Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group
Abstract
:Simple Summary
Abstract
1. Introduction
2. Phylum Spirochaetes
2.1. Pathogenicity of Phylum Spirochaetes
- Family Brachyspiraceae, Genus Brachispira: Spirochetosis can be associated with mild mucosal inflammation. In detail, Brachyspira sp. colonization was associated to mucus barrier failure. Brachyspira aalbongi was found in some resected appendages with possible implication in inflammation [11]. Brachyspira pilosicoli and B. aalborgi have been identified in the colonic mucosa of patients with diarrhea from irritable bowel syndrome (IBS), but not in healthy individuals [12].
- Family Brevinemataceae, Genus Brevinema: Brevinema andersoni can infect the short-tailed shrew (Blarina brevicauda) and the white-footed mouse (Peromyscus leucopus) [13]. Infections in humans are not known; however, Peromyscus leucopus is one of the main reservoirs of Borrelia burgdorferi s.l., which infects humans by Ixodes ticks.
- Family Leptospiraceae, Genus Leptospira includes both pathogenic and nonpathogenic species [14,15]. The spectrum of human diseases is extremely wide, ranging from subclinical infection to a severe syndrome of multiorgan infection with high mortality. The syndrome, icteric leptospirosis (Serogroup Icterohaemorrhagiae—Leptospira interrogans) can also cause renal failure [16]. It was first reported in 1886 by Adolf Weil [17]. In 1915, Inada and Ido published the first article on the discovery of the new species of Spirochaetae of Weil’s disease, which they isolated by culture [18].
- Family Spirochaetaceae: in this family, only Treponema Genus can be pathogenic. Among the treponematoses (diseases transmitted by Treponema sp.) the best known is syphilis, which is due to Treponema pallidum. The name of this venereal disease was given by Girolamo Fracastoro, poet and doctor from Verona, in his work “Syphilis sive Morbus Gallicus” of 1530, where the shepherd Sifilo was punished by Apollo with a disease, which rapidly spreads. From the name of that shepherd the name Syphilis was derived. The disease was likely imported from America during the travels of Cristoforo Colombo in 1492. In 1495, the disease involved the army of Charles VIII when the French king invaded Naples, and then spread rapidly throughout Europe and the world. Fritz Richard Schaudinn discovered Spirochaeta pallida (Treponema pallidum) with Paul Erich Hoffmann. Only recently it has been possible to cultivate this spirochaete [19]. There are other endemic nonvenereal treponematoses [20], as reported in Table 2.
2.2. Spirochetosis from the Clinic to Culture Isolation
3. Borreliaceae Family
3.1. Cristispira Genus
3.2. Borrelia Genus
- Microbiological features;
- Vector;
- Epidemiology;
- Clinical manifestations in humans.
4. Lyme Group Borrelia
4.1. Ecology
- Reservoir hosts that participate significantly in the circulation of spirochetes in nature. Ticks that feed on these animals become infected and the spirochetes multiply, disseminate in the body and persist there for a considerable period.
- Non-reservoir hosts, such as humans, where spirochetes circulation in blood is very low and ticks that feed on them do not contract spirochetosis.
4.2. Ticks Vector of BL Group
4.3. Reservoir and Occasional Hosts
4.4. Epidemiology
5. Microbiology
5.1. Genetic Characteristics of B. burgdorferi Sensu Lato
5.2. Genome of Borrelia Lyme Group
- The intraspecific lineages of B. burgdorferi s.s. can be differentiated by 16S-23S ribosomal RNA spacer (IGS) and outer surface protein C gene (ospC) sequences of the plasmids.
- Multi-locus sequence typing (MLST) is used to characterize genetic variations of natural populations of a bacterial pathogen.
- Molecular phylogeny.
