Associations of Hyperactivity and Inattention Scores with Theta and Beta Oscillatory Dynamics of EEG in Stop-Signal Task in Healthy Children 7–10 Years Old
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stop-Signal Task
2.3. Electroencephalography (EEG) Records
2.4. Data Analysis
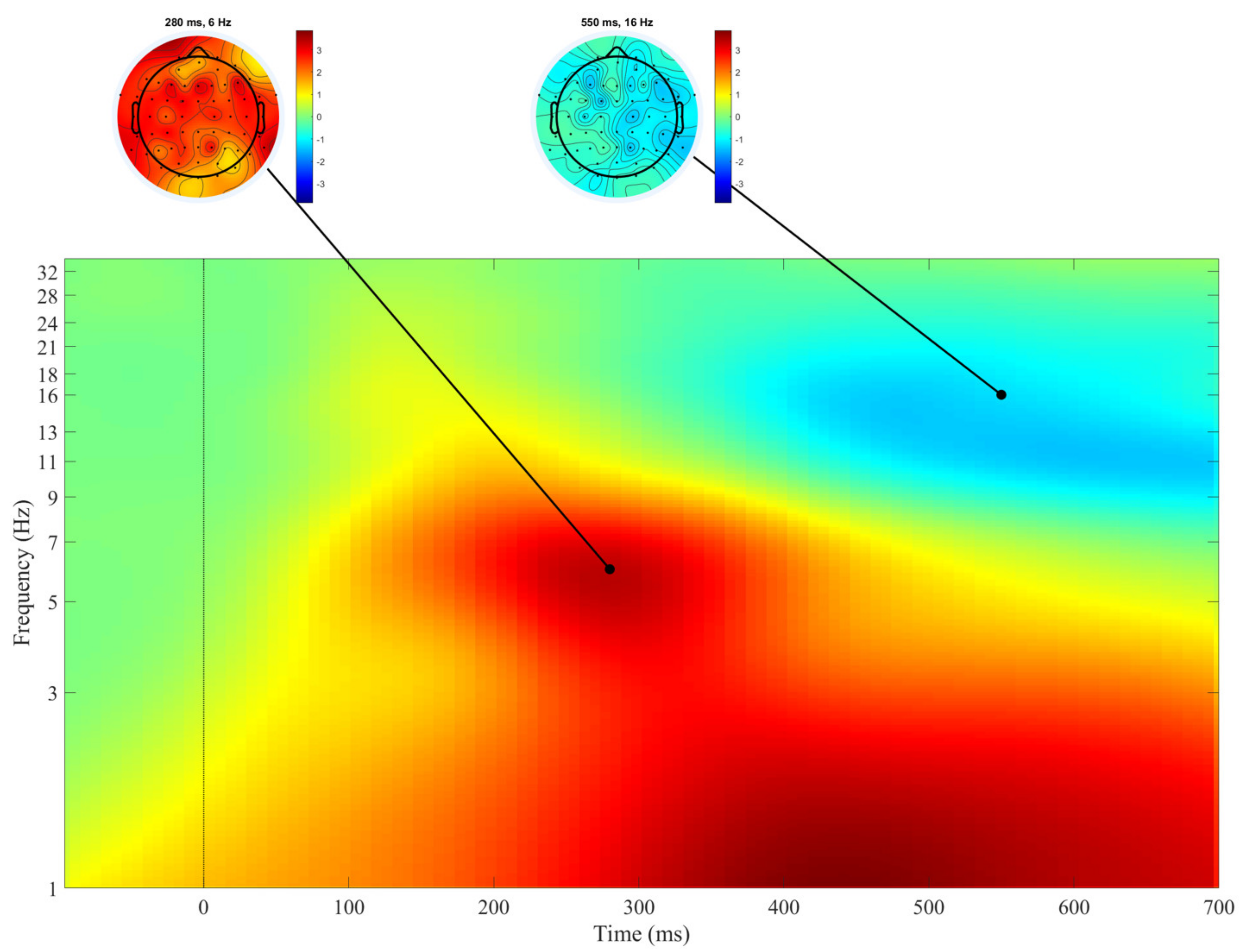
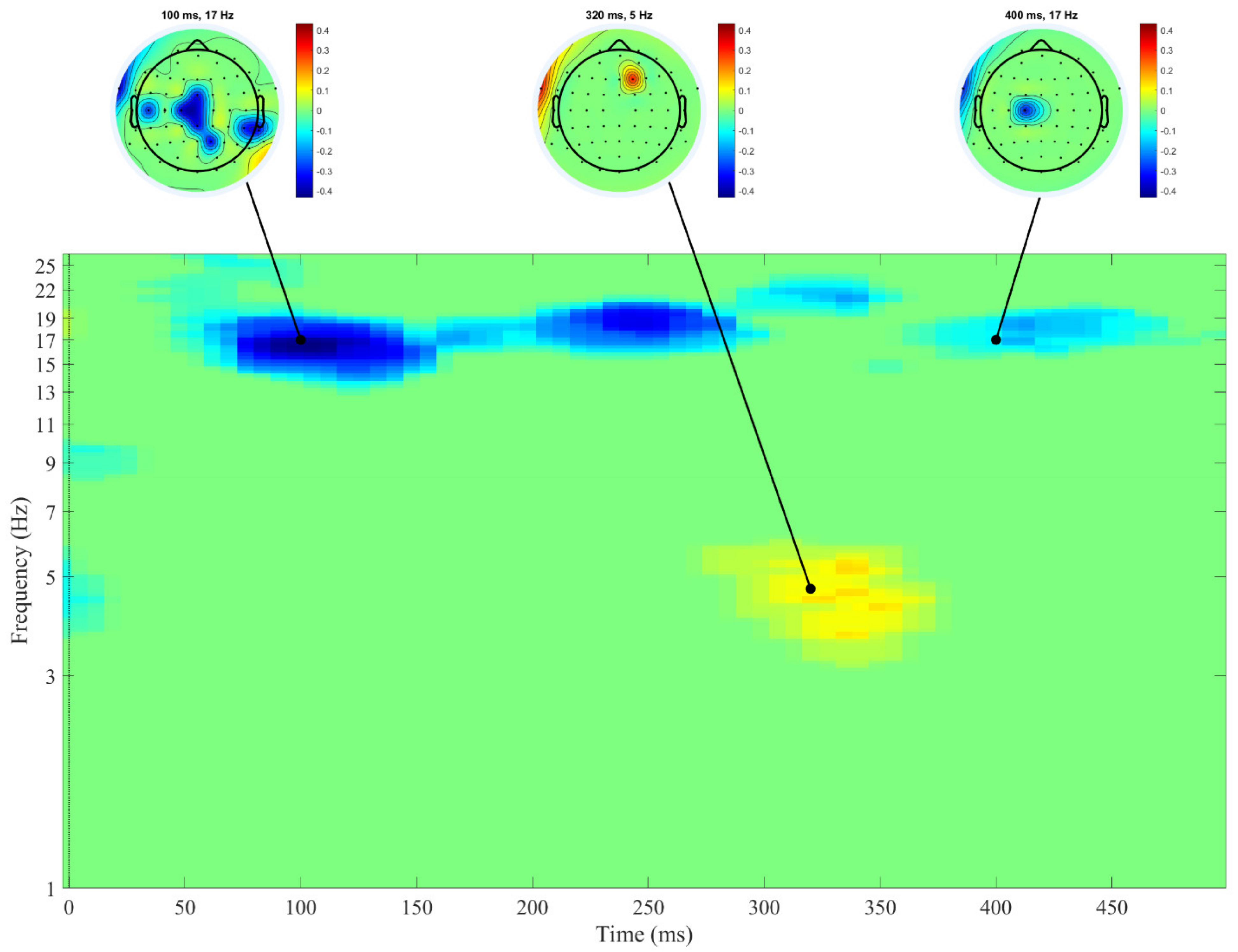
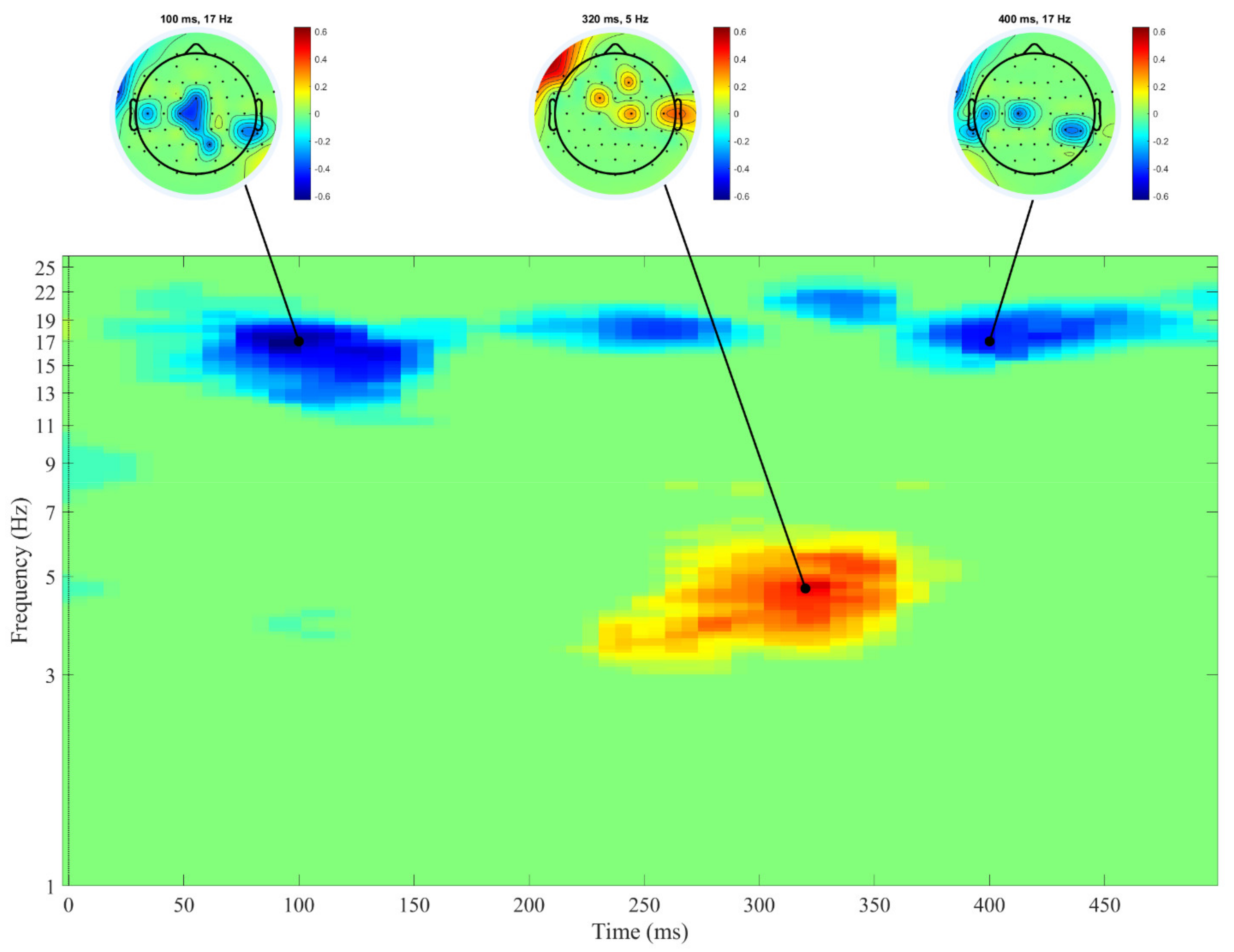
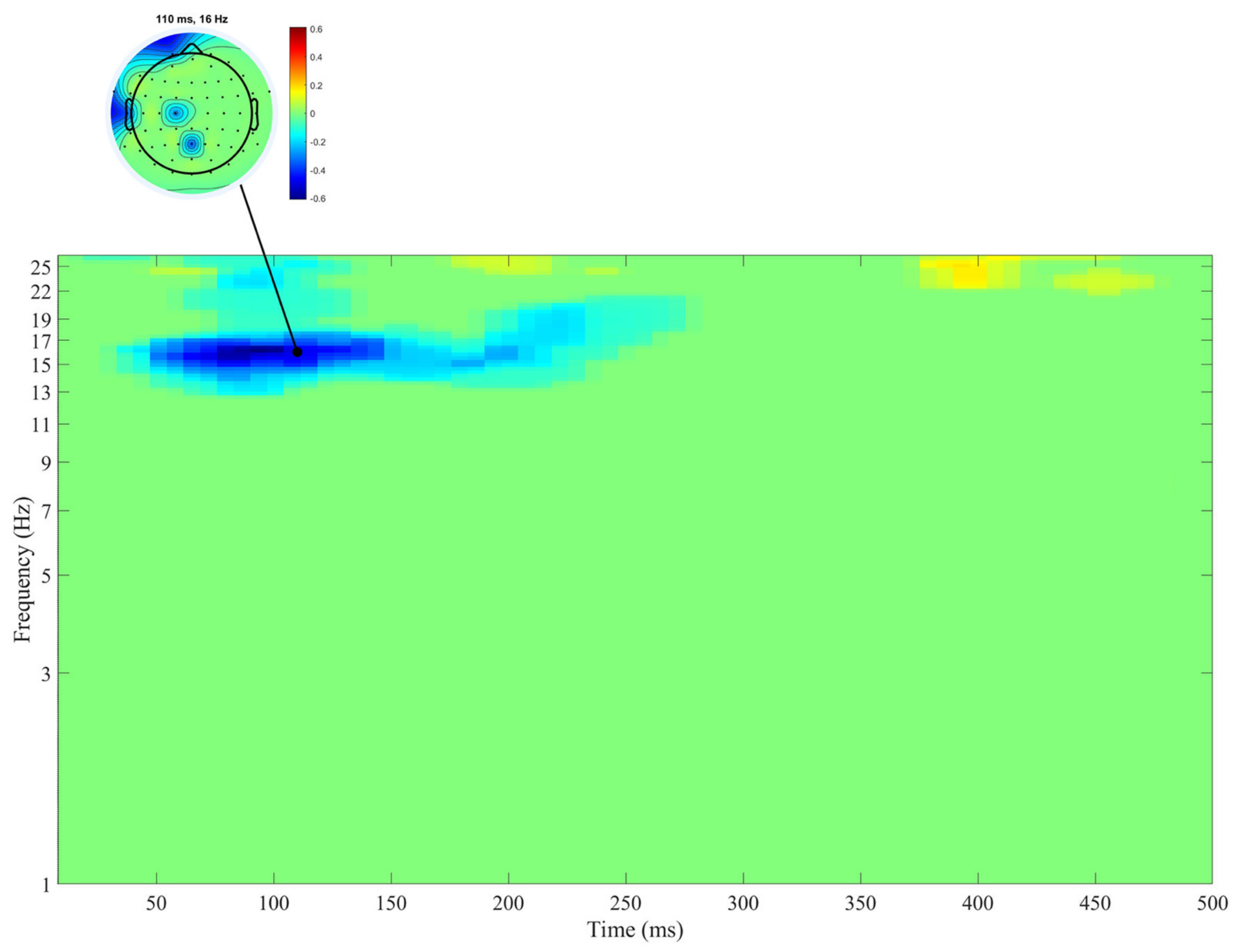
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rader, R.; McCauley, L.; Callen, E.C. Current strategies in the diagnosis and treatment of childhood attention-deficit/hyperactivity disorder. Am. Fam. Physician 2009, 79, 657–665. [Google Scholar]
- Barry, R.J.; Johnstone, S.J.; Clarke, A.R. A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clin. Neurophysiol. 2003, 114, 184–198. [Google Scholar] [CrossRef]
- Bocharov, A.V.; Knyazev, G.G.; Savostyanov, A.N.; Tamozhnikov, S.S.; Saprygin, A.E.; Bairova, N.B.; Slobodskaya, H.R. Correlation of emotional problems and hyperactivity with components of event related potencial in oddball paradigm in children. Zhurnal Vysshei Nervnoi Deyatelnosti Imeni Ip Pavlova 2019, 69, 314–324. [Google Scholar]
- Einziger, T.; Ben-Shachar, M.S.; Devor, T.; Shmueli, M.; Auerbach, J.G.; Berger, A. “My Brain Can Stop”: An ERP Study of Longitudinal Prediction of Inhibitory Control in Adolescence. Brain Sci. 2021, 11, 100. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Du, Y.; Zhai, G.; Xiong, H.; Yao, D.; Li, F. Altered Functional Connectivity in Children with ADHD Revealed by Scalp EEG: An ERP Study. Neural Plast. 2021, 2021, 1–9. [Google Scholar]
- Suwazono, S.; Machado, L.; Knight, R.T. Predictive value of novel stimuli modifies visual event-related potentials and behavior. Clin. Neurophysiol. 2000, 111, 29–39. [Google Scholar] [CrossRef]
- Senderecka, M.; Grabowska, A.; Gerc, K.; Szewczyk, J.; Chmylak, R. Event-related potentials in children with attention deficit hyperactivity disorder: An investigation using an auditory oddball task. Int. J. Psychophys. 2012, 85, 106–115. [Google Scholar] [CrossRef]
