COVID-19 Vaccines: Current Conditions and Future Prospects
Abstract
:Simple Summary
Abstract
1. Introduction
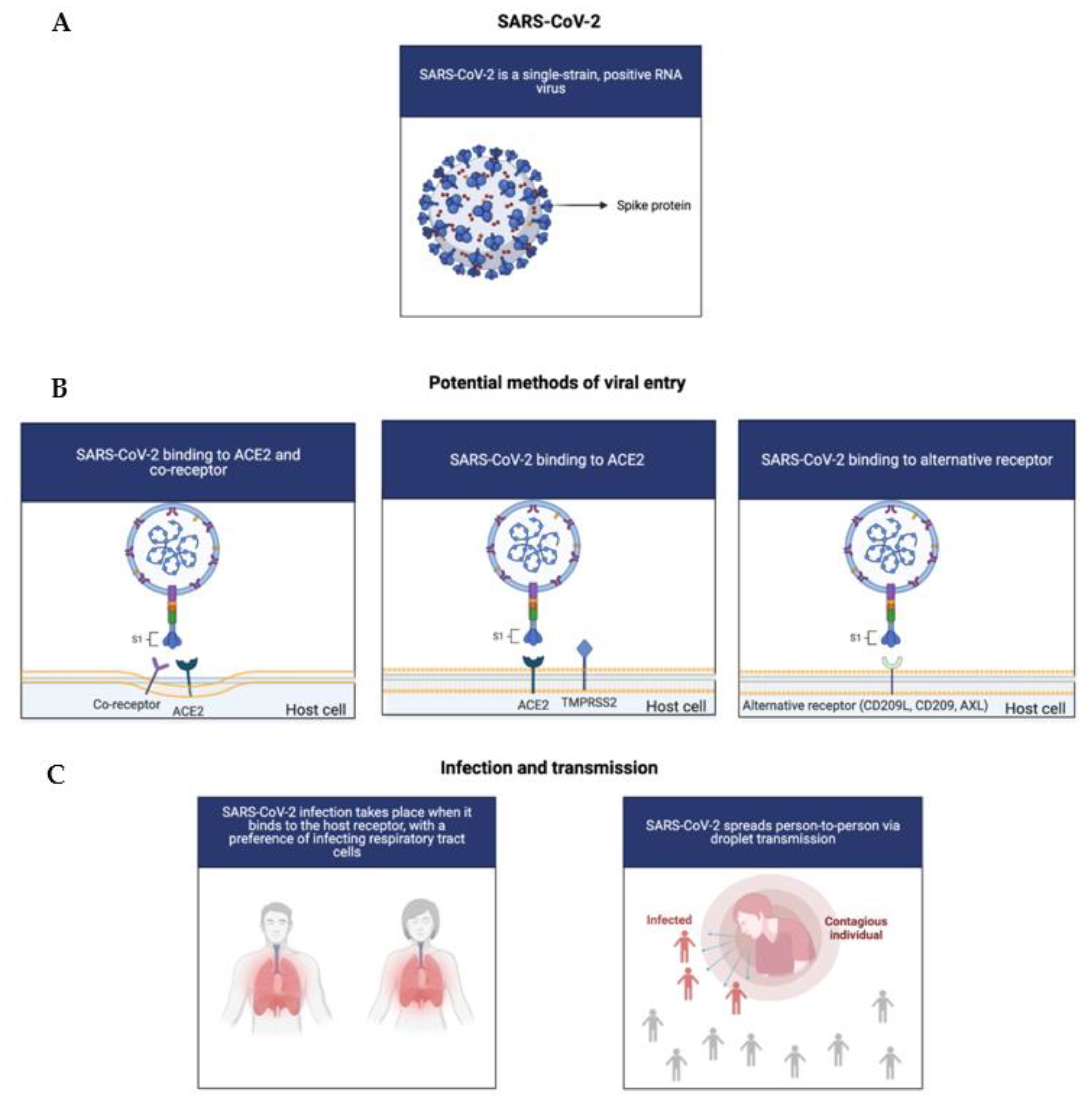
1.1. The COVID-19 Virus: Infection and Symptoms
1.2. Treatment and Vaccine Approaches for COVID-19
1.3. The Role of Antibody and T Cell Responses in Fighting COVID-19
1.4. T-Cells and Pre-Existing Cross-Reactive Immunity
1.5. Current and Most Common COVID-19 Vaccines
1.6. Additional COVID-19 Vaccines
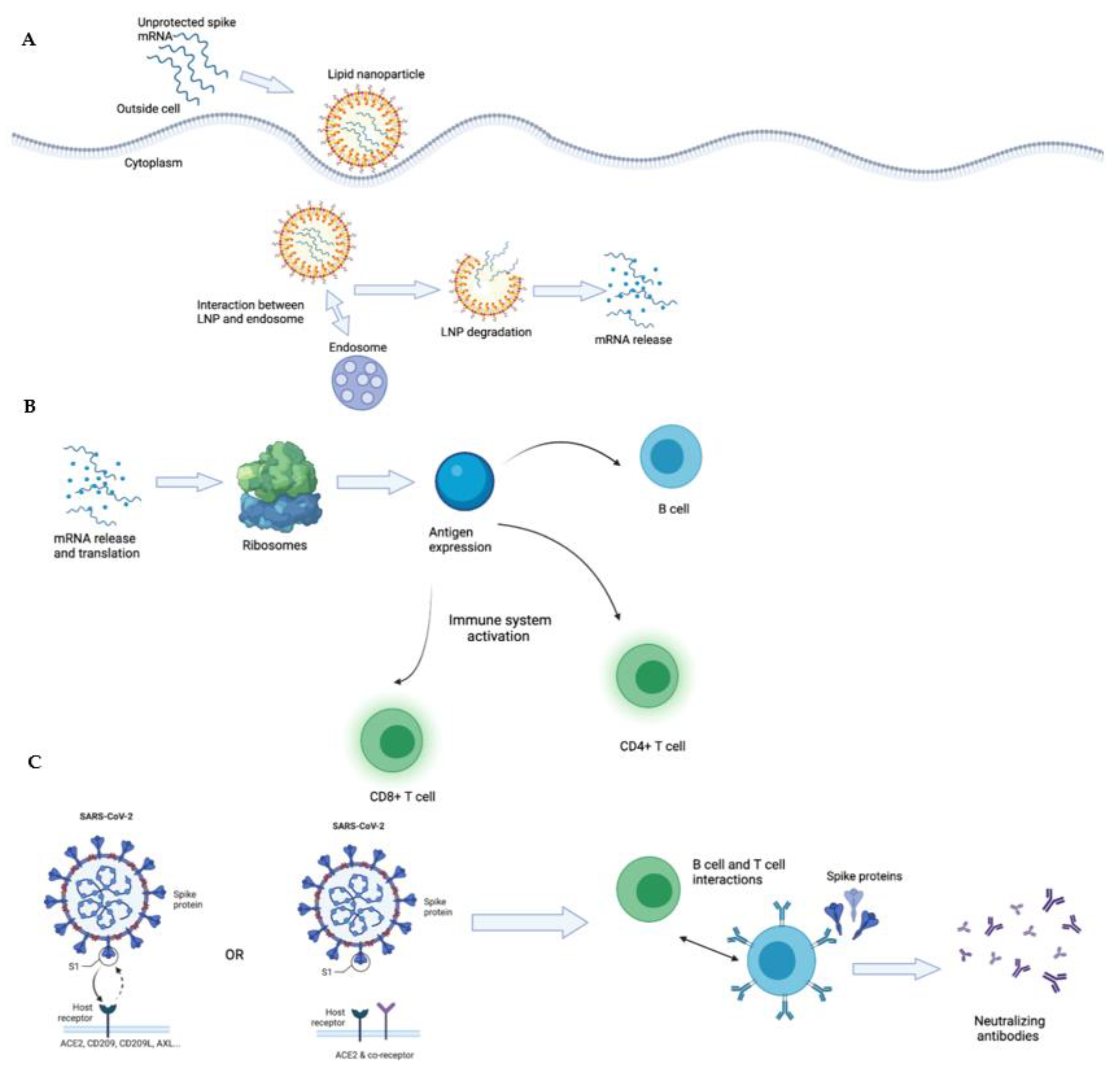
1.7. Lipid Nanoparticles and the Lipid mRNA Vaccine of COVID-19
1.8. The Unique Pharmacokinetic/Pharmacodynamic (PK/PD) Property of Lipid mRNA Particles
1.9. The Function and Potential Antagonist Risks of ACE2
1.10. Technological and Dosage Regimen of Current COVID-19 Vaccines
1.11. The Immune System and Immune Response to the COVID-19 mRNA Vaccines
1.12. Concerns and Controversies Regarding Current COVID-19 Vaccines
1.13. Currently Unknown Data for COVID-19 Vaccines
1.14. Future Considerations for the COVID-19 Vaccines
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [Green Version]
- John Hopkins University of Medicine. Coronavirus Resource Center. 2021. Available online: https://coronavirus.jhu.edu/ (accessed on 30 August 2021).
