The Role of Satellite Cells in Skeletal Muscle Regeneration—The Effect of Exercise and Age
Abstract
:Simple Summary
Abstract
1. History of Studies on Satellite Cells
2. Pax7 Characterizes Inactive mSCs
3. Inflammatory Processes Involved in Muscle Regeneration
4. Presence of MyoD1 and Myf5 Is Characteristic of Proliferating Satellite Cells
5. Myogenin and Myf6 have Essential Roles in mSCs Differentiation
6. Fusion Is the Final Stage of Muscle Fiber Regeneration
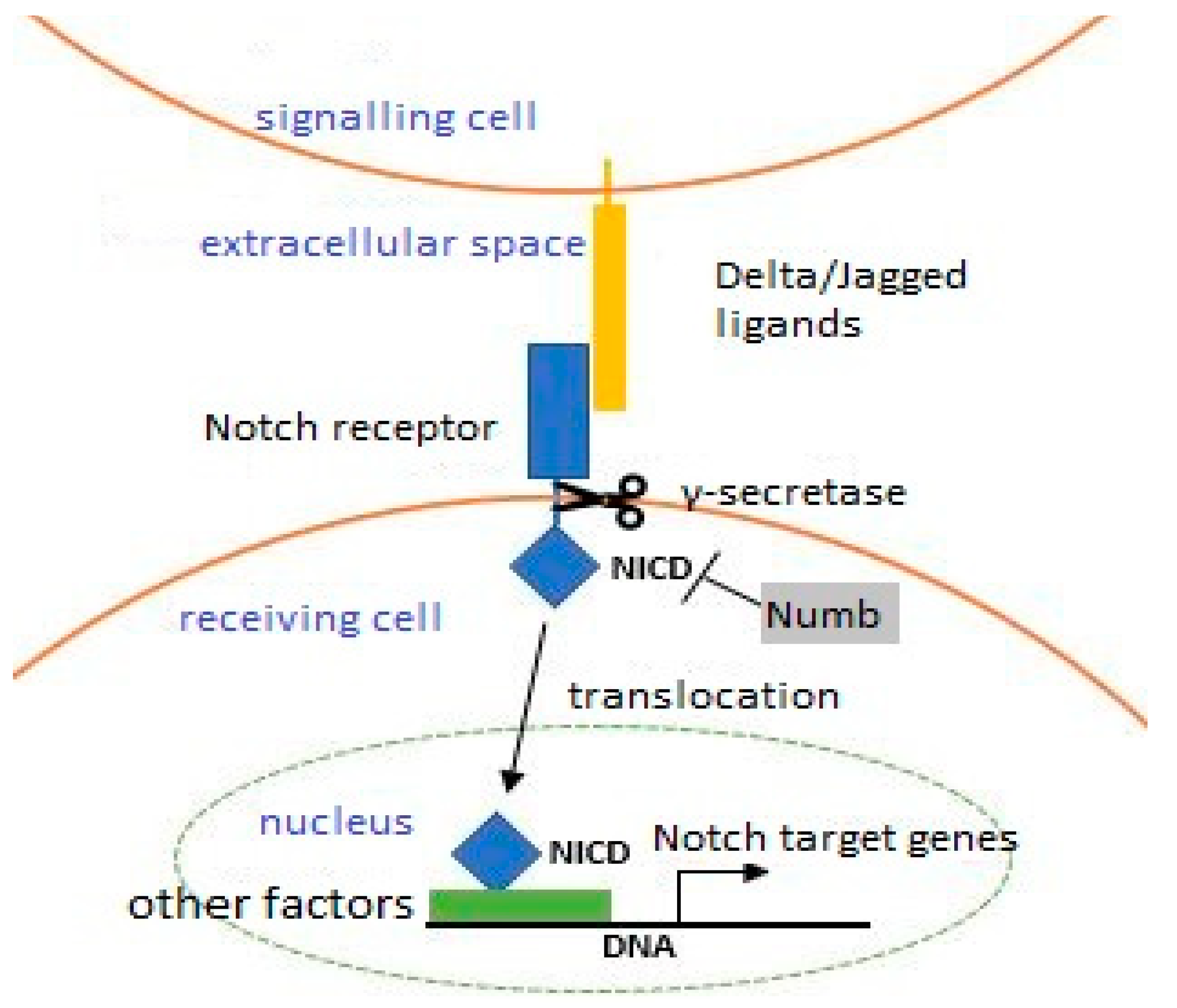
7. Satellite Cell Self-Renewal
8. Satellite Cells and Physical Activity
9. Satellite Cells and Age
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Cell Biol. 1961, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Katz, B. The terminations of the afferent nerve fiber in the muscle spindle of the frog. Philos. Transac. R. Soc. Lond. B Biol. Sci. 1961, 243, 221–240. [Google Scholar]
- Ishikawa, H. Electron microscopic observations of satellite cells with special reference to the development of mammalian skeletal muscles. Brain Struct. Funct. 1966, 125, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.M.; Zacks, S.I. The histogenesis of rat intercostal muscle. J. Cell Biol. 1969, 42, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Moss, F.P.; Leblond, C.P. Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1971, 170, 421–435. [Google Scholar] [CrossRef]
- Shultz, E. A quantitative study of the satellite cell population in postnatal mouse lumbrical muscle. Anat. Rec. 1974, 180, 589–595. [Google Scholar] [CrossRef]
- Konigsberg, U.R.; Lipton, B.H.; Konigsberg, I.R. The regenerative response of single mature muscle fibers isolated in vitro. Dev. Biol. 1975, 45, 260–275. [Google Scholar] [CrossRef]
- Bischoff, R. A satellite cell mitogen from crushed adult muscle. Dev. Biol. 1986, 115, 140–147. [Google Scholar] [CrossRef]
- Allen, R.E.; Boxhorn, L.K. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J. Cell. Physiol. 1987, 133, 567–572. [Google Scholar] [CrossRef]
- Cornelison, D.; Wold, B.J. Single-Cell Analysis of Regulatory Gene Expression in Quiescent and Activated Mouse Skeletal Muscle Satellite Cells. Dev. Biol. 1997, 191, 270–283. [Google Scholar] [CrossRef] [Green Version]
- Maroto, M.; Reshef, R.; Munsterberg, A.; Koester, S.; Goulding, M.; Lassar, A.B. Ectopic Pax-3 Activates MyoD and Myf-5 Expression in Embryonic Mesoderm and Neural Tissue. Cell 1997, 89, 139–148. [Google Scholar] [CrossRef]
- Bischoff, R. Interaction between satellite cells and skeletal muscle fibers. Development 1990, 109, 943–952. [Google Scholar] [CrossRef]
- Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric Self-Renewal and Commitment of Satellite Stem Cells in Muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef] [Green Version]
- Przewoźniak, M.; Brzóska, E. Białka Pax w różnicowaniu komórek i organogenezie. Post. Biol. Kom. 2008, 2, 229–242. [Google Scholar]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 Is Required for the Specification of Myogenic Satellite Cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Relaix, F.; Montarras, D.; Zaffran, S.; Gayraud-Morel, B.; Rocancourt, D.; Tajbakhsh, S.; Mansouri, A.; Cumano, A.; Buck-ingham, M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J. Cell Biol. 2006, 17, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.; Chargé, S.B.; Seale, P.; Huh, M.; Rudnicki, M.A. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J. Cell Biol. 2006, 172, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmalbruch, H.; Lewis, D. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve 2000, 23, 617–626. [Google Scholar] [CrossRef]