5.2.1. Flagellum
5.2.2. Extracellular Matrix Ligands (ECM)
5.2.3. Outer Surface Protein (Osp)
5.2.4. Heat Shock Proteins
5.2.5. Other Proteins
5.2.6. VlsE (Variable Major Protein-Like Sequence Expressed)
5.3. Antigenic Heterogenecity of Borrelia burgdorferi
6. Species of Borreliae Lyme Group
6.1. Isolated Strains of B. burgdorferi and Species Present in Italy
6.2. Clinic
7. Borrelia Lyme Group with High Spirochaetemia—Borrelia mayonii
7.1. Clinical and Microbiological Characteristics
7.2. Vectors and Reservoirs
7.3. Genome
7.4. Clinical Manifestations
8. Borrelia Lyme Group—Baggio–Yoshinari Group (BYS): The Brazilian Lyme-Disease-like Illness
Genetic Characteristics of BYS
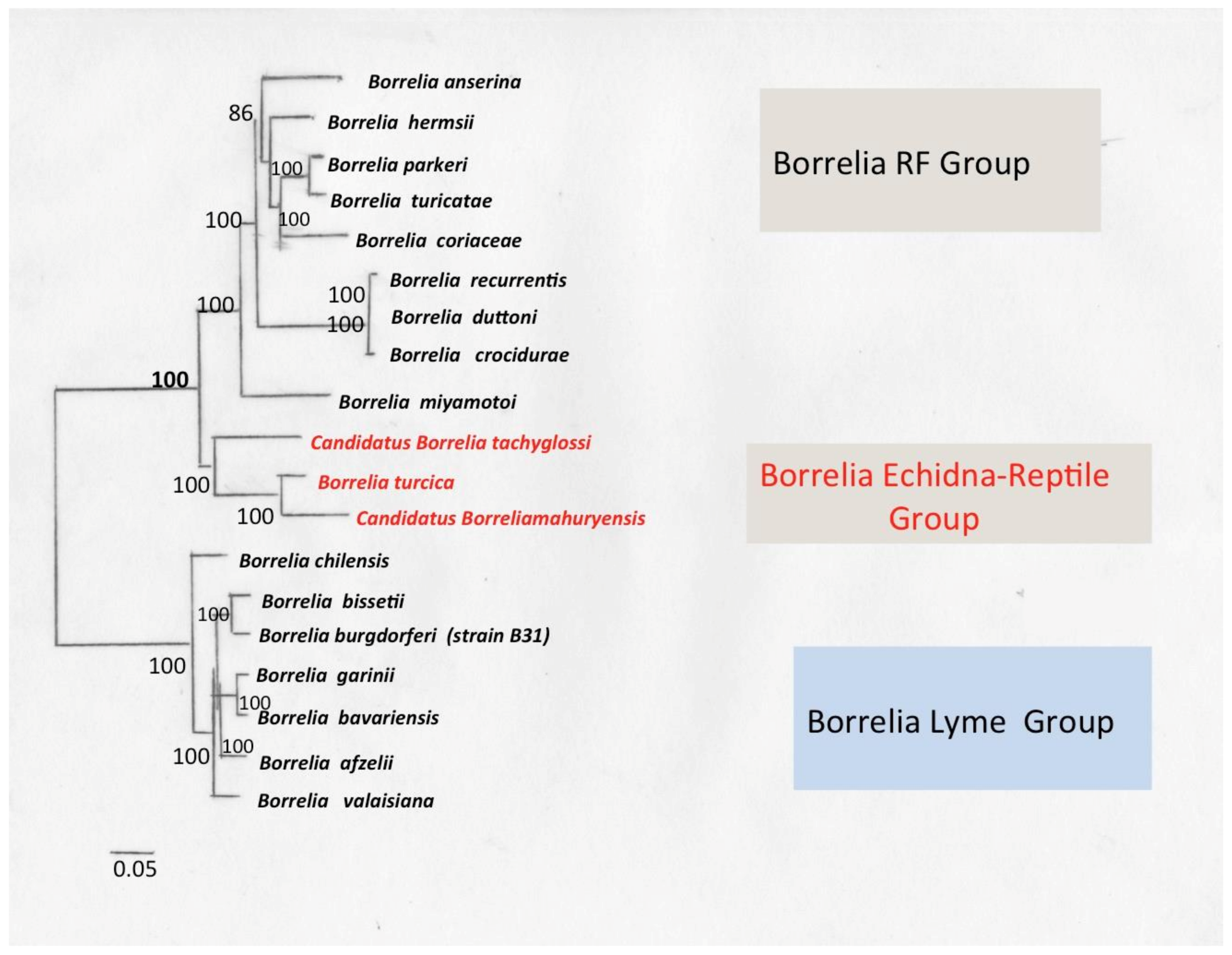
9. Borrelia Echidna-Reptile Group
9.1. Borrelia turcica
9.2. Borrelia Tachyglossi
9.3. Borrelia mahuryensis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, R.S.; Mahmood, S.; Adeolu, M. A phylogenomic and molecular signature based approach for characterization of the phylum Spirochaetes and its major clades: Proposal for a taxonomic revision of the phylum. Front. Microbiol. 2013, 4, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renesto, P.; Lorvellec-Guillon, K.; Drancourt, M.; Raoult, D. rpoB gene analysis as a novel strategy for identification of spirochetes from the genera Borrelia, Treponema, and Leptospira. J. Clin. Microbiol. 2000, 38, 2200–2203. [Google Scholar] [CrossRef] [PubMed]
- Coulter, P.; Lema, C.; Flayhart, D.; Linhardt, A.S.; Aucott, J.N.; Auwaerter, P.G.; Dumler, J.S. Two-year evaluation of Borrelia burgdorferi culture and supplemental tests for definitive diagnosis of Lyme disease. J. Clin. Microbiol. 2005, 43, 5080–5084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.C. The spirochetes. Annu. Rev. Microbiol. 1977, 31, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Paster, B.J. Phylum XV. Spirochaetes Garrity and Holt 2001. In Bergey’s Manual® of Systematic Bacteriology: Volume Four The Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes; Krieg, N.R., Staley, J.T., Brown, D.R., Hedlund, B.P., Paster, B.J., Ward, N.L., Ludwig, W., Whitman, W.B., Eds.; Springer: New York, NY, USA, 2010; pp. 471–566. [Google Scholar] [CrossRef]
- Paster, B.J.; Canale-Parola, E. Treponema saccharophilum sp. nov., a large pectinolytic spirochete from the bovine rumen. Appl. Environ. Microbiol. 1985, 50, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Stanton, T.B.; Canale-Parola, E. Treponema bryantii sp. nov., a rumen spirochete that interacts with cellulolytic bacteria. Arch. Microbiol. 1980, 127, 145–156. [Google Scholar] [CrossRef]
- Newbrook, K.; Staton, G.J.; Clegg, S.R.; Birtles, R.J.; Carter, S.D.; Evans, N.J. Treponema ruminis sp. nov., a spirochaete isolated from the bovine rumen. Int. J. Syst. Evol. Microbiol. 2017, 67, 1349–1354. [Google Scholar] [CrossRef]