- Villa, S.; Lombardi, A.; Mangioni, D.; Bozzi, G.; Bandera, A.; Gori, A.; Raviglione, M. The COVID-19 pandemic preparedness or lack thereof: From China to Italy. Glob. Health Med. 2020, 2, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Zhang, T.; Song, C.; Shen, S.; Jiang, Y.; Zhang, X. The Coupled Impact of Emergency Responses and Population Flows on the COVID-19 Pandemic in China. Geohealth 2020, 4, e2020GH000332. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Gouripeddi, R.; Facelli, J.C. Human activity pattern implications for modeling SARS-CoV-2 transmission. Comput. Methods Programs Biomed. 2021, 199, 105896. [Google Scholar] [CrossRef]
- Buchanan, N.D.; Aslaner, D.M.; Adelstein, J.; MacKenzie, D.M.; Wold, L.E.; Gorr, M.W. Remote Work during the COVID-19 Pandemic: Making the Best of It. Physiology 2021, 36, 2–4. [Google Scholar] [CrossRef]
- Freppel, W.; Merindol, N.; Rallu, F.; Bergevin, M. Efficient SARS-CoV-2 detection in unextracted oro-nasopharyngeal specimens by rRT-PCR with the Seegene Allplex™ 2019-nCoV assay. Virol. J. 2020, 17, 196. [Google Scholar] [CrossRef]
- Bolcato, M.; Aurilio, M.; Aprile, A.; Di Mizio, G.; Della Pietra, B.; Feola, A. Take-Home Messages from the COVID-19 Pandemic: Strengths and Pitfalls of the Italian National Health Service from a Medico-Legal Point of View. Healthcare 2021, 9, 17. [Google Scholar] [CrossRef]
- Bolcato, M.; Rodriguez, D.; Feola, A.; Di Mizio, G.; Bonsignore, A.; Ciliberti, R.; Tettamanti, C.; Aurillo, M.; Aprile, A. COVID-19 Pandemic and Equal Access to Vaccines. Vaccines 2021, 9, 538. [Google Scholar] [CrossRef]
- WHO. Tracking SARS-CoV-2 Variants; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Chams, N.; Chams, S.; Badran, R.; Shams, A.; Araji, A.; Raad, M.; Mukhopadhyay, S.; Stroberg, E.; Duval, E.; Barton, L.; et al. COVID-19: A Multidisciplinary Review. Front. Public Health 2020, 8, 383. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef] [Green Version]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heineman, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Z.; Hou, Y.; Deng, X.; Xu, W.; Zheng, T.; Wu, P.; Xie, S.; Bian, W.; Zhang, C.; et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021, 31, 126–140. [Google Scholar] [CrossRef]
- Lukassen, S.; Chua, R.L.; Trefzer, T.; Kahn, N.C.; Schneider, M.A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A.; et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020, 39, e105114. [Google Scholar] [CrossRef]
- Amraei, R.; Yin, W.; Napoleon, M.A.; Suder, E.L.; Berrigan, J.; Zhao, Q.; Olejnik, J.; Chandler, K.; Xia, C.; Feldman, J.; et al. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2. bioRxiv 2021, 7, 1156–1165. [Google Scholar]
- Cuervo, N.Z.; Grandvaux, N. ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. Elife 2020, 9, e61390. [Google Scholar] [CrossRef]
- Edwards, D.; Hickey, A.; Batycky, R.; Griel, L.; Lipp, M.; Dehaan, W.; Clarke, R.; Hava, D.; Perry, J.; Laurenzi, B.; et al. A New Natural Defense Against Airborne Pathogens. QRB Discov. 2020, 1, e5. [Google Scholar] [CrossRef]
- Chen, Z.; Wherry, E.J. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020, 20, 529–536. [Google Scholar] [CrossRef]
- Golubev, A.G. COVID-19: A Challenge to Physiology of Aging. Front. Physiol. 2020, 11, 584248. [Google Scholar] [CrossRef]
- Wang, B.; Li, R.; Lu, Z.; Huang, Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging 2020, 12, 6049–6057. [Google Scholar] [CrossRef]
- Harapan, H.; Itoh, N.; Yufika, A.; Winardi, W.; Keam, S.; Te, H.; Megawati, D.; Hayati, Z.; Wagner, A.; Mudatsir, M.; et al. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health. 2020, 13, 667–673. [Google Scholar] [CrossRef]
- Hazlett, C.; Wulf, D.A.; Pasaniuc, B.; Arah, O.A.; Erlandson, K.M.; Montague, B.T. Credible learning of hydroxychloroquine and dexamethasone effects on COVID-19 mortality outside of randomized trials. medRxiv 2020. [Google Scholar] [CrossRef]
- Singh, A.K.; Majumdar, S.; Singh, R.; Misra, A. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician’s perspective. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 971–978. [Google Scholar] [CrossRef]
- Hossen, M.S.; Barek, M.A.; Jahan, N.; Safiqul Islam, M. A Review on Current Repurposing Drugs for the Treatment of COVID-19: Reality and Challenges. SN Compr. Clin. Med. 2020, 2, 1777–1789. [Google Scholar] [CrossRef]
- Corey, L.; Mascola, J.R.; Fauci, A.S.; Collins, F.S. A strategic approach to COVID-19 vaccine R&D. Science 2020, 368, 948–950. [Google Scholar]
- Chowdhury, F.R.; Hoque, A.; Chowdhury, F.U.H.; Amin, M.R.; Rahim, A.; Rahman, M.M.; Yasmin, R.; Amin, M.R.; Miah, M.T.; Kalam, M.A.; et al. Convalescent plasma transfusion therapy in severe COVID-19 patients- a safety, efficacy and dose response study: A structured summary of a study protocol of a phase II randomized controlled trial. Trials 2020, 21, 883. [Google Scholar] [CrossRef]
- Lee, W.T.; Goo, T.T.; Lim, W.W.; Toh, H.C.; Yasai, Y. Hospital Seeing More Personal Mobility Device Accidents and Serious Injuries Despite Active Mobility Act. J. Emerg. Trauma Shock 2020, 13, 274–278. [Google Scholar] [CrossRef]
- Maor, Y.; Cohen, D.; Paran, N.; Israely, T.; Ezra, V.; Axelrod, O.; Shinar, E.; Izak, M.; Rahav, G.; Rahimi-Levene, N.; et al. Compassionate use of convalescent plasma for treatment of moderate and severe pneumonia in COVID-19 patients and association with IgG antibody levels in donated plasma. EClinicalMedicine 2020, 26, 100525. [Google Scholar] [CrossRef]