- Bansal, D.; Miyake, K.; Vogel, S.; Groh, S.; Chen, C.-C.; Williamson, R.A.; McNeil, P.L.; Campbell, K. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nat. Cell Biol. 2003, 423, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Mellgren, R.L.; Miyake, K.; Kramerova, I.; Spencer, M.J.; Bourg, N.; Bartoli, M.; Richard, I.; Greer, P.A.; McNeil, P.L. Cal-cium-dependent plasma membrane repair requires m- or μ-calpain, but not calpain-3, the proteasome, or caspases. Biochim. Biophys. Acta 2009, 1793, 1886–1893. [Google Scholar] [CrossRef] [Green Version]
- Ciciliot, S.; Schiaffino, S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr. Pharma. Des. 2010, 16, 906–914. [Google Scholar] [CrossRef] [Green Version]
- Chargé, S.B.P.; Rudnicki, M. Cellular and Molecular Regulation of Muscle Regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orimo, S.; Hiyamuta, E.; Arahata, K.; Sugita, H. Analysis of inflammatory cells and complement C3 in bupivacaine-induced myonecrosis. Muscle Nerve 1991, 14, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Manfredi, T.J.; Ding, W.; Fiatarone, M.A.; Evans, W.J.; Cannon, J.G. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1993, 265, R166–R172. [Google Scholar] [CrossRef] [PubMed]
- Ceafalan, L.C.; Popescu, B.O.; Hinescu, M.E. Cellular Players in Skeletal Muscle Regeneration. BioMed Res. Int. 2014, 2014, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sonnet, C.; Lafuste, P.; Arnold, L.; Brigitte, M.; Poron, F.; Authier, F.J.; Chrétien, F.; Gherardi, R.K.; Chazaud, B. Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. J. Cell Sci. 2006, 119, 2497–2507. [Google Scholar] [CrossRef] [Green Version]
- Lescaudron, L.; Peltékian, E.; Fontaine-Pérus, J.; Paulin, D.; Zampieri, M.; Garcia, L.; Parrish, E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul. Disord. 1999, 9, 72–80. [Google Scholar] [CrossRef]
- Schultz, E.; Jaryszak, D.L.; Valliere, C.R. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve 1985, 8, 217–222. [Google Scholar] [CrossRef]
- Alfaro, L.A.S.; Dick, S.A.; Siegel, A.L.; Anonuevo, A.S.; McNagny, K.; Megeney, L.; Cornelison, D.D.; Rossi, F.M. CD34 Promotes Satellite Cell Motility and Entry into Proliferation to Facilitate Efficient Skeletal Muscle Regeneration. Stem Cells 2011, 29, 2030–2041. [Google Scholar] [CrossRef] [Green Version]
- Hughes, S.; Blau, H.M. Migration of myoblasts across basal lamina during skeletal muscle development. Nat. Cell Biol. 1990, 345, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Koishi, K.; Masuda, K.; Nabeshima, Y. Cell heterogeneity upon myogenic differentiation: Down-regulation of MyoD and Myf-5 generates ‘reserve cells’. J. Cell Sci. 1998, 111, 769–779. [Google Scholar] [CrossRef]
- Yablonka-Reuveni, Z.; Rudnicki, M.A.; Rivera, A.J.; Primig, M.; Anderson, J.E.; Natanson, P. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Develop. Biol. 1999, 210, 440–455. [Google Scholar] [CrossRef] [Green Version]