- Kaiser, A.; Aggensteiner, P.M.; Baumeister, S.; Holz, N.E.; Banaschewski, T.; Brandeis, D. Earlier versus later cognitive event-related potentials (ERPs) in attention-deficit/hyperactivity disorder (ADHD): A meta-analysis. Neurosci. Biobehav. Rev. 2020, 112, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin. Neurophys. 2003, 114, 171–183. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; McCarthy, R.; Selikowitz, M. Age and sex effects in the EEG: Differences in two subtypes of attention-deficit/hyperactivity disorder. Clin. Neurophys. 2001, 112, 815–826. [Google Scholar] [CrossRef]
- Deiber, M.P.; Hasler, R.; Colin, J.; Dayer, A.; Aubry, J.M.; Baggio, S.; Perroud, N.; Ros, T. Linking alpha oscillations, attention and inhibitory control in adult ADHD with EEG neurofeedback. NeuroImage Clin. 2020, 25, 102145. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Eckstrand, K.; Sharp, W.; Blumenthal, J.; Lerch, J.P.; Greenstein, D.; Clasen, A.E.; Giedd, J.; Rapoport, J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19649–19654. [Google Scholar] [CrossRef]
- Gasser, T.; Verleger, R.; Bächer, P.; Sroka, L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalogr. Clin. Neurophysiol. 1988, 69, 91–99. [Google Scholar] [CrossRef]
- Fernández, A.; Quintero, J.; Hornero, R.; Zuluaga, P.; Navas, M.; Gómez, C.; Escudero, J.; García-Campos, N.; Biederman, J.; Ortiz, T. Complexity analysis of spontaneous brain activity in attention-deficit/hyperactivity disorder: Diagnostic implications. Biol. Psychiatry 2009, 65, 571–577. [Google Scholar] [CrossRef]
- Liechti, M.D.; Valko, L.; Müller, U.C.; Döhnert, M.; Drechsler, R.; Steinhausen, H.C.; Brandeis, D. Diagnostic value of resting electroencephalogram in attention-deficit/hyperactivity disorder across the lifespan. Brain Topogr. 2013, 26, 135–151. [Google Scholar] [CrossRef]
