016, 10, 97–110. [Google Scholar] [CrossRef]
- Huffman, L.S.; O’Connell, L.A.; Hofmann, H.A. Aromatase regulates aggression in the African cichlid fish Astatotilapia burtoni. Physiol. Behav. 2013, 112–113, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.N.; Jiang, X.L.; Xie, Q.P.; Yuan, J.; Huang, B.F.; Tao, W.J.; Zhou, L.Y.; Nagahama, Y.; Wang, D.S. Transdifferentiation of differentiated ovary into functional testis by long-term treatment of aromatase inhibitor in Nile tilapia. Endocrinology 2014, 155, 1476–1488. [Google Scholar] [CrossRef]
- Huang, S.; Ye, L.; Chen, H. Sex determination and maintenance: The role of DMRT1 and FOXL2. Asian J. Androl. 2017, 19, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Wang, Y.; Li, Z.; Zhou, L.; Gui, J.F. Sequential, Divergent, and Cooperative Requirements of Foxl2a and Foxl2b in Ovary Development and Maintenance of Zebrafish. Genetics 2017, 205, 1551–1572. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, K.H.; Eicher, E.M. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev. Biol. 2001, 240, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, P.; Mahlapuu, M. Forkhead Transcription Factors: Key Players in Development and Metabolism. Dev. Biol. 2002, 250, 1–23. [Google Scholar] [CrossRef]
- Baron, D.; Cocquet, J.; Xia, X.; Fellous, M.; Guiguen, Y.; Veitia, R.A. An evolutionary and functional analysis of FoxL2 in rainbow trout gonad differentiation. J. Mol. Endocrinol. 2004, 33, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.; Batista, F.; Chaffaux, S.; Cocquet, J.; Cotinot, C.; Cribiu, E.; De Baere, E.; Baeree, E.; Guiguen, Y.; Jaubert, F.; et al. Foxl2 gene and the development of the ovary: A story about goat, mouse, fish and woman. Reprod. Nutr. Dev. 2005, 45, 377–382. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Li, M.; Ma, H.; Liu, X.; Shi, H.; Li, M.; Wang, D. Mutation of foxl2 or cyp19a1a Results in Female to Male Sex Reversal in XX Nile Tilapia. Endocrinology 2017, 158, 2634–2647. [Google Scholar] [CrossRef]
- Boulanger, L.; Pannetier, M.; Gall, L.; Allais-Bonnet, A.; Elzaiat, M.; Le Bourhis, D.; Daniel, N.; Richard, C.; Cotinot, C.; Ghyselinck, N.B.; et al. FOXL2 is a female sex-determining gene in the goat. Curr. Biol. CB 2014, 24, 404–408. [Google Scholar] [CrossRef]
- Xie, Q.P.; He, X.; Sui, Y.N.; Chen, L.L.; Sun, L.N.; Wang, D.S. Haploinsufficiency of SF-1 Causes Female to Male Sex Reversal in Nile Tilapia, Oreochromis niloticus. Endocrinology 2016, 157, 2500–2514. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Davies, H.; Sims, L.P.; Levy, S.E.; Dean, J. Ovarian gene expression in the absence of FIGLA, an oocyte-specific transcription factor. BMC Dev. Biol. 2007, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, W.; Zhang, N.; Shao, C.; Zhu, Y.; Dong, Z.; Wang, N.; Jia, X.; Xu, H.; Chen, S. Two Figla homologues have disparate functions during sex differentiation in half-smooth tongue sole (Cynoglossus semilaevis). Sci. Rep. 2016, 6, 28219. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Zhang, Z.; Song, W.; Wong, Q.W.-L.; Chen, W.; Shirgaonkar, N.; Ge, W. Roles of Figla/figla in Juvenile Ovary Development and Follicle Formation during Zebrafish Gonadogenesis. Endocrinology 2018, 159, 3699–3722. [Google Scholar] [CrossRef]
- Liang, L.-f.; Soyal, S.M.; Dean, J. FIGalpha, a germ cell specific transcription factor involved in the coordinate expression of the zona pellucida genes. Development 1997, 124, 4939–4947. [Google Scholar] [CrossRef]
- Qiu, Y.; Sun, S.; Charkraborty, T.; Wu, L.; Sun, L.; Wei, J.; Nagahama, Y.; Wang, D.; Zhou, L. Figla Favors Ovarian Differentiation by Antagonizing Spermatogenesis in a Teleosts, Nile Tilapia (Oreochromis niloticus). PLoS ONE 2015, 10, e0123900. [Google Scholar] [CrossRef]