- Megeney, L.A.; Kablar, B.; Garrett, K.; Anderson, J.E.; Rudnicki, M.A. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996, 10, 1173–1183. [Google Scholar] [CrossRef] [Green Version]
- Sabourin, L.A.; Girgis-Gabardo, A.; Seale, P.; Asakura, A.; Rudnicki, M.A. Reduced Differentiation Potential of Primary MyoD−/− Myogenic Cells Derived from Adult Skeletal Muscle. J. Cell Biol. 1999, 144, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Cornelison, D.D.; Olwin, B.B.; Rudnicki, M.A.; Wolb, B.J. MyoD(−/−) satellite cells in single- fiber culture are differentiation defective and MRF4 deficient. Develop. Biol. 2000, 224, 122–137. [Google Scholar] [CrossRef] [Green Version]
- Asakura, A.; Hirai, H.; Kablar, B.; Morita, S.; Ishibashi, J.; Piras, B.A.; Christ, A.J.; Verma, M.; Vineretsky, K.A.; Rudnicki, M.A. Increased survival of muscle stem cells lacking the MyoD gene after transplantation into regenerating skeletal muscle. Proc. Natl. Acad. Sci. USA 2007, 104, 16552–16557. [Google Scholar] [CrossRef] [Green Version]
- Gayraud-Morel, B.; Chrétien, F.; Flamant, P.; Gomès, D.; Zammit, P.; Tajbakhsh, S. A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev. Biol. 2007, 312, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Gensch, N.; Borchardt, T.; Schneider, A.; Riethmacher, D.; Braun, T. Different autonomous myogenic cell populations revealed by ablation of Myf5-expressing cells during mouse embryogenesis. Development 2008, 135, 1597–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kablar, B.; Rudnicki, M.A. Skeletal muscle development in the mouse embryo. Histol. Histopathol. 2000, 15, 649–656. [Google Scholar] [PubMed]
- Li, S.; Czubryt, M.; McAnally, J.; Bassel-Duby, R.; Richardson, J.A.; Wiebel, F.F.; Nordheim, A.; Olson, E.N. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1082–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- L’Honoré, A.; Rana, V.; Arsic, N.; Franckhauser, C.; Lamb, N.J.; Fernandez, A. Identification of a New Hybrid Serum Response Factor and Myocyte Enhancer Factor 2-binding Element in MyoD Enhancer Required for MyoD Expression during Myogenesis. Mol. Biol. Cell 2007, 18, 1992–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.-M.; Wei, Q.; Zhao, X.; Paterson, B.M. Coupling of the cell cycle and myogenesis through the cyclin D1-dependent interaction of MyoD with cdk4. EMBO J. 1999, 18, 926–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitzmann, M.; Fernández, A. Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell. Mol. Life Sci. 2001, 58, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Berkes, C.A.; Tapscott, S.J. MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 2005, 16, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, K.K.; Marinescu, V.D.; Ramoni, M.F.; Sanoudou, D.; Montanaro, F.; Han, M.; Kunkel, L.M.; Kohane, I.S.; Beggs, A.H. Expression profiling and identification of novel genes involved in myogenic differentiation. FASEB J. 2004, 18, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yablonka–Reuveni, Z.; Paterson, B.M. MyoD and Myogenin Expression Patterns in Cultures of Fetal and Adult Chicken Myoblasts. J. Histochem. Cytochem. 2001, 49, 455–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, D.; Gorospe, J.R.; Brenman, J.E.; Rafael, J.A.; Peters, M.F.; Froehner, S.C.; Hoffman, E.; Chamberlain, J.S.; Bredt, D.S. Selective loss of sarcolemmal nitric oxide synthase in Becker muscular dystrophy. J. Exp. Med. 1996, 184, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Kumar, R.M.; Penn, B.H.; Berkes, C.A.; Kooperberg, C.; Boyer, L.A.; Young, R.A.; Tapscott, S.J. Global and gene-specific analyses show distinct roles for Myod and Myog at a common set of promoters. EMBO J. 2006, 25, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Meadows, E.; Cho, J.-H.; Flynn, J.M.; Klein, W.H. Myogenin regulates a distinct genetic program in adult muscle stem cells. Dev. Biol. 2008, 322, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, U.; Martin, B.; Link, D.; Witt, K.; Zeitler, R.; Reinhard, S.; Starzinski-Powitz, A. M-cadherin and its sisters in de-velopment of striated muscle. Cell Tiss. 1999, 296, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.; Walsh, F. The cell adhesion molecule M-cadherin is specifically expressed in developing and regenerating, but not denervated skeletal muscle. Development 1993, 117, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, R.J.; Deasy, B.M.; Cao, B.; Gates, C.; Huard, J. The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J. Cell Sci. 2002, 115, 4361–4374. [Google Scholar] [CrossRef] [Green Version]
- Hollnagel, A.; Grund, C.; Franke, W.W.; Arnold, H.H. The cell adhesion molecule M-cadherin is not essential for muscle de-velopment and regeneration. Mol. Cell. Biol. 2002, 22, 4760–4770. [Google Scholar] [CrossRef] [Green Version]
- Brzoska, E.; Ciemerych, M.A.; Przewozniak, M.; Zimowska, M. Regulation of muscle stem cells activation: The role of growth factors and extracellular matrix. Vitam. Horm. 2011, 87, 239–276. [Google Scholar]
- Brustis, J.J.; Elamrani, N.; Balcerzak, D.; Safwate, A.; Soriano, M.; Poussard, S.; Cottin, P.; Ducastaing, A. Rat myoblast fusion requires exteriorized m-calpain activity. Eur. J. Cell Biol. 1994, 64, 320–327. [Google Scholar]
- Smythe, G.; Davies, M.; Paulin, D.; Grounds, M. Absence of desmin slightly prolongs myoblast proliferation and delays fusion in vivo in regenerating grafts of skeletal muscle. Cell Tissue Res. 2001, 304, 287–294. [Google Scholar] [CrossRef]
- Zammit, P.S.; Heslop, L.; Hudon, V.; Rosenblatt, J.D.; Tajbakhsh, S.; Buckingham, M.E.; Beauchamp, J.R.; Partridge, T.A. Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp. Cell Res. 2002, 281, 39–49. [Google Scholar] [CrossRef]
- Czerwińska, A.M.; Streminska, W.; Ciemerych, M.A.; Grabowska, I. Mouse gastrocnemius muscle regeneration after me-chanical or cardiotoxin injury. Folia Histochem. Cytobiol. 2012, 50, 144–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, E.; McCormick, K.M. Skeletal muscle satellite cells. Rev. Physiol. Biochem. Pharmacol. 1994, 123, 213–257. [Google Scholar] [PubMed]
- Moyer, A.L.; Wagner, K.R. Regeneration versus fibrosis in skeletal muscle. Curr. Opin. Rheumatol. 2011, 23, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.V.; Pardal, R.; Morrison, S.J. Diverse mechanisms regulate stem cell self-renewal. Curr. Opin. Cell Biol. 2004, 16, 700–707. [Google Scholar] [CrossRef]
- Lark, K.G.; Consigli, R.A.; Minocha, H.C. Segregation of Sister Chromatids in Mammalian Cells. Science 1966, 154, 1202–1205. [Google Scholar] [CrossRef]
- Conboy, M.J.; Karasov, A.O.; Rando, T.A. High incidence of non-random template strand regregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 2007, 5, e102. [Google Scholar]