- Liu, S.T.H.; Lin, H.M.; Baine, I.; Wajnberg, A.; Gumprecht, J.P.; Rahman, F.; Rodriguez, D.; Tandon, P.; assily-Marcus, A.; Bander, J.; et al. Convalescent plasma treatment of severe COVID-19: A propensity score-matched control study. Nat. Med. 2020, 26, 1708–1713. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [Green Version]
- Hinman, A. Eradication of vaccine-preventable diseases. Annu. Rev. Public Health 1999, 20, 211–229. [Google Scholar] [CrossRef]
- Silveira, M.M.; Moreira, G.M.S.G.; Mendonça, M. DNA vaccines against COVID-19: Perspectives and challenges. Life Sci. 2021, 267, 118919. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Lu, J.; Lu, G.; Tan, S.; Xia, J.; Xiong, H.; Yu, X.; Qi, Q.; Yu, X.; Li, L.; Yu, H.; et al. A COVID-19 mRNA vaccine encoding SARS-CoV-2 virus-like particles induces a strong antiviral-like immune response in mice. Cell Res. 2020, 30, 936–939. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Kalita, P.; Padhi, A.K.; Zhang, K.Y.J.; Tripathi, T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb. Pathog. 2020, 145, 104236. [Google Scholar] [CrossRef]
- Abbasi, J. COVID-19 and mRNA Vaccines—First Large Test for a New Approach. JAMA 2020, 324, 1125–1127. [Google Scholar] [CrossRef]
- Huang, Q.; Zeng, J.; Yan, J. COVID-19 mRNA vaccines. J. Genet. Genom. 2021, 48, 107–114. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.A.; Hossain, N.; Kashem, M.A.; Shahid, M.A.; Alam, A. Immune response in COVID-19: A review. J. Infect. Public Health 2020, 13, 1619–1629. [Google Scholar] [CrossRef]
- Nakanaga, K.; Yamanouchi, K.; Fujiwara, K. Protective effect of monoclonal antibodies on lethal mouse hepatitis virus infection in mice. J. Virol. 1986, 59, 168–171. [Google Scholar] [CrossRef] [Green Version]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Coughlin, M.; Lou, G.; Martinez, O.; Masterman, S.K.; Olsen, O.A.; Moksa, A.A.; Farzan, M.; Babcook, J.S.; Prabhakar, B.S. Generation and characterization of human monoclonal neutralizing antibodies with distinct binding and sequence features against SARS coronavirus using XenoMouse®. Virology 2007, 361, 93–102. [Google Scholar] [CrossRef] [Green Version]
- To, K.K.; Tsang, O.T.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Liu, L.; Kou, G.; Zheng, Y.; Ding, Y.; Ni, W.; Wang, Q.; Tan, L.; Wu, W.; Tang, S.; et al. Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020, 58, 6. [Google Scholar] [CrossRef] [Green Version]
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.C.; Deng, K.; Chen, Y.K.; Liao, P.; Qui, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020, 41, 355–359. [Google Scholar] [CrossRef]
- Duan, J.; Yan, X.; Guo, X.; Cao, W.; Han, W.; Qi, C.; Feng, J.; Yang, D.; Gao, G.; Jin, G.; et al. A human SARS-CoV neutralizing antibody against epitope on S2 protein. Biochem. Biophys. Res. Commun. 2005, 333, 186–193. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef]
- Nanda, A.; Vura, N.V.R.K.; Gravenstein, S. COVID-19 in older adults. Aging Clin. Exp. Res. 2020, 32, 1199–1202. [Google Scholar] [CrossRef]
- Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.S.; Mathew, D.; Baxter, A.E.; Vella, L.A.; et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020, 5, eabd7114. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Author Correction: Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 448. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef]
- Sariol, A.; Perlman, S. Lessons for COVID-19 Immunity from Other Coronavirus Infections. Immunity 2020, 53, 248–263. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Saponaro, F.; Rutigliano, G.; Sestito, S.; Bandini, L.; Storti, B.; Bizzarri, R.; Zucchi, R. ACE2 in the Era of SARS-CoV-2: Controversies and Novel Perspectives. Front. Mol. Biosci. 2020, 7, 588618. [Google Scholar] [CrossRef]
- Chagla, Z. The BNT162b2 (BioNTech/Pfizer) vaccine had 95% efficacy against COVID-19 ≥7 days after the 2nd dose. Ann. Intern. Med. 2021, 174, JC15. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Vaccine wagers on coronavirus surface protein pay off. Science 2020, 370, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, C.; Nussenzweig, M.C. All eyes on a hurdle race for a SARS-CoV-2 vaccine. Nature 2020, 586, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Brussow, H. COVID-19: Vaccination Problems. Environ. Microbiol. 2021, 23, 2878–2890. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [PubMed]
- Pacheco, T.J.A.; da Silva, V.C.M.; de Souza, D.G. Nano COVID-19 Vaccines: The firsts RNA lipid nanoparticle vaccines being approved from history—Review. Res. Soc. Dev. 2020, 9, 12. [Google Scholar] [CrossRef]
- Knoll, M.D.; Wonodi, C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Oxford vaccine is up to 90% effective, interim analysis indicates. BMJ 2020, 371, m4564. [Google Scholar] [CrossRef]
- Arashkia, A.; Jalilvand, S.; Mohajel, N.; Afchangi, A.; Azadmanesh, K.; Salehi-Vaziri, M.; Fazlalipour, M.; Pouriayevali, M.H.; Jalali, T.; Nasab, S.D.M.; et al. Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects. Rev. Med. Virol. 2020, 3.e2183. [Google Scholar] [CrossRef]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, D.W.; Bellamy, D.; Bittaye, M.; Dold, C.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef]
- Jones, I.; Roy, P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet 2021, 397, 642–643. [Google Scholar] [CrossRef]
- Prevention CfDCa. Understanding Viral Vector COVID-19 Vaccines. CDC. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/viralvector.html (accessed on 25 June 2021).