- Dohle, G.; Smit, M.; Weber, R. Androgens and male fertility. World J. Urol. 2003, 21, 341–345. [Google Scholar] [CrossRef]
- Aluru, N.; Renaud, R.; Leatherland, J.F.; Vijayan, M.M. Ah receptor-mediated impairment of interrenal steroidogenesis involves StAR protein and P450scc gene attenuation in rainbow trout. Toxicol. Sci. 2005, 84, 260–269. [Google Scholar] [CrossRef]
- Arukwe, A. Steroidogenic acute regulatory (StAR) protein and cholesterol side-chain cleavage (P450scc)-regulated steroidogenesis as an organ-specific molecular and cellular target for endocrine disrupting chemicals in fish. Cell Biol. Toxicol. 2008, 24, 527–540. [Google Scholar] [CrossRef]
- Chen, S.-L.; Deng, S.-P.; Ma, H.-Y.; Tian, Y.-S.; Xu, J.-Y.; Yang, J.-F.; Wang, Q.-Y.; Ji, X.-S.; Shao, C.-W.; Wang, X.-L. Molecular marker-assisted sex control in half-smooth tongue sole (Cynoglossus semilaevis). Aquaculture 2008, 283, 7–12. [Google Scholar] [CrossRef]
- Baker, P.J.; Sha, J.A.; McBride, M.W.; Peng, L.; Payne, A.H.; O’Shaughnessy, P.J. Expression of 3β-hydroxysteroid dehydrogenase type I and type VI isoforms in the mouse testis during development. Eur. J. Biochem. 1999, 260, 911–917. [Google Scholar] [CrossRef]
- Bauer, M.; Bridgham, J.; Langenau, D.; Johnson, A.; Goetz, F. Conservation of steroidogenic acute regulatory (StAR) protein structure and expression in vertebrates. Mol. Cell. Endocrinol. 2000, 168, 119–125. [Google Scholar] [CrossRef]
- Barannikova, I.; Dyubin, V.; Bayunova, L.; Semenkova, T. Steroids in the control of reproductive function in fish. Neurosci. Behav. Physiol. 2002, 32, 141–148. [Google Scholar] [CrossRef]
- Imai, T.; Saino, K.; Matsuda, M. Mutation of Gonadal soma-derived factor induces medaka XY gonads to undergo ovarian development. Biochem. Biophys. Res. Commun. 2015, 467, 109–114. [Google Scholar] [CrossRef]
- Rodríguez-Marí, A.; Yan, Y.-L.; BreMiller, R.A.; Wilson, C.; Canestro, C.; Postlethwait, J.H. Characterization and expression pattern of zebrafish anti-Müllerian hormone (amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr. Patterns 2005, 5, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Aoki, Y.; Saito, D.; Kuroki, Y.; Fujiyama, A.; Naruse, K.; Tanaka, M. Sox9b/sox9a2-EGFP transgenic medaka reveals the morphological reorganization of the gonads and a common precursor of both the female and male supporting cells. Mol. Reprod. Dev. Inc. Gamete Res. 2008, 75, 472–476. [Google Scholar] [CrossRef]
- Yokoi, H.; Kobayashi, T.; Tanaka, M.; Nagahama, Y.; Wakamatsu, Y.; Takeda, H.; Araki, K.; Morohashi, K.I.; Ozato, K. Sox9 in a teleost fish, medaka (Oryzias latipes): Evidence for diversified function of Sox9 in gonad differentiation. Mol. Reprod. Dev. Inc. Gamete Res. 2002, 63, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Suzuki, A.; Matsuda, M.; Nagahama, Y.; Shibata, N. Testicular type Sox9 is not involved in sex determination but might be in the development of testicular structures in the medaka, Oryzias latipes. Biochem. Biophys. Res. Commun. 2005, 333, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y. Ontogeny and plasticity of sex determination/gonadal differentiation in fishes. In Proceedings of the Biology of Reproduction; Soc Study Reproduction: Madison, WI, USA, 2002; pp. 75–76. [Google Scholar]
- Guiguen, Y.; Jalabert, B.; Thouard, E.; Fostier, A. Changes in plasma and gonadal steroid hormones in relation to the reproductive cycle and the sex inversion process in the protandrous seabass, Lates calcarifer. Gen. Comp. Endocrinol. 1993, 92, 327–338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guiguen, Y.; Baroiller, J.F.; Ricordel, M.J.; Iseki, K.; McMeel, O.; Martin, S.A.M.; Fostier, A. Involvement of estrogens in the process of sex differentiation in two fish species: The rainbow trout (Oncorhynchus mykiss) and a tilapia (Oreochromis niloticus). Mol. Reprod. Dev. Inc. Gamete Res. 1999, 54, 154–162. [Google Scholar] [CrossRef]
- Chang, C.F.; Lee, M.F.; Chen, G.R. Estradiol-17β associated with the sex reversal in protandrous black porgy, Acanthopagrus schlegeli. J. Exp. Zool. 1994, 268, 53–58. [Google Scholar] [CrossRef]
- Piferrer, F.; Zanuy, S.; Carrillo, M.; Solar, I.I.; Devlin, R.H.; Donaldson, E.M. Brief treatment with an aromatase inhibitor during sex differentiation causes chromosomally female salmon to develop as normal, functional males. J. Exp. Zool. 1994, 270, 255–262. [Google Scholar] [CrossRef]
- Piferrer, F. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 2001, 197, 229–281. [Google Scholar] [CrossRef]
- Tsai, Y.-J.; Lee, M.-F.; Chen, C.-Y.; Chang, C.-F. Development of gonadal tissue and aromatase function in the protogynous orange-spotted grouper Epinephelus coioides. Zool. Stud. 2011, 50, 693–704. [Google Scholar]
- Murata, R.; Kobayashi, Y.; Karimata, H.; Kishimoto, K.; Kimura, M.; Nakamura, M. Transient sex change in the immature Malabar grouper, Epinephelus malabaricus, androgen treatment. Biol. Reprod. 2014, 91, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Guiguen, Y.; Fostier, A.; Herpin, A. Sex Determination and Differentiation in Fish: Genetic, Genomic, and Endocrine Aspects. In Sex Control in Aquaculture; Willey: Hoboken, NJ, USA, 2018; pp. 35–63. [Google Scholar]
- Taranger, G.L.; Carrillo, M.; Schulz, R.W.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.-A.; Dufour, S.; Karlsen, Ø.; Norberg, B. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 2010, 165, 483–515. [Google Scholar] [CrossRef]
- Singh, A.K. Introduction of modern endocrine techniques for the production of monosex population of fishes. Gen. Comp. Endocrinol. 2013, 181, 146–155. [Google Scholar] [CrossRef]
- Pandian, T.; Sheela, S. Hormonal induction of sex reversal in fish. Aquaculture 1995, 138, 1–22. [Google Scholar] [CrossRef]
- Hoga, C.A.; Almeida, F.L.; Reyes, F.G.R. A review on the use of hormones in fish farming: Analytical methods to determine their residues. CyTA J. Food 2018, 16, 679–691. [Google Scholar] [CrossRef]
- Díaz, N.; Piferrer, F. Estrogen exposure overrides the masculinizing effect of elevated temperature by a downregulation of the key genes implicated in sexual differentiation in a fish with mixed genetic and environmental sex determination. BMC Genom. 2017, 18, 973. [Google Scholar] [CrossRef]
- Filby, A.L.; Thorpe, K.L.; Maack, G.; Tyler, C.R. Gene expression profiles revealing the mechanisms of anti-androgen- and estrogen-induced feminization in fish. Aquat. Toxicol. 2007, 81, 219–231. [Google Scholar] [CrossRef]
- Bull, J.J. Evolution of Sex Determining Mechanisms; The Benjamin/Cummings Publishing Company, Inc.: San Francisco, CA, USA, 1983. [Google Scholar]
- Sullivan, J.A.; Schultz, R.J. Genetic and environmental basis of variablesex ratios in laboratory strains of Poeciliopsis lucida. Evolution 1986, 40, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Conover, D.; Demond, S. Absence of temperature-dependent sex determination in northern populations of two cyprinodontid fishes. Can. J. Zool. 1991, 69, 530–533. [Google Scholar] [CrossRef]
- Fernandino, J.I.; Hattori, R.S.; Acosta, O.D.M.; Strüssmann, C.A.; Somoza, G.M. Environmental stress-induced testis differentiation: Androgen as a by-product of cortisol inactivation. Gen. Comp. Endocrinol. 2013, 192, 36–44. [Google Scholar] [CrossRef]
- Fernandino, J.I.; Hattori, R.; Strüssmann, C.; Yamamoto, Y.; Somoza, G.M. Sex determination in fish: Odontesthes spp.(Atherinopsidae) as experimental models. Anim. Reprod. 2018, 12, 24–27. [Google Scholar]
- Karube, M.; Fernandino, J.I.; Strobl-Mazzulla, P.; Strussmann, C.A.; Yoshizaki, G.; Somoza, G.M.; Patino, R. Characterization and expression profile of the ovarian cytochrome P-450 aromatase (cyp19A1) gene during thermolabile sex determination in Pejerrey, Odontesthes bonariensis. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2007, 307, 625–636. [Google Scholar] [CrossRef]
- Kitano, T.; Takamune, K.; Kobayashi, T.; Nagahama, Y.; Abe, S.I. Suppression of P450 aromatase gene expression in sex-reversed males produced by rearing genetically female larvae at a high water temperature during a period of sex differentiation in the Japanese flounder (Paralichthys olivaceus). J. Mol. Endocrinol. 1999, 23, 167–176. [Google Scholar] [CrossRef]
- Kitano, T.; Yoshinaga, N.; Shiraishi, E.; Koyanagi, T.; Abe, S.-I. Tamoxifen induces masculinization of genetic females and regulates P450 aromatase and Müllerian inhibiting substance mRNA expression in Japanese flounder (Paralichthys olivaceus). Mol. Reprod. Dev. 2007, 74, 1171–1177. [Google Scholar] [CrossRef]
- D’Cotta, H.; Fostier, A.; Guiguen, Y.; Govoroun, M.; Baroiller, J.F. Aromatase plays a key role during normal and temperature-induced sex differentiation of tilapia Oreochromis niloticus. Mol. Reprod. Dev. 2001, 59, 265–276. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yamaguchi, S.; Hirai, T.; Kitano, T. Follicle-stimulating hormone signaling and Foxl2 are involved in transcriptional regulation of aromatase gene during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochem. Biophys. Res. Commun. 2007, 359, 935–940. [Google Scholar] [CrossRef]
- Benjamín Barón, S.; Fernando Bückle, R.; Espina, S. Environmental factors and sexual differentiation in Poecilia sphenops Valenciennes (Pisces: Poeciliidae). Aquac. Res. 2002, 33, 615–619. [Google Scholar] [CrossRef]
- Horbe, A.M.C.; Queiroz, M.M.d.A.; Moura, C.A.V.; Toro, M.A.G. Geoquímica das águas do médio e baixo rio Madeira e seus principais tributários-Amazonas-Brasil. Acta Amaz. 2013, 43, 489–504. [Google Scholar] [CrossRef][Green Version]
- Silva de Morais, I.d.; Reis, V.R.; de Almeida, F.L. The influence of the water pH on the sex ratio of tambaqui colossoma macropomum (CUVIER, 1818). Aquac. Rep. 2020, 17, 100334. [Google Scholar] [CrossRef]
- Heiligenberg, W. Colour polymorphism in the males of an African cichlid fish. In Proceedings of the Zoological Society of London; Blackwell Publishing, Ltd.: Oxford, UK, 1965; pp. 95–97. [Google Scholar]
- Nwadiaro, C. The distribution and food habits of the dwarf African cichlid, Pelvicachromis pulcher in the River Sombreiro, Nigeria. Hydrobiologia 1985, 121, 157–164. [Google Scholar] [CrossRef]
- Mota, V.C.; Martins, C.I.M.; Eding, E.H.; Canário, A.V.M.; Verreth, J.A.J. Cortisol and testosterone accumulation in a low pH recirculating aquaculture system for rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2017, 48, 3579–3588. [Google Scholar] [CrossRef]
- Baroiller, J.-F.; Guiguen, Y.; Fostier, A. Endocrine and environmental aspects of sex differentiation in fish. Cell. Mol. Life Sci. CMLS 1999, 55, 910–931. [Google Scholar] [CrossRef]
- Baroiller, J.-F.; d’Cotta, H. Environment and sex determination in farmed fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 399–409. [Google Scholar] [CrossRef]
- Francis, R.C.; Barlow, G.W. Social control of primary sex differentiation in the Midas cichlid. Proc. Natl. Acad. Sci. USA 1993, 90, 10673. [Google Scholar] [CrossRef]
- Liew, W.C.; Bartfai, R.; Lim, Z.; Sreenivasan, R.; Siegfried, K.R.; Orban, L. Polygenic sex determination system in zebrafish. PLoS ONE 2012, 7, e34397. [Google Scholar]