- Conboy, I.M.; Rando, T.A. The Regulation of Notch Signaling Controls Satellite Cell Activation and Cell Fate Determination in Postnatal Myogenesis. Dev. Cell 2002, 3, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Mourikis, P.; Sambasivan, R.; Castel, D.; Rocheteau, P.; Bizzarro, V.; Tajbakhsh, S. A Critical Requirement for Notch Signaling in Maintenance of the Quiescent Skeletal Muscle Stem Cell State. Stem Cells 2012, 30, 243–252. [Google Scholar] [CrossRef]
- Zammit, P.S.; Golding, J.P.; Nagata, Y.; Hudon, V.; Partridge, T.A.; Beauchamp, J.R. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J. Cell Biol. 2004, 166, 347–357. [Google Scholar] [CrossRef]
- Day, K.; Shefer, G.; Richardson, J.B.; Enikolopov, G.; Yablonka-Reuveni, Z. Nestin-GFP reporter expression defines the quies-cent state of skeletal muscle satellite cells. Dev. Biol. 2007, 304, 246–259. [Google Scholar] [CrossRef] [Green Version]
- Day, K.; Shefer, G.; Shearer, A.; Yablonka-Reuveni, Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev. Biol. 2010, 340, 330–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bąkowski, P.; Musielak, B.; Sip, P.; Biegański, G. Wpływ masażu na powysiłkową bolesność mięśni. Chir. Narz. Ruchu Ortop. Pol. 2008, 73, 261–265. [Google Scholar]
- Barnett, A. Using Recovery Modalities between Training Sessions in Elite Athletes. Sports Med. 2006, 36, 781–796. [Google Scholar] [CrossRef]
- Proske, U.; Morgan, D.L. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 2001, 537, 333–345. [Google Scholar] [CrossRef]
- McCormick, K.M.; Thomas, D.P. Exercise-induced satellite cell activation in senescent soleus muscle. J. Appl. Physiol. 1992, 72, 888–893. [Google Scholar] [CrossRef]
- Kadi, F.; Thornell, L.-E. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem. Cell Biol. 2000, 113, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kadi, F.; Charifi, N.; Denis, C.; Lexell, J.; Andersen, J.L.; Schjerling, P.; Olsen, S.; Kjaer, M. The behaviour of satellite cells in response to exercise: What have we learnd from human studies? Pflug. Arch. 2005, 451, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Nederveen, J.P.; Snijders, T.; Joanisse, S.; Wavell, C.G.; Mitchell, C.J.; Johnston, L.M.; Baker, S.K.; Phillips, S.M.; Parise, G. Altered muscle satellite cell activation following 16 wk of resistance training in young men. Am. J. Physiol. Integr. Comp. Physiol. 2017, 312, R85–R92. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, R.D.; Olson, T.; Welling, T.; Groscost, L.; Parcell, A.C. Satellite cell activity is differentially affected by contraction mode in human muscle following a work-matched bout of exercise. Front. Physiol. 2014, 5, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farup, J.; Rahbek, S.K.; Riis, S.; Vendelbo, M.H.; de Paoli, F.; Vissing, K. Influence of exercise contraction mode and protein supplementation on human skeletal muscle satellite cell content and muscle fiber growth. J. Appl. Physiol. 2014, 117, 898–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babcock, L.; Escano, M.; D’Lugos, A.; Todd, K.; Murach, K.; Luden, N. Concurrent aerobic exercise interferes with the satellite cell response to acute resistance exercise. Am. J. Physiol. Integr. Comp. Physiol. 2012, 302, R1458–R1465. [Google Scholar] [CrossRef]