- Cinco, M.; Coghlan, J.D.; Matthews, P.R. Isolation and classification of sixteen strains of saprophytic leptospires. J. Hyg. 1980, 84, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Gofton, A.W.; Margos, G.; Fingerle, V.; Hepner, S.; Loh, S.M.; Ryan, U.; Irwin, P.; Oskam, C.L. Genome-wide analysis of Borrelia turcica and ‘Candidatus Borrelia tachyglossi’ shows relapsing fever-like genomes with unique genomic links to Lyme disease Borrelia. Infect. Genet. Evol. 2018, 66, 72–81. [Google Scholar] [CrossRef]
- Brooke, C.J.; Riley, T.V.; Hampson, D.J. Comparison of prevalence and risk factors for faecal carriage of the intestinal spirochaetes Brachyspira aalborgi and Brachyspira pilosicoli in four Australian populations. Epidemiol. Infect. 2006, 134, 627–634. [Google Scholar] [CrossRef]
- Jabbar, K.S.; Dolan, B.; Eklund, L.; Wising, C.; Ermund, A.; Johansson, A.; Tornblom, H.; Simren, M.; Hansson, G.C. Association between Brachyspira and irritable bowel syndrome with diarrhoea. Gut 2021, 70, 1117–1129. [Google Scholar] [CrossRef]
- Defosse, D.L.; Johnson, R.C.; Paster, B.J.; Dewhirst, F.E.; Fraser, G.J. Brevinema andersonii gen. nov., sp. nov., an infectious spirochete isolated from the short-tailed shrew (Blarina brevicauda) and the white-footed mouse (Peromyscus leucopus). Int. J. Syst. Bacteriol. 1995, 45, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Paz, L.N.; Dias, C.S.; Carvalho, V.M.P.; Muramoto, C.; Estrela-Lima, A.; Pinna, M.H. Unusual case of polyarthritis and hepatorenal syndrome associated with Leptospira interrogans infection in a dog: A case report. Res. Vet. Sci. 2021, 134, 186–190. [Google Scholar] [CrossRef]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef] [Green Version]
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Mohd Khalid, M.K.N.; Amran, F.; Masuzawa, T.; et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl. Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef] [Green Version]
- Alston, J.M.; Brown, H.C. The Epidemiology of Weil’s Disease: (Section of Epidemiology and State Medicine). Proc. R. Soc. Med. 1937, 30, 741–756. [Google Scholar]
- Kobayashi, Y. Discovery of the causative organism of Weil’s disease: Historical view. J. Infect. Chemother. 2001, 7, 10–15. [Google Scholar] [CrossRef]
- Edmondson, D.G.; Hu, B.; Norris, S.J. Long-term in vitro culture of the syphilis spirochete treponema pallidum subsp. Pallidum. mBio 2018, 9, e01153-18. [Google Scholar] [CrossRef] [Green Version]
- Antal, G.M.; Lukehart, S.A.; Meheus, A.Z. The endemic treponematoses. Microbes Infect. 2002, 4, 83–94. [Google Scholar] [CrossRef]
- Kawahata, T.; Kojima, Y.; Furubayashi, K.; Shinohara, K.; Shimizu, T.; Komano, J.; Mori, H.; Motomura, K. Bejel, a Nonvenereal Treponematosis, among Men Who Have Sex with Men, Japan. Emerg. Infect. Dis. 2019, 25, 1581–1583. [Google Scholar] [CrossRef] [Green Version]
- Mitja, O.; Asiedu, K.; Mabey, D. Yaws. Lancet 2013, 381, 763–773. [Google Scholar] [CrossRef]
- Mitja, O.; Smajs, D.; Bassat, Q. Advances in the diagnosis of endemic treponematoses: Yaws, bejel, and pinta. PLoS Negl. Trop. Dis. 2013, 7, e2283. [Google Scholar] [CrossRef]