- Ricke, D.O. Two Different Antibody-Dependent Enhancement (ADE) Risks for SARS-CoV-2 Antibodies. Front. Immunol. 2021, 12, 640093. [Google Scholar] [CrossRef]
- Administration UFD. FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine. 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine (accessed on 30 June 2021).
- Burki, T.K. The Russian vaccine for COVID-19. Lancet Respir. Med. 2020, 8, e85–e86. [Google Scholar] [CrossRef]
- Balakrishnan, V.S. The arrival of Sputnik, V. Lancet Infect. Dis. 2020, 20, 1128. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Russian vaccine efficacy is 91.6%, show phase III trial results. BMJ 2021, 372, n309. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Sofia Mouthinho, M.W. Is Russia’s COVID-19 Vaccine Safe? Brazil’s Veto of Sputnik V Sparks Lawsuit Threat and Confusion Science. 2021. Available online: https://www.science.org/news/2021/04/russias-covid-19-vaccine-safe-brazils-veto-sputnik-v-sparks-lawsuit-threat-and (accessed on 10 May 2021).
- Johnson, J. A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study to Assess the Efficacy and Safety of Ad26.COV2.S for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adults Aged 18 Years and Older. 2020. Available online: https://www.jnj.com/coronavirus/ensemble-1-study-protocol (accessed on 5 May 2021).
- Brüssow, H. Efforts towards a COVID-19 vaccine. Environ. Microbiol. 2020, 22, 4071–4084. [Google Scholar] [CrossRef]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588. [Google Scholar] [CrossRef]
- Lavigne, S.E. Vaccine hesitancy: Root causes and possible solutions. Can. J. Dent. Hyg. 2021, 55, 79–82. [Google Scholar] [PubMed]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Baraniuk, C. What do we know about China’s covid-19 vaccines? BMJ 2021, 373, n912. [Google Scholar] [CrossRef]
- Hotez, P.J.; Nuzhath, T.; Callaghan, T.; Colwell, B. COVID-19 Vaccine Decisions: Considering the Choices and Opportunities. Microbes Infect. 2021, 23, 104811. [Google Scholar] [CrossRef] [PubMed]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Nusair, M.A.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. China’s COVID vaccines are going global—But questions remain. Nature 2021, 593, 178–179. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Bangaru, S.; Ozorowski, G.; Turner, H.L.; Antanasijevic, A.; Huang, D.; Wang, X.; Torres, J.; Diedrich, J.K.; Tian, J.-H.; Portnoff, A.D.; et al. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science 2020, 370, 1089–1094. [Google Scholar] [CrossRef]
- Novavax. Recombinant Nanoparticle Vaccine Technology. 2021. Available online: https://www.novavax.com/our-unique-technology (accessed on 27 June 2021).
- Chung, Y.H.; Beiss, V.; Fiering, S.N.; Steinmetz, N.F. COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano 2020, 14, 12522–12537. [Google Scholar] [CrossRef]
- Callaway, E.; Mallapaty, S. Novavax offers first evidence that COVID vaccines protect people against variants. Nat. Cell Biol. 2021, 590, 17. [Google Scholar]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- Baraniuk, C. Covid-19: What do we know about Sputnik V and other Russian vaccines? BMJ 2021, 372, n743. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.L.; MacIntyre, C.R.; McIntyre, P.B.; Nelson, M.R. SARS-CoV-2 Vaccines: Where Are We Now? J. Allergy Clin. Immunol. Pract. 2021. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [Green Version]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; Lopez-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [Green Version]
- Guevara, M.L.; Persano, F.; Persano, S. Advances in Lipid Nanoparticles for mRNA-Based Cancer Immunotherapy. Front. Chem. 2020, 8, 589959. [Google Scholar] [CrossRef]
- Chatzikleanthous, D.; O’Hagan, D.T.; Adamo, R. Lipid-Based Nanoparticles for Delivery of Vaccine Adjuvants and Antigens: Toward Multicomponent Vaccines. Mol. Pharm 2021, 18, 2867–2888. [Google Scholar] [CrossRef]
- Przybytkowski, E.; Behrendt, M.; Dubois, D.; Maysinger, D. Nanoparticles can induce changes in the intracellular metabolism of lipids without compromising cellular viability. FEBS J. 2009, 276, 6204–6217. [Google Scholar] [CrossRef] [Green Version]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Moss, K.H.; Popova, P.; Hadrup, S.R.; Astakhova, K.; Taskova, M. Lipid Nanoparticles for Delivery of Therapeutic RNA Oligonucleotides. Mol. Pharm. 2019, 16, 2265–2277. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Asteroid treasure, COVID vaccine and public peer review. Nature 2020, 588, 543. [CrossRef] [PubMed]