- Snijders, T.; Verdijk, L.; Smeets, J.; McKay, B.R.; Senden, J.M.G.; Hartgens, F.; Parise, G.; Greenhaff, P.; Van Loon, L.J.C. The skeletal muscle satellite cell response to a single bout of resistance-type exercise is delayed with aging in men. Age 2014, 36, 1–15. [Google Scholar] [CrossRef]
- Verney, J.; Kadi, F.; Charifi, N.; Féasson, L.; Saafi, M.A.; Castells, J.; Piehl-Aulin, K.; Denis, C. Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve 2008, 38, 1147–1154. [Google Scholar] [CrossRef]
- Smith, H.K.; Merry, T.L. Voluntary resistance wheel exercise during post-natal growth in rats enhances skeletal muscle satellite cell and myonuclear content at adulthood. Acta Physiol. 2012, 204, 393–402. [Google Scholar] [CrossRef]
- Bruusgaard, J.C.; Johansen, I.B.; Egner, I.M.; Rana, Z.A.; Gundersen, K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc. Natl. Acad. Sci. USA 2010, 107, 15111–15116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppeler, H.; Howald, H.; Conley, K.; Lindstedt, S.L.; Claassen, H.; Vock, P.; Weibel, E.R. Endurance training in humans: Aerobic capacity and structure of skeletal muscle. J. Appl. Physiol. 1985, 59, 320–327. [Google Scholar] [CrossRef]
- Martin, N.R.; Lewis, M.P. Satellite cell activation and number following acute and chronic exercise: A mini review. Cell. Mol. Exerc. Physiol. 2012, 1, e3. [Google Scholar] [CrossRef]
- Shefer, G.; Rauner, G.; Yablonka-Reuveni, Z.; Benayahu, D. Reduced Satellite Cell Numbers and Myogenic Capacity in Aging Can Be Alleviated by Endurance Exercise. PLoS ONE 2010, 5, e13307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murach, K.A.; Walton, R.G.; Fry, C.S.; Michaelis, S.L.; Groshong, J.S.; Finlin, B.S.; Kern, P.A.; Peterson, C.A. Cycle training modulates satellite cell and transcriptional responses to a bout of resistance exercise. Physiol. Rep. 2016, 4, e12973. [Google Scholar] [CrossRef] [Green Version]
- Kadi, F.; Charifi, N.; Henriksson, J. The number of satellite cells in slow and fast fibres from human vastus lateralis muscle. Histochem. Cell Biol. 2006, 126, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Verdijk, L.B.; Beelen, M.; McKay, B.R.; Parise, G.; Kadi, F.; van Loon, L.J. A single bout of exercise activates skeletal muscle satellite cells during subsequent overnight recovery. Exp. Physiol. 2012, 97, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Koopman, R.; Schaart, G.; Meijer, K.; Savelberg, H.H.; van Loon, L.J. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am. J. Physiol. Endocrinol. Metab. 2007, 292, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Verdijk, L.B.; Gleeson, B.G.; Jonkers, R.A.M.; Meijer, K.; Savelberg, H.H.C.M.; Dendale, P.; van Loon, L.J. Skeletal Muscle Hypertrophy Following Resistance Training Is Accompanied by a Fiber Type-Specific Increase in Satellite Cell Content in Elderly Men. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2009, 64, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Snijders, T.; McKay, B.R.; Parise, G.; Verdijk, L.B.; Tarnopolsky, M.A.; Gibala, M.J.; Loon, L.J. Eccentric exercise increases satellite cell content in type II muscle fibers. Med. Sci. Sports Exerc. 2013, 45, 230–237. [Google Scholar] [CrossRef]