- Markovska-Simoska, S.; Pop-Jordanova, N. Quantitative EEG in children and adults with attention deficit hyperactivity disorder: Comparison of absolute and relative power spectra and theta/beta ratio. Clin. EEG Neurosci. 2017, 48, 20–32. [Google Scholar] [CrossRef] [PubMed]
- McVoy, M.; Lytle, S.; Fulchiero, E.; Aebi, M.E.; Adeleye, O.; Sajatovic, M. A systematic review of quantitative EEG as a possible biomarker in child psychiatric disorders. Psychiatry Res. 2019, 279, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Coolidge, F.L.; Starkey, M.T.; Cahill, B.S. Comparison of a parent-rated DSM-IV measure of attention-deficit/hyperactivity disorder and quantitative EEG parameters in an outpatient sample of children. J. Clin. Neurophys. 2007, 24, 348–351. [Google Scholar] [CrossRef]
- Baijot, S.; Cevallos, C.; Zarka, D.; Leroy, A.; Slama, H.; Colin, C.; Cheron, G. EEG dynamics of a go/nogo task in children with ADHD. Brain Sci. 2017, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Lenartowicz, A.; Delorme, A.; Walshaw, P.D.; Cho, A.L.; Bilder, R.M.; McGough, J.J.; McCracken, J.T.; Makeig, S.; Loo, S.K. Electroencephalography correlates of spatial working memory deficits in attention-deficit/hyperactivity disorder: Vigilance, encoding, and maintenance. J. Neurosci. 2014, 34, 1171–1182. [Google Scholar] [CrossRef]
- Rommel, A.S.; James, S.N.; McLoughlin, G.; Brandeis, D.; Banaschewski, T.; Asherson, P.; Kuntsi, J. Altered EEG spectral power during rest and cognitive performance: A comparison of preterm-born adolescents to adolescents with ADHD. Eur. Child Adolesc. Psychiatry 2017, 26, 1511–1522. [Google Scholar] [CrossRef]
- Yordanova, J.; Heinrich, H.; Kolev, V.; Rothenberger, A. Increased event-related theta activity as a psychophysiological marker of comorbidity in children with tics and attention-deficit/hyperactivity disorders. NeuroImage 2006, 32, 940–955. [Google Scholar] [CrossRef]