- Weledji, E.P.; Njong, S. Cancrum Oris (Noma): The Role of Nutrition in Management. J. Am. Coll. Clin. Wound Spec. 2015, 7, 50–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Sardana, K.; Gautam, R.K. An unusual case of noma caused by Klebsiella pnuemoniae and its management. Trop. Doct. 2018, 48, 230–232. [Google Scholar] [CrossRef]
- Feller, L.; Khammissa, R.A.G.; Altini, M.; Lemmer, J. Noma (cancrum oris): An unresolved global challenge. Periodontology 2000 2019, 80, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, J.F., Jr.; Rocas, I.N. Treponema species associated with abscesses of endodontic origin. Oral Microbiol. Immunol. 2004, 19, 336–339. [Google Scholar] [CrossRef]
- Ng, H.M.; Slakeski, N.; Butler, C.A.; Veith, P.D.; Chen, Y.Y.; Liu, S.W.; Hoffmann, B.; Dashper, S.G.; Reynolds, E.C. The Role of Treponema denticola Motility in Synergistic Biofilm Formation with Porphyromonas gingivalis. Front. Cell. Infect. Microbiol. 2019, 9, 432. [Google Scholar] [CrossRef] [Green Version]
- Craigie, D. Notice of a febrile disorder which has prevailed at Edinburgh during the Summer of 1843. Edinb. Med. Surg. J. 1843, 60, 410–418. [Google Scholar]
- Obermeier, V.D. Ueber das wiederkehrende Fieber. Arch. Pathol. Anat. Physiol. Klin. Med. 1869, 47, 428–472. [Google Scholar] [CrossRef]
- Ross, P.H.; Milne, A.D. “Tick Fever”. Br. Med. J. 1904, 2, 1453–1454. [Google Scholar] [CrossRef]
- Lechat, M.F. The Dutton-Todd Expedition to the Congo (1903–1905). Deboma at Coquilhatville (September 1903–July 1904). Ann. Soc. Belges. Med. Trop. Parasitol. Mycol. 1964, 44, 493–511. [Google Scholar]
- Novy, F.G.; Knapp, R.E. The cultivation of Spirillium Obermeieri. Preliminary Note. J. Am. Med. Assoc. 1906, 47, 2152–2154. [Google Scholar] [CrossRef] [Green Version]
- Borrel, A.; Marchoux, E. Argas et spirilles. CR Seances Soc. Biol. Fil. 1905, 58, 362–364. [Google Scholar]
- Borrel, A.; Burnet, E. Developement initial in vitro du spirille de la poule. CR Seances Soc. Biol. Fil. 1906, 60, 540–542. [Google Scholar]
- Swellengrebel, N.H. Sur la cytologie apparaissent les spirochetes et les Spirilles. Ann. Inst. Past. 1907, 21, 562–584. [Google Scholar]
- Wright, D.J. Borrel’s accidental legacy. Clin. Microbiol. Infect. 2009, 15, 397–399. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, H. The Pure Cultivation of Spirochaeta Duttoni, Spirochaeta Kochi, Spirochaeta Obermeieri, and Spirochaeta Novyi. J. Exp. Med. 1912, 16, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Barbour, A.G. Cultivation of Borrelia: A historical overview. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1986, 263, 11–14. [Google Scholar] [CrossRef]
- Kelly, R. Cultivation of Borrelia hermsi. Science 1971, 173, 443–444. [Google Scholar] [CrossRef]
- Stoenner, H.G. Biology of Borrelia hermsii in Kelly medium. Appl. Microbiol. 1974, 28, 540–543. [Google Scholar] [CrossRef]