- Mackey, A.; Magnan, M.; Chazaud, B.; Kjaer, M. Human skeletal muscle fibroblasts stimulate in vitro myogenesis and in vivo muscle regeneration. J. Physiol. 2017, 595, 5115–5127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.M.; Lawson, J.A.; Mathew, S.; Hutcheson, D.A.; Kardon, G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 2011, 138, 3625–3637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, J.L.; Aagaard, P.; Bech, R.D.; Nygaard, T.; Hvid, L.G.; Wernbom, M.; Suetta, C.; Frandsen, U. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J. Physiol. 2012, 590, 4351–4361. [Google Scholar] [CrossRef]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef]
- Faulkner, J.A.; Larkin, L.M.; Claflin, D.R.; Brooks, S.V. Age-related changes in the structure and function of skeletal muscles. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef]
- Jang, Y.C.; van Remmen, H. Age-associated alterations of the neuromuscular junction. Exp. Gerontol. 2011, 46, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Erim, Z.; Beg, M.F.; Burke, D.T.; De Luca, C.J. Effects of Aging on Motor-Unit Control Properties. J. Neurophysiol. 1999, 82, 2081–2091. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2000, 50, 500–509. [Google Scholar] [CrossRef]
- Lexell, J. Evidence for Nervous System Degeneration with Advancing Age. J. Nutr. 1997, 127, 1011S–1013S. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.B.; Wittert, G.A. Endocrinology of the aging male. Best Pr. Res. Clin. Endocrinol. Metab. 2011, 25, 303–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryall, J.; Schertzer, J.D.; Lynch, G.S. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 2008, 9, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M.V.; Davis, B.S.; Booth, F.W. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J. Appl. Physiol. 2000, 89, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Shefer, G.; Van de Mark, D.P.; Richardson, J.B.; Yablonka-Reuveni, Z. Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle. Dev. Biol. 2006, 294, 50–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, N.E.; Schuenke, M.D.; Hikida, R.S. No change in skeletal muscle satellite cells in young and aging rat soleus muscle. J. Physiol. Sci. 2009, 59, 465–471. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nat. Cell Biol. 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Smythe, G.M.; Rando, T.A. Notch-Mediated Restoration of Regenerative Potential to Aged Muscle. Science 2003, 302, 1575–1577. [Google Scholar] [CrossRef]
- Waś, H.; Czarnecka, J. Komórki macierzyste a starzenie. Post. Biochem. 2014, 60, 161–176. [Google Scholar]
- Koga, H.; Kaushik, S.; Cuervo, A.M. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res. Rev. 2011, 10, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Conley, K.E.; Amara, C.E.; Jubrias, S.A.; Marcinek, D.J. Mitochondrial function, fibre types and ageing: New insights from human muscle in vivo. Exp. Physiol. 2007, 92, 333–339. [Google Scholar] [CrossRef] [PubMed]
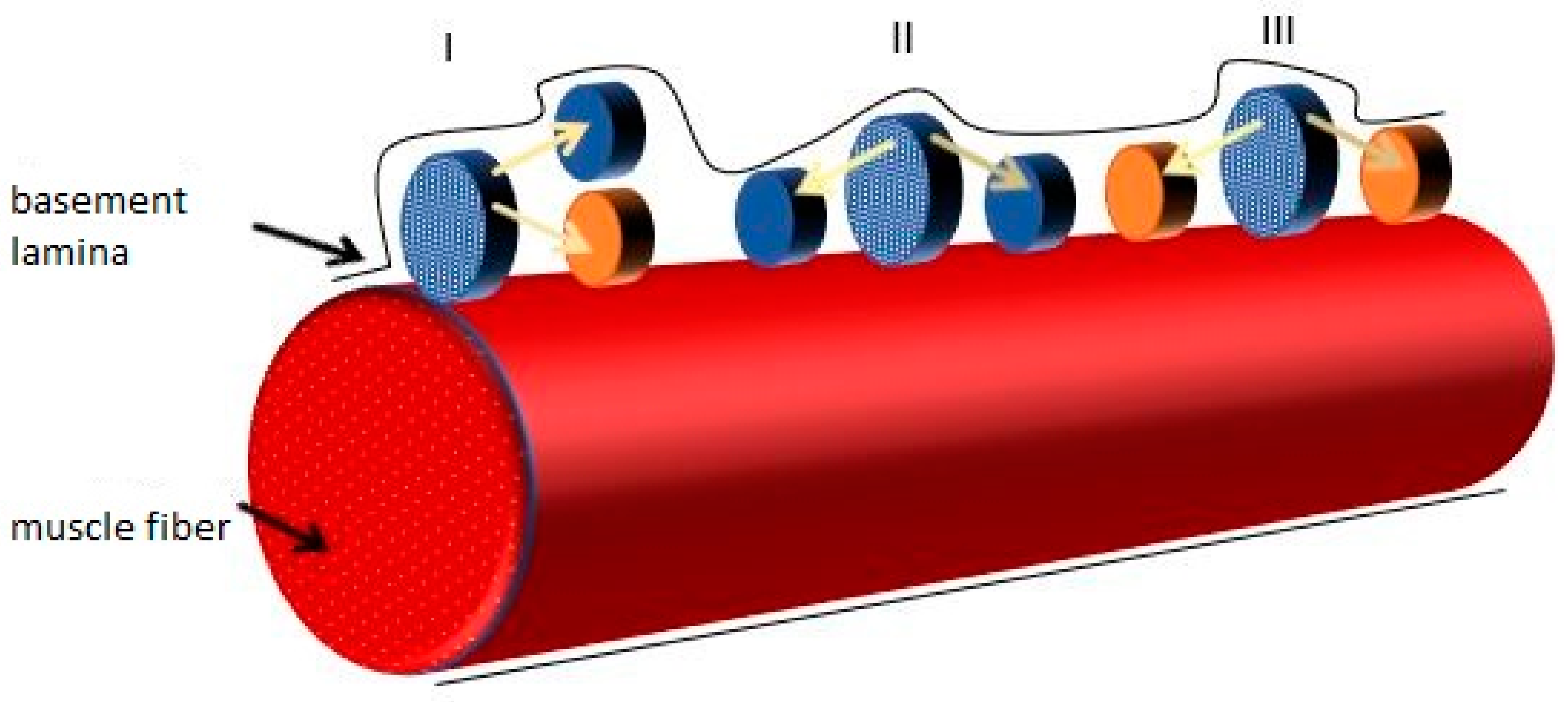
 —quiescent mSC;
—quiescent mSC;  —nuclei;
—nuclei;  —active mSCs;
—active mSCs;  —myofiber damage;
—myofiber damage;  —inflammatory cells.
—inflammatory cells.
 —quiescent mSC;
—quiescent mSC;  —nuclei;
—nuclei;  —active mSCs;
—active mSCs;  —myofiber damage;
—myofiber damage;  —inflammatory cells.