- Eygeris, Y.; Patel, S.; Jozic, A.; Sahay, G. Deconvoluting Lipid Nanoparticle Structure for Messenger RNA Delivery. Nano Lett. 2020, 20, 4543–4549. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef]
- Messengers of hope. Nat. Biotechnol. 2021, 39, 1. [CrossRef]
- Park, K.S.; Sun, X.; Aikins, M.E.; Moon, J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021, 169, 137–151. [Google Scholar] [CrossRef]
- Mocan, T.; Matea, C.T.; Iancu, C.; Agoston-Coldea, L.; Mocan, L.; Orasan, R. Hypersensitivity and nanoparticles: Update and research trends. Clujul. Med 2016, 89, 216–219. [Google Scholar] [CrossRef] [Green Version]
- Moghimi, S.M. Allergic Reactions and Anaphylaxis to LNP-Based COVID-19 Vaccines. Mol. Ther. 2021, 29, 898–900. [Google Scholar] [CrossRef]
- Sellaturay, P.; Nasser, S.; Islam, S.; Gurugama, P.; Ewan, P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin. Exp. Allergy 2021, 51, 861–863. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cataldi, M.; Vigliotti, C.; Mosca, T.; Cammarota, M.; Capone, D. Emerging Role of the Spleen in the Pharmacokinetics of Monoclonal Antibodies, Nanoparticles and Exosomes. Int. J. Mol. Sci. 2017, 18, 1249. [Google Scholar] [CrossRef] [Green Version]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol. Rep. 2019, 71, 156–166. [Google Scholar] [CrossRef]
- Patel, D.K.; Kesharwani, R.; Kumar, V. Lipid Nanoparticle Topical and Transdermal Delivery: A Review on Production, Penetration Mechanism to Skin. Int. J. Pharm. Investig. 2019, 9, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Meng, C.; Chen, Z.; Li, G.; Welte, T.; Shen, H. Nanoparticles for mRNA Therapeutics. Adv. Therap. 2021, 4, 2000099. [Google Scholar] [CrossRef]
- Wang, R.; Luo, X.; Liu, F.; Luo, S. Confronting the threat of SARS-CoV-2: Realities, challenges and therapeutic strategies (Review). Exp. Ther. Med. 2021, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Hrkach, J.; Langer, R. From micro to nano: Evolution and impact of drug delivery in treating disease. Drug. Deliv. Transl. Res. 2020, 10, 567–570. [Google Scholar] [CrossRef]
- Prub, B.M. Current State of the First COVID-19 Vaccines. Vaccines 2021, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef]
- Speiser, D.E.; Bachmann, M.F. COVID-19: Mechanisms of Vaccination and Immunity. Vaccines 2020, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.J.; Hiscox, J.A.; Hooper, N.M. ACE2: From vasopeptidase to SARS virus receptor. Trends Pharm. Sci. 2004, 25, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, P.; Liu, K.; Guo, F.; Zhang, Y.; Zhang, G.; Jiang, C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008, 18, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Prevention CfDCa. Moderna CDC. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html (accessed on 1 July 2021).
- Prevention CfDCa. Pfizer-BioNTech COVID-19 Overview and Safety: CDC. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html (accessed on 1 July 2021).
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and T. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Nogrady, B. Mounting evidence suggests Sputnik COVID vaccine is safe and effective. Nat. Cell Biol. 2021, 595, 339–340. [Google Scholar]
- CDC. Janssen COVID-19 Vaccine (Johnson & Johnson). 2021. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/janssen/index.html (accessed on 1 July 2021).
- WHO. The Oxford/AstraZeneca COVID-19 Vaccine: What You Need to Know. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/the-oxford-astrazeneca-covid-19-vaccine-what-you-need-to-know (accessed on 10 July 2021).
- Health NIo. U.S. Clinical Trial Results Show Novavax Vaccine Is Safe and Prevents COVID-19. NIH. 2021. Available online: https://www.nih.gov/news-events/news-releases/us-clinical-trial-results-show-novavax-vaccine-safe-prevents-covid-19 (accessed on 10 July 2021).
- Sharma, K.; Koirala, A.; Nicolopoulos, K.; Chiu, C.; Wood, N.; Britton, P.N. Vaccines for COVID-19: Where do we stand in 2021? Paediatr. Respir. Rev. 2021, 39, 22–31. [Google Scholar] [CrossRef]
- Diseases NIoAaI. Vaccine Types. NIH. 2019. Available online: https://www.niaid.nih.gov/research/vaccine-types (accessed on 10 July 2021).
- ClinicalTrials.gov. Study of the Safety, Reactogenicity and Immunogenicity of “EpiVacCorona” Vaccine for the Prevention of COVID-19 (EpiVacCorona) 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04527575 (accessed on 10 July 2021).