- Tillman, C.M.; Thorell, L.B.; Brocki, K.C.; Bohlin, G. Motor response inhibition and execution in the stop-signal task: Development and relation to ADHD behaviors. Child Neuropsychol. 2007, 14, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Kofler, M.J.; Rapport, M.D.; Sarver, D.E.; Raiker, J.S.; Orban, S.A.; Friedman, L.M.; Kolomeyer, E.G. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clin. Psychol. Rev. 2013, 33, 795–811. [Google Scholar] [CrossRef]
- Weigard, A.; Heathcote, A.; Matzke, D.; Huang-Pollock, C. Cognitive modeling suggests that attentional failures drive longer stop-signal reaction time estimates in attention deficit/hyperactivity disorder. Clin. Psychol. Sci. 2019, 7, 856–872. [Google Scholar] [CrossRef] [PubMed]
- Logan, G.D.; Cowan, W.B.; Davis, K.A. On the ability to inhibit simple and choice reaction time responses: A model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984, 10, 276. [Google Scholar] [CrossRef]
- van Tol, M.J.; van der Wee, N.J.; van den Heuvel, O.A.; Nielen, M.M.A.; Demenescu, L.R.; Aleman, A.; Renken, R.; Van Buchem, M.A.; Zitman, F.G.; Veltman, D.J. Regional brain volume in depression and anxiety disorders. Arch. Gen. Psychiatry 2010, 67, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, B.N. The role of RDoC in future classification of mental disorders. Dialogues Clin. Neurosci. 2020, 22, 81–85. [Google Scholar]
- Güntekin, B.; Başar, E. A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia 2014, 58, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Del Percio, C.; Vecchio, F.; Sebastiano, F.; Di Gennaro, G.; Quarato, P.P.; Morace, R.; Pavone, L.; Soricelli, A.; Noce, G.; et al. Alpha, beta and gamma electrocorticographic rhythms in somatosensory, motor, premotor and prefrontal cortical areas differ in movement execution and observation in humans. Clin. Neurophys. 2016, 127, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Del Percio, C.; Lopez, S.; Di Gennaro, G.; Quarato, P.P.; Pavone, L.; Morace, R.; Soricelli, A.; Noce, G.; Esposito, V.; et al. Frontal functional connectivity of electrocorticographic delta and theta rhythms during action execution versus action observation in humans. Front. Behav. Neurosci. 2017, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R. Psychometric properties of the strengths and difficulties questionnaire. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 1337–1345. [Google Scholar] [CrossRef]