—inflammatory cells.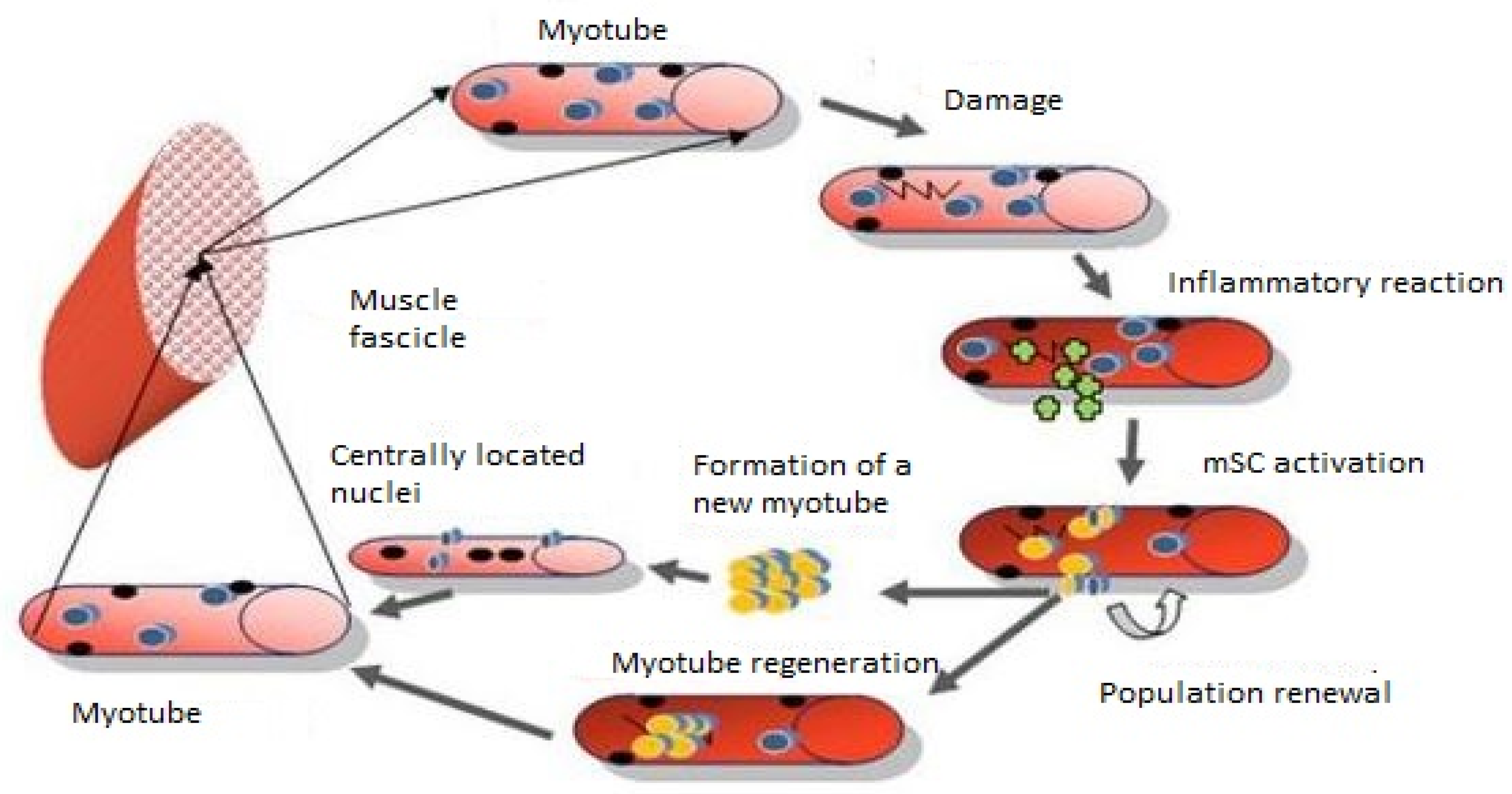
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, A.; Kaczmarek, M.; Ciałowicz, M.; Clemente, F.M.; Wolański, P.; Badicu, G.; Murawska-Ciałowicz, E. The Role of Satellite Cells in Skeletal Muscle Regeneration—The Effect of Exercise and Age. Biology 2021, 10, 1056. https://doi.org/10.3390/biology10101056
Kaczmarek A, Kaczmarek M, Ciałowicz M, Clemente FM, Wolański P, Badicu G, Murawska-Ciałowicz E. The Role of Satellite Cells in Skeletal Muscle Regeneration—The Effect of Exercise and Age. Biology. 2021; 10(10):1056. https://doi.org/10.3390/biology10101056
Chicago/Turabian StyleKaczmarek, Agnieszka, Mateusz Kaczmarek, Maria Ciałowicz, Filipe Manuel Clemente, Paweł Wolański, Georgian Badicu, and Eugenia Murawska-Ciałowicz. 2021. "The Role of Satellite Cells in Skeletal Muscle Regeneration—The Effect of Exercise and Age" Biology 10, no. 10: 1056. https://doi.org/10.3390/biology10101056
APA StyleKaczmarek, A., Kaczmarek, M., Ciałowicz, M., Clemente, F. M., Wolański, P., Badicu, G., & Murawska-Ciałowicz, E. (2021). The Role of Satellite Cells in Skeletal Muscle Regeneration—The Effect of Exercise and Age. Biology, 10(10), 1056. https://doi.org/10.3390/biology10101056