- WHO. The Sinopharm COVID-19 Vaccine: What You Need to Know. WHO. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/the-sinopharm-covid-19-vaccine-what-you-need-to-know (accessed on 10 July 2021).
- Nicholson, L.B. The immune system. Essays Biochem. 2016, 60, 275–301. [Google Scholar] [CrossRef] [Green Version]
- Weiskopf, D.; Weinberger, B.; Grubeck-Loebenstein, B. The aging of the immune system. Transpl. Int. 2009, 22, 1041–1050. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Peter, D. Gray’s Anatomy. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering, 39th ed.; Stranding, S., Livingstone, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 327–358. [Google Scholar]
- Wiendl, H.; Lautwein, A.; Mitsdörffer, M.; Krause, S.; Erfurth, S.; Wienhold, W.; Morgalla, M.; Weber, E.; Overkleeft, H.S.; Lochmuller, H.; et al. Antigen processing and presentation in human muscle: Cathepsin S is critical for MHC class II expression and upregulated in inflammatory myopathies. J. Neuroimmunol. 2003, 138, 132–143. [Google Scholar] [CrossRef]
- Florindo, H.F.; Kleiner, R.; Vaskovich-Koubi, D.; Acúrcio, R.C.; Carreira, B.; Yeini, E.; Tiram, G.; Liubomirski, Y.; Satchi-Fainaro, R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020, 15, 630–645. [Google Scholar] [CrossRef]
- Pušnik, J.; Richter, E.; Schulte, B.; Dolscheid-Pommerich, R.; Bode, C.; Putensen, C.; Hartmann, G.; Alter, G.; Streeck, H. Memory B cells targeting SARS-CoV-2 spike protein and their dependence on CD4. Cell Rep. 2021, 35, 109320. [Google Scholar] [CrossRef]
- Prevention CfDCa. Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Pfizer-BioNTech COVID-19 Vaccine. 2020. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html (accessed on 23 August 2021).
- BioNTech. Update on Our COVID-19 Vaccine Development Program with BNT162b2. 2020. Available online: https://investors.biontech.de/static-files/53f0968a-279b-4f82-a2fc-d67dcb6e4e91 (accessed on 30 June 2021).
- Wise, J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ 2021, 372, n699. [Google Scholar] [CrossRef]
- Blauenfeldt, R.A.; Kristensen, S.R.; Ernstsen, S.L.; Kristensen, C.C.H.; Simonsen, C.Z.; Hvas, A.-M. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J. Thromb. Haemost. 2021, 19, 1771–1775. [Google Scholar] [CrossRef]
- Agency, E.M. EMA’s Safety Committee Continues Investigation of COVID-19 Vaccine AstraZeneca and Thromboembolic Events. 2021. Available online: https://www.ema.europa.eu/en/news/emas-safety-committee-continues-investigation-covid-19-vaccine-astrazeneca-thromboembolic-events (accessed on 1 June 2021).
- Ledford, H. COVID vaccines and blood clots: Five key questions. Nature 2021, 592, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Callway, E. Oxford COVID Vaccine Results Puzzle Scientists. Nature 2020, 588, 16–18. [Google Scholar] [CrossRef]
- Agency MHPR. Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting. 2021. Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (accessed on 1 June 2021).
- Bucci, E.; Andreev, K.; Björkman, A.; Calogero, R.A.; Carafoli, E.; Carninci, P.; Castagnoli, P.; Cossarizza, A.; Mussini, C.; Guerin, P.; et al. Safety and efficacy of the Russian COVID-19 vaccine: More information needed. Lancet 2020, 396, e53. [Google Scholar] [CrossRef]
- Cyranoski, D. Why emergency COVID-vaccine approvals pose a dilemma for scientists. Nat. Cell Biol. 2020, 588, 18–19. [Google Scholar]
- Mahase, E. Covid-19: What have we learnt about the new variant in the UK? BMJ 2020, 371, m4944. [Google Scholar] [CrossRef]
- Nagy, Á.; Pongor, S.; Győrffy, B. Different mutations in SARS-CoV-2 associate with severe and mild outcome. Int. J. Antimicrob. Agents 2021, 57, 106272. [Google Scholar] [CrossRef]
- Iyengar, K.P.; Jain, V.K.; Ish, P. COVID-19 reinfection—An enigmatic public health threat. Monaldi Arch. Chest Dis. 2020, 90, 90. [Google Scholar] [CrossRef]
- Raghav, S.; Ghosh, A.; Turuk, J.; Kumar, S.; Jha, A.; Madhulika, S.; Priyadarshini, M.; Biswas, V.K.; Shyamli, P.S.; Singh, B.; et al. Analysis of Indian SARS-CoV-2 Genomes Reveals Prevalence of D614G Mutation in Spike Protein Predicting an Increase in Interaction With TMPRSS2 and Virus Infectivity. Front. Microbiol. 2020, 11, 594928. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A. COVID-19 Genomics UK (COG-UK) Consortium.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Prevention CfDCa. SARS-CoV-2 Variant Classifications and Definitions 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html (accessed on 26 August 2021).