- Stoenner, H.G.; Dodd, T.; Larsen, C. Antigenic variation of Borrelia hermsii. J. Exp. Med. 1982, 156, 1297–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benach, J.L.; Bosler, E.M.; Hanrahan, J.P.; Coleman, J.L.; Habicht, G.S.; Bast, T.F.; Cameron, D.J.; Ziegler, J.L.; Barbour, A.G.; Burgdorfer, W.; et al. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 1983, 308, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Grodzicki, R.L.; Kornblatt, A.N.; Craft, J.E.; Barbour, A.G.; Burgdorfer, W.; Schmid, G.P.; Johnson, E.; Malawista, S.E. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 1983, 308, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G.; Burgdorfer, W.; Hayes, S.F.; Péter, O.; Aeschlimann, A. Isolation of a cultivable spirochete fromIxodes ricinus ticks of Switzerland. Curr. Microbiol. 1983, 8, 123–126. [Google Scholar] [CrossRef]
- Barbour, A.G. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 1984, 57, 521–525. [Google Scholar] [PubMed]
- Johnson, S.E.; Klein, G.C.; Schmid, G.P.; Bowen, G.S.; Feeley, J.C.; Schulze, T. Lyme disease: A selective medium for isolation of the suspected etiological agent, a spirochete. J. Clin. Microbiol. 1984, 19, 81–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duray, P.H.; Johnson, R.C. The histopathology of experimentally infected hamsters with the Lyme disease spirochete, Borrelia burgdorferi. Proc. Soc. Exp. Biol. Med. 1986, 181, 263–269. [Google Scholar] [CrossRef]
- Fukunaga, M.; Sohnaka, M.; Takahashi, Y.; Nakao, M.; Miyamoto, K. Antigenic and genetic characterization of Borrelia species isolated from Ixodes persulcatus in Hokkaido, Japan. J. Clin. Microbiol. 1993, 31, 1388–1391. [Google Scholar] [CrossRef] [Green Version]
- Fukunaga, M.; Koreki, Y. The flagellin gene of Borrelia miyamotoi sp. nov. and its phylogenetic relationship among Borrelia species. FEMS Microbiol. Lett. 1995, 134, 255–258. [Google Scholar] [CrossRef]
- Fukunaga, M.; Takahashi, Y.; Tsuruta, Y.; Matsushita, O.; Ralph, D.; McClelland, M.; Nakao, M. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int. J. Syst. Bacteriol. 1995, 45, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Wagemakers, A.; Oei, A.; Fikrig, M.M.; Miellet, W.R.; Hovius, J.W. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Pettenkofer medium, and is resistant to human complement. Parasit. Vectors 2014, 7, 418. [Google Scholar] [CrossRef] [Green Version]
- Replogle, A.J.; Sexton, C.; Young, J.; Kingry, L.C.; Schriefer, M.E.; Dolan, M.; Johnson, T.L.; Connally, N.P.; Padgett, K.A.; Petersen, J.M. Isolation of Borrelia miyamotoi and other Borreliae using a modified BSK medium. Sci. Rep. 2021, 11, 1926. [Google Scholar] [CrossRef]
- Margulis, L.; Nault, L.; Sieburth, J.M. Cristispira from oyster styles: Complex morphology of large symbiotic spirochetes. Symbiosis 1991, 11, 1–17. [Google Scholar]
- Takano, A.; Goka, K.; Une, Y.; Shimada, Y.; Fujita, H.; Shiino, T.; Watanabe, H.; Kawabata, H. Isolation and characterization of a novel Borrelia group of tick-borne Borreliae from imported reptiles and their associated ticks. Environ. Microbiol. 2010, 12, 134–146. [Google Scholar] [CrossRef]