- Goodman, R.; Slobodskaya, H.; Knyazev, G. Russian child mental health a cross-sectional study of prevalence and risk factors. Eur. Child Adolesc. Psychiatry 2005, 14, 28–33. [Google Scholar] [CrossRef]
- Knyazev, G.G.; Levin, E.A.; Savostyanov, A.N. Impulsivity, anxiety, and individual differences in evoked and induced brain oscillations. Int. J. Psychophys. 2008, 68, 242–254. [Google Scholar] [CrossRef]
- Bocharov, A.V.; Savostyanov, A.N.; Tamozhnikov, S.S.; Saprigyn, A.E.; Proshina, E.A.; Astakhova, T.N.; Knyazev, G.G. Impact of polymorphisms in the serotonin transporter gene on oscillatory dynamics during inhibition of planned movement in children. Brain Sci. 2019, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, G.; Pani, P.; Paré, M.; Ferraina, S. Inhibitory control of reaching movements in humans. Exp. Brain Res. 2006, 174, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, A.; Picton, T.W. EEG spectral dynamics during discrimination of auditory and visual targets. Cogn. Brain Res. 2005, 24, 81–96. [Google Scholar] [CrossRef]
- Başar-Eroglu, C.; Başar, E.; Demiralp, T.; Schürmann, M. P300-response: Possible psychophysiological correlates in delta and theta frequency channels. A review. Int. J. Psychophysiol. 1992, 13, 161–179. [Google Scholar] [CrossRef]
- Calmels, C.; Holmes, P.; Jarry, G.; Lévêque, J.M.; Hars, M.; Stam, C.J. Cortical activity prior to, and during, observation and execution of sequential finger movements. Brain Topogr. 2006, 19, 77–88. [Google Scholar] [CrossRef][Green Version]
- Satterfield, J.H.; Dawson, M.E. Electrodermal correlates of hyperactivity in children. Psychophysiology 1971, 8, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, J. The cognitive-energetic model: An empirical approach to attention-deficit hyperactivity disorder. Neurosci. Biobehav. Rev. 2000, 24, 7–12. [Google Scholar] [CrossRef]