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A. Vaccination NSGfC-. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. South Africa suspends use of AstraZeneca’s COVID-19 vaccine after it fails to clearly stop virus variant. Science 2021, 10. [Google Scholar] [CrossRef]
- Callaway, E. Delta coronavirus variant: Scientists brace for impact. Nat. Cell Biol. 2021, 595, 17–18. [Google Scholar]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Prevention CfDCa. Delta Variant: What We Know About the Science. CDC. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html (accessed on 5 August 2021).
- Bernal, J.L.; Andrews, N.; Gower, C.; Phil, D.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Sanderson, K. COVID vaccines protect against Delta, but their effectiveness wanes. Nat. Cell Biol. 2021. [Google Scholar] [CrossRef]
- Packham, H.; Nina, G.D.; Charles, R.; Radziszewska, A.; Ciurtin, C.; Wedderburn, R.L.; Rosser, E.C.; Deakin, C.; Webb, K. Sex-bias in COVID-19: A meta-analysis and review of sex differences in disease and immunity. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.-K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Scully, E.P.; Haverfield, J.; Ursin, R.L.; Tannenbaum, C.; Klein, S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020, 20, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Bouman, A.; Heineman, M.J.; Faas, M.M. Sex hormones and the immune response in humans. Hum. Reprod. Update 2005, 11, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Ecker, J.W.; Kirchenbaum, G.A.; Pierce, S.R.; Skarlupka, A.L.; Abreu, R.B.; Cooper, R.E.; Taylor-Mulneix, D.; Ross, T.M.; Sautto, G.A. High-Yield Expression and Purification of Recombinant Influenza Virus Proteins from Stably-Transfected Mammalian Cell Lines. Vaccines 2020, 8, 462. [Google Scholar] [CrossRef]
- Reshma, N.J.; Istvan, T.; Maruisz, S. 12-Peptide-Based Vaccines. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 327–358. [Google Scholar]
| Company | Required Doses | Technological and Dosage Overview |
|---|---|---|
| Exposure method: RNA (mRNA) | ||
  Pfizer & BioNTech |  | The Pfizer BioNTech vaccine dosage is 0.3 mL and requires 2 doses, 21 days apart and the Moderna vaccine dosage is 0.5 mL and requires 2 doses, 20 days apart [133,134].Lipid nanoparticles encapsulate mRNA, allowing for the precise delivery of the genetic components of the vaccine, optimizing the translation of the proteins [135]. |
 Moderna |  | |
| Exposure method: Viral Vector | ||
 Sputnik V (recombinant adenovirus type 26 and 5) |  | The SputnikV vaccine dosage is 0.5 mL with 2 vaccine doses, 21 days apart [136]. The Johnson & Johnson vaccine dosage is 0.5 mL and requires 1 vaccine dose [137]. The Oxford AstraZeneca vaccine dosage is 0.5 mL with 2 vaccine doses, 8–12 weeks apart [138].Utilizes an adenovirus vector to elicit spike proteins on cell surfaces resulting in immune responses through the activation of antibodies [75]. |
 Johnson & Johnson (recombinant adenovirus type 26 vector) |  | |
 Oxford AstraZeneca (adenovirus) |  | |
| Exposure method: Protein based | ||
 Novavax |  | Utilizes the S protein from SARS-CoV-2 with recombinant protein nanoparticles and adjuvant MatrixM to elicit desired immune responses, and requires 2 vaccine doses, 21 days apart [139]. |
 Zifivax |  | The vaccine is composed of antigens and viral particles of SARS-CoV-2 that will generate an immune response and requires 3 vaccine doses, 0.5 mL each, over the course of 2 months [140,141]. |
| Exposure method: Peptide-antigen based | ||
 EpiVacCorona |  | Three peptide antigens for SARS-CoV-2 are synthesized consisting of the spike protein and a chimeric protein, an aluminum hydroxide adjuvant is utilized to synthesize the vaccine, and requires 2 vaccine doses, 0.5 mL each, over the course of 21–28 days [142]. |
| Exposure Method: Inactivated Virus | ||
 WIBP-CorV Sinopharm |  | Inactive whole virus technology has been widely studied and is effective for individuals with impaired immune systems and requires 2 vaccine doses, 0.5 mL each, with an interval of 3–4 weeks [132,143]. |
 BBIBP-CorV Sinopharm |  | Inactive whole virus technology has been widely studied and is effective for individuals with impaired immune systems and requires 2 vaccine doses, 0.5 mL each, with an interval of 3–4 weeks [132,143]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieneldien, T.; Kim, J.; Cao, J.; Cao, C. COVID-19 Vaccines: Current Conditions and Future Prospects. Biology 2021, 10, 960. https://doi.org/10.3390/biology10100960
Zieneldien T, Kim J, Cao J, Cao C. COVID-19 Vaccines: Current Conditions and Future Prospects. Biology. 2021; 10(10):960. https://doi.org/10.3390/biology10100960
Chicago/Turabian StyleZieneldien, Tarek, Janice Kim, Jessica Cao, and Chuanhai Cao. 2021. "COVID-19 Vaccines: Current Conditions and Future Prospects" Biology 10, no. 10: 960. https://doi.org/10.3390/biology10100960
APA StyleZieneldien, T., Kim, J., Cao, J., & Cao, C. (2021). COVID-19 Vaccines: Current Conditions and Future Prospects. Biology, 10(10), 960. https://doi.org/10.3390/biology10100960