- Binetruy, F.; Garnier, S.; Boulanger, N.; Talagrand-Reboul, E.; Loire, E.; Faivre, B.; Noel, V.; Buysse, M.; Duron, O. A novel Borrelia species, intermediate between Lyme disease and relapsing fever groups, in neotropical passerine-associated ticks. Sci. Rep. 2020, 10, 10596. [Google Scholar] [CrossRef]
- Cutler, S.J.; Moss, J.; Fukunaga, M.; Wright, D.J.; Fekade, D.; Warrell, D. Borrelia recurrentis characterization and comparison with relapsing-fever, Lyme-associated, and other Borrelia spp. Int. J. Syst. Bacteriol. 1997, 47, 958–968. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.C.; Porcella, S.F.; Raffel, S.J.; Schwan, T.G.; Barbour, A.G. Large linear plasmids of Borrelia species that cause relapsing fever. J. Bacteriol. 2013, 195, 3629–3639. [Google Scholar] [CrossRef] [Green Version]
- Yabsley, M.J.; Parsons, N.J.; Horne, E.C.; Shock, B.C.; Purdee, M. Novel relapsing fever Borrelia detected in African penguins (Spheniscus demersus) admitted to two rehabilitation centers in South Africa. Parasitol. Res. 2012, 110, 1125–1130. [Google Scholar] [CrossRef]
- Adeolu, M.; Gupta, R.S. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: The emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek 2014, 105, 1049–1072. [Google Scholar] [CrossRef]
- Stevenson, B.; Fingerle, V.; Wormser, G.P.; Margos, G. Public health and patient safety concerns merit retention of Lyme borreliosis-associated spirochetes within the genus Borrelia, and rejection of the genus novum Borreliella. Ticks Tick Borne Dis. 2019, 10, 1–4. [Google Scholar] [CrossRef]
- Margos, G.; Gofton, A.; Wibberg, D.; Dangel, A.; Marosevic, D.; Loh, S.M.; Oskam, C.; Fingerle, V. The genus Borrelia reloaded. PLoS ONE 2018, 13, e0208432. [Google Scholar] [CrossRef]
- Adamek, M.; Alanjary, M.; Sales-Ortells, H.; Goodfellow, M.; Bull, A.T.; Winkler, A.; Wibberg, D.; Kalinowski, J.; Ziemert, N. Comparative genomics reveals phylogenetic distribution patterns of secondary metabolites in Amycolatopsis species. BMC Genom. 2018, 19, 426. [Google Scholar] [CrossRef] [Green Version]
- Qin, Q.L.; Xie, B.B.; Zhang, X.Y.; Chen, X.L.; Zhou, B.C.; Zhou, J.; Oren, A.; Zhang, Y.Z. A proposed genus boundary for the prokaryotes based on genomic insights. J. Bacteriol. 2014, 196, 2210–2215. [Google Scholar] [CrossRef] [Green Version]
- Guner, E.S.; Watanabe, M.; Hashimoto, N.; Kadosaka, T.; Kawamura, Y.; Ezaki, T.; Kawabata, H.; Imai, Y.; Kaneda, K.; Masuzawa, T. Borrelia turcica sp. nov., isolated from the hard tick Hyalomma aegyptium in Turkey. Int. J. Syst. Evol. Microbiol. 2004, 54, 1649–1652. [Google Scholar] [CrossRef]
- Loh, S.M.; Gofton, A.W.; Lo, N.; Gillett, A.; Ryan, U.M.; Irwin, P.J.; Oskam, C.L. Novel Borrelia species detected in echidna ticks, Bothriocroton concolor, in Australia. Parasit. Vectors 2016, 9, 339. [Google Scholar] [CrossRef] [Green Version]
- Takano, A.; Fujita, H.; Kadosaka, T.; Konnai, S.; Tajima, T.; Watanabe, H.; Ohnishi, M.; Kawabata, H. Characterization of reptile-associated Borrelia sp. in the vector tick, Amblyomma geoemydae, and its association with Lyme disease and relapsing fever Borrelia spp. Environ. Microbiol. Rep. 2011, 3, 632–637. [Google Scholar] [CrossRef]