- Hegerl, U.; Hensch, T. The vigilance regulation model of affective disorders and ADHD. Neurosci. Biobehav. Rev. 2014, 44, 45–57. [Google Scholar] [CrossRef]
- Grønli, J.; Rempe, M.J.; Clegern, W.C.; Schmidt, M.; Wisor, J.P. Beta EEG reflects sensory processing in active wakefulness and homeostatic sleep drive in quiet wakefulness. J. Sleep Res. 2016, 25, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, B.; Haegens, S. Beyond the status quo: A role for beta oscillations in endogenous content (re) activation. Eneuro 2017, 4, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A. Beta activity: A carrier for visual attention. Acta Neurobiol. Exp. 2000, 60, 247–260. [Google Scholar]
- Ogrim, G.; Kropotov, J.; Hestad, K. The quantitative EEG theta/beta ratio in attention deficit/hyperactivity disorder and normal controls: Sensitivity, specificity, and behavioral correlates. Psychiatry Res. 2012, 198, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Wiersema, R.; Van Der Meere, J.; Roeyers, H.; Van Coster, R.; Baeyens, D. Event rate and event-related potentials in ADHD. J. Child Psychol. Psychiatry 2006, 47, 560–567. [Google Scholar] [CrossRef]
- Huycke, P.; Verbeke, P.; Boehler, C.N.; Verguts, T. Theta and alpha power across fast and slow timescales in cognitive control. Eur. J. Neurosci. 2021, 54, 4581–4594. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.S.; Eling, P.A.; Hopman, M.T.; Coenen, A.M. Mental and physical effort affect vigilance differently. Int. J. Psychophys. 2005, 57, 211–217. [Google Scholar] [CrossRef]
- Ward, A.R.; Sibley, M.H.; Musser, E.D.; Campez, M.; Bubnik-Harrison, M.G.; Meinzer, M.C. Relational impairments, sluggish cognitive tempo, and severe inattention are associated with elevated self-rated depressive symptoms in adolescents with ADHD. ADHD Atten. Deficit Hyperact. Disord. 2019, 11, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, G. Inhibitory control and impulsive responses in neurodevelopmental disorders. Dev. Med. Child. Neurol. 2021, 63, 520–526. [Google Scholar] [CrossRef] [PubMed]
- van Hulst, B.M.; de Zeeuw, P.; Vlaskamp, C.; Rijks, Y.; Zandbelt, B.B.; Durston, S. Children with ADHD symptoms show deficits in reactive but not proactive inhibition, irrespective of their formal diagnosis. Psychol. Med. 2018, 48, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Zandbelt, B.B.; Vink, M. On the role of the striatum in response inhibition. PLoS ONE 2010, 5, e13848. [Google Scholar] [CrossRef]
- Mancini, C.; Cardona, F.; Baglioni, V.; Panunzi, S.; Pantano, P.; Suppa, A.; Mirabella, G. Inhibition is impaired in children with obsessive-compulsive symptoms but not in those with tics. Mov. Disord. 2018, 33, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, G.; Mancini, C.; Valente, F.; Cardona, F. Children with primary complex motor stereotypies show impaired reactive but not proactive inhibition. Cortex 2020, 124, 250–259. [Google Scholar] [CrossRef]
| Min | Max | Mean | SD | Median | QR | 95% CI Lower Bound | 95% CI Upper Bound | |
|---|---|---|---|---|---|---|---|---|
| Age | 7 | 10 | 8.6 | 1.1 | 8.6 | 2 | 8.4 | 8.8 |
| Hyperactivity/inattention SDQ scores | 0 | 10 | 4.4 | 2.5 | 4 | 4 | 3.8 | 4.9 |
| Separated Hyperactivity SDQ scores | 0 | 4 | 1.4 | 1.3 | 1 | 2 | 1.1 | 1.7 |
| Separated Inattention SDQ scores | 0 | 6 | 3 | 1.6 | 3 | 2 | 2.6 | 3.3 |
| Mean Go reaction time (ms) | 537 | 702 | 619.6 | 36 | 622 | 55.5 | 612 | 627 |
| SD of Go reaction time (ms) | 34 | 129 | 80.6 | 15.8 | 80.5 | 19 | 77.4 | 83.9 |
| Mean Stop-failure reaction time (ms) | 481 | 709 | 593.3 | 55.1 | 594 | 71.5 | 581 | 604 |
| SD of Stop-failure reaction time (ms) | 2 | 146 | 77.1 | 26.9 | 76 | 28.5 | 71.5 | 82.7 |
| Successful “Stop” | 22% | 94% | 63.5% | 14.4 | 65 | 20.3 | 60.5 | 66.4 |
| Successful “Go” | 34% | 100% | 66.7% | 19.7 | 67.5 | 33.3 | 62.6 | 70.7 |
| Go Condition | ||||||
|---|---|---|---|---|---|---|
| SD RT | Beta | Theta | ||||
| r | p | r | p | r | p | |
| Hyperactivity/inattention SDQ scores | 0.23 | 0.027 | –0.21 | 0.049 | ns | ns |
| Separated hyperactivity SDQ scores | ns | ns | ns | ns | ns | ns |
| Separated inattention SDQ scores | 0.227 | 0.03 | –0.23 | 0.027 | 0.24 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocharov, A.V.; Savostyanov, A.N.; Slobodskaya, H.R.; Tamozhnikov, S.S.; Levin, E.A.; Saprigyn, A.E.; Proshina, E.A.; Astakhova, T.N.; Merkulova, E.A.; Knyazev, G.G. Associations of Hyperactivity and Inattention Scores with Theta and Beta Oscillatory Dynamics of EEG in Stop-Signal Task in Healthy Children 7–10 Years Old. Biology 2021, 10, 946. https://doi.org/10.3390/biology10100946
Bocharov AV, Savostyanov AN, Slobodskaya HR, Tamozhnikov SS, Levin EA, Saprigyn AE, Proshina EA, Astakhova TN, Merkulova EA, Knyazev GG. Associations of Hyperactivity and Inattention Scores with Theta and Beta Oscillatory Dynamics of EEG in Stop-Signal Task in Healthy Children 7–10 Years Old. Biology. 2021; 10(10):946. https://doi.org/10.3390/biology10100946
Chicago/Turabian StyleBocharov, Andrey V., Alexander N. Savostyanov, Helena R. Slobodskaya, Sergey S. Tamozhnikov, Evgeny A. Levin, Alexander E. Saprigyn, Ekaterina A. Proshina, Tatiana N. Astakhova, Ekaterina A. Merkulova, and Gennady G. Knyazev. 2021. "Associations of Hyperactivity and Inattention Scores with Theta and Beta Oscillatory Dynamics of EEG in Stop-Signal Task in Healthy Children 7–10 Years Old" Biology 10, no. 10: 946. https://doi.org/10.3390/biology10100946
APA StyleBocharov, A. V., Savostyanov, A. N., Slobodskaya, H. R., Tamozhnikov, S. S., Levin, E. A., Saprigyn, A. E., Proshina, E. A., Astakhova, T. N., Merkulova, E. A., & Knyazev, G. G. (2021). Associations of Hyperactivity and Inattention Scores with Theta and Beta Oscillatory Dynamics of EEG in Stop-Signal Task in Healthy Children 7–10 Years Old. Biology, 10(10), 946. https://doi.org/10.3390/biology10100946