- Ribas, L.; Valdivieso, A.; Díaz, N.; Piferrer, F. Appropriate rearing density in domesticated zebrafish to avoid masculinization: Links with the stress response. J. Exp. Biol. 2017, 220, 1056–1064. [Google Scholar] [CrossRef]
- Shang, E.H.; Yu, R.M.; Wu, R.S. Hypoxia affects sex differentiation and development, leading to a male-dominated population in zebrafish (Danio rerio). Environ. Sci. Technol. 2006, 40, 3118–3122. [Google Scholar] [CrossRef] [PubMed]
- Baroiller, J.-F.; d’Cotta, H. The reversible sex of gonochoristic fish: Insights and consequences. Sex. Dev. 2016, 10, 242–266. [Google Scholar] [CrossRef] [PubMed]
- Fernandino, J.I.; Hattori, R.S.; Kishii, A.; Strüssmann, C.A.; Somoza, G.M. The cortisol and androgen pathways cross talk in high temperature-induced masculinization: The 11β-hydroxysteroid dehydrogenase as a key enzyme. Endocrinology 2012, 153, 6003–6011. [Google Scholar] [CrossRef] [PubMed]
- Hattori, R.S.; Fernandino, J.I.; Kishii, A.; Kimura, H.; Kinno, T.; Oura, M.; Somoza, G.M.; Yokota, M.; Strüssmann, C.A.; Watanabe, S. Cortisol-induced masculinization: Does thermal stress affect gonadal fate in pejerrey, a teleost fish with temperature-dependent sex determination? PLoS ONE 2009, 4, e6548. [Google Scholar] [CrossRef]
- Kitano, T.; Hayashi, Y.; Shiraishi, E.; Kamei, Y. Estrogen rescues masculinization of genetically female medaka by exposure to cortisol or high temperature. Mol. Reprod. Dev. 2012, 79, 719–726. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yoshinaga, N.; Yazawa, T.; Gen, K.; Kitano, T. Cortisol is involved in temperature-dependent sex determination in the Japanese flounder. Endocrinology 2010, 151, 3900–3908. [Google Scholar] [CrossRef]
- Ni, M.; Wen, H.; Li, J.; Chi, M.; Bu, Y.; Ren, Y.; Zhang, M.; Song, Z.; Ding, H. The physiological performance and immune responses of juvenile Amur sturgeon (Acipenser schrenckii) to stocking density and hypoxia stress. Fish Shellfish Immunol. 2014, 36, 325–335. [Google Scholar] [CrossRef]
- Gardner, L.; Anderson, T.; Place, A.R.; Dixon, B.; Elizur, A. Sex change strategy and the aromatase genes. J. Steroid Biochem. Mol. Biol. 2005, 94, 395–404. [Google Scholar] [CrossRef]
- Chu, L.; Li, J.; Liu, Y.; Cheng, C.H. Gonadotropin signaling in zebrafish ovary and testis development: Insights from gene knockout study. Mol. Endocrinol. 2015, 29, 1743–1758. [Google Scholar] [CrossRef]
- Xie, Y.; Chu, L.; Liu, Y.; Sham, K.W.; Li, J.; Cheng, C.H. The highly overlapping actions of Lh signaling and Fsh signaling on zebrafish spermatogenesis. J. Endocrinol. 2017, 234, 233–246. [Google Scholar] [CrossRef]
- Chu, L.; Li, J.; Liu, Y.; Hu, W.; Cheng, C.H. Targeted gene disruption in zebrafish reveals noncanonical functions of LH signaling in reproduction. Mol. Endocrinol. 2014, 28, 1785–1795. [Google Scholar] [CrossRef]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.-F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar] [CrossRef]
- Nakamura, M.; Nagahama, Y. Differentiation and development of Leydig cells, and changes of testosterone levels during testicular differentiation in tilapia Oreochromis niloticus. Fish Physiol. Biochem. 1989, 7, 211–219. [Google Scholar] [CrossRef]
- Le Page, Y.; Diotel, N.; Vaillant, C.; Pellegrini, E.; Anglade, I.; Mérot, Y.; Kah, O. Aromatase, brain sexualization and plasticity: The fish paradigm. Eur. J. Neurosci. 2010, 32, 2105–2115. [Google Scholar] [CrossRef]
- Silva, A.C.; Zubizarreta, L.; Quintana, L. A teleost fish model to understand hormonal mechanisms of non-breeding territorial behavior. Front. Endocrinol. 2020, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qi, X.; Guo, Y.; Li, S.; Zhang, Y.; Liu, X.; Lin, H. Molecular identification of GnIH/GnIHR signal and its reproductive function in protogynous hermaphroditic orange-spotted grouper (Epinephelus coioides). Gen. Comp. Endocrinol. 2015, 216, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhou, W.; Lu, D.; Wang, Q.; Zhang, H.; Li, S.; Liu, X.; Zhang, Y.; Lin, H. Sexual Dimorphism of Steroidogenesis Regulated by GnIH in the Goldfish, Carassius auratus. Biol. Reprod. 2013, 88. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Parhar, I. Structural and Functional Divergence of Gonadotropin-Inhibitory Hormone (GnIH) from Jawless Fish to Mammals. Front. Endocrinol. 2014, 5, 177. [Google Scholar] [CrossRef]
- Selvaraj, S.; Kitano, H.; Ohga, H.; Yamaguchi, A.; Matsuyama, M. Expression changes of mRNAs encoding kisspeptins and their receptors and gonadotropin-releasing hormones during early development and gonadal sex differentiation periods in the brain of chub mackerel (Scomber japonicus). Gen. Comp. Endocrinol. 2015, 222, 20–32. [Google Scholar] [CrossRef]
- Kim, N.N.; Shin, H.S.; Choi, Y.J.; Choi, C.Y. Kisspeptin regulates the hypothalamus–pituitary–gonad axis gene expression during sexual maturation in the cinnamon clownfish, Amphiprion melanopus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 168, 19–32. [Google Scholar] [CrossRef]

| Gene Name | Full Gene Name | Chromosome Location | Species | References |
|---|---|---|---|---|
| amhr2 | Anti-Müllerian hormone receptor type 2 | Autosomal | Grass puffer (Takifugu rubripes) | [4] |
| amhy | Y-linked anti-Müllerian hormone | Y chromosome | Nile tilapia (Oreochromis niloticus) | [5] |
| Cobaltcap silverside (Hypoatherina tsurugae) | [33] | |||
| Northern pike (Esox lucius) | [34] | |||
| Rockfish (Sebastes schlegelii) | [35] | |||
| dmrt1 | Doublesex and mab-3 related transcription factor | Autosomal/Sex chromosome | Spotted scat (Scatophagus argus) | [6] |
| Chinese tongue sole (Cynoglossus semilaevis) | [36] | |||
| dmy | DM-domain on the Y-chromosome | Y chromosome | Japanese medaka (Oryzias latipes) | [7] |
| gdf6Y | Growth differentiation factor 6 on the Y-chromosome | Y chromosome | Turquoise killifish (Nothobranchius furzeri) | [8] |
| gsdf | Gonadal soma derived factor | Autosomal | Rainbow trout (Oncorhynchus mykiss) | [9] |
| gsdfY | Gonadal soma derived factor on the Y-chromosome | Y chromosome | Philippine medaka (Oryzias luzonensis) | [10] |
| sdY | Sexually dimorphic on the Y-chromosome | Y chromosome | Rainbow trout (Oncorhynchus mykiss) | [37] |
| Atlantic salmon (Salmon salar) | [11] | |||
| Brown trout (Salmo trutta) | [11] | |||
| Arctic charr (Salvelinus alpinus) | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendiran, P.; Jaafar, F.; Kar, S.; Sudhakumari, C.; Senthilkumaran, B.; Parhar, I.S. Sex Determination and Differentiation in Teleost: Roles of Genetics, Environment, and Brain. Biology 2021, 10, 973. https://doi.org/10.3390/biology10100973
Rajendiran P, Jaafar F, Kar S, Sudhakumari C, Senthilkumaran B, Parhar IS. Sex Determination and Differentiation in Teleost: Roles of Genetics, Environment, and Brain. Biology. 2021; 10(10):973. https://doi.org/10.3390/biology10100973
Chicago/Turabian StyleRajendiran, Preetha, Faizul Jaafar, Sonika Kar, Chenichery Sudhakumari, Balasubramanian Senthilkumaran, and Ishwar S. Parhar. 2021. "Sex Determination and Differentiation in Teleost: Roles of Genetics, Environment, and Brain" Biology 10, no. 10: 973. https://doi.org/10.3390/biology10100973
APA StyleRajendiran, P., Jaafar, F., Kar, S., Sudhakumari, C., Senthilkumaran, B., & Parhar, I. S. (2021). Sex Determination and Differentiation in Teleost: Roles of Genetics, Environment, and Brain. Biology, 10(10), 973. https://doi.org/10.3390/biology10100973