- Trevisan, G.; Cinco, M. Lyme disease. A general survey. Int. J. Dermatol. 1990, 29, 1–8. [Google Scholar] [CrossRef]
- Sala, V.; De Faveri, E. Epidemiology of lyme disease in domestic and wild animals. Open Dermatol. J. 2016, 10, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Balashov, Y.S. Significance of ixodid tick (Parasitiformes, Ixodidae) population structure for maintenance of natural foci of infection. Biol. Bull. 2010, 37, 677–683. [Google Scholar] [CrossRef]
- Ostfeld, R.; Keesing, F. Effects of Host Diversity on Infectious Disease. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 157–182. [Google Scholar] [CrossRef]
- Roche, B.; Rohani, P.; Dobson, A.P.; Guegan, J.F. The impact of community organization on vector-borne pathogens. Am. Nat. 2013, 181, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perronne, C. Lyme and associated tick-borne diseases: Global challenges in the context of a public health threat. Front. Cell. Infect. Microbiol. 2014, 4, 74. [Google Scholar] [CrossRef] [PubMed]
- Rollend, L.; Fish, D.; Childs, J.E. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations. Ticks Tick Borne Dis. 2013, 4, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Pal, U.; Kitsou, C.; Drecktrah, D.; Yas, O.B.; Fikrig, E. Interactions Between Ticks and Lyme Disease Spirochetes. Curr. Issues. Mol. Biol. 2021, 42, 113–144. [Google Scholar] [CrossRef]
- Swei, A.; Kwan, J.Y. Tick microbiome and pathogen acquisition altered by host blood meal. ISME J. 2017, 11, 813–816. [Google Scholar] [CrossRef]
- Brown, R.N.; Lane, R.S. Lyme disease in California: A novel enzootic transmission cycle of Borrelia burgdorferi. Science 1992, 256, 1439–1442. [Google Scholar] [CrossRef]
- Cerar, T.; Strle, F.; Stupica, D.; Ruzic-Sabljic, E.; McHugh, G.; Steere, A.C.; Strle, K. Differences in Genotype, Clinical Features, and Inflammatory Potential of Borrelia burgdorferi sensu stricto Strains from Europe and the United States. Emerg. Infect. Dis. 2016, 22, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Brisson, D.; Drecktrah, D.; Eggers, C.H.; Samuels, D.S. Genetics of Borrelia burgdorferi. Annu. Rev. Genet. 2012, 46, 515–536. [Google Scholar] [CrossRef] [Green Version]
- Rosa, P.A.; Tilly, K.; Stewart, P.E. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat. Rev. Microbiol. 2005, 3, 129–143. [Google Scholar] [CrossRef]
- Kurtenbach, K.; Hanincova, K.; Tsao, J.I.; Margos, G.; Fish, D.; Ogden, N.H. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat. Rev. Microbiol. 2006, 4, 660–669. [Google Scholar] [CrossRef]
- Zawada, S.G.; von Fricken, M.E.; Weppelmann, T.A.; Sikaroodi, M.; Gillevet, P.M. Optimization of tissue sampling for Borrelia burgdorferi in white-footed mice (Peromyscus leucopus). PLoS ONE 2020, 15, e0226798. [Google Scholar] [CrossRef] [Green Version]
- Gern, L.; Siegenthaler, M.; Hu, C.M.; Leuba-Garcia, S.; Humair, P.F.; Moret, J. Borrelia burgdorferi in rodents (Apodemus flavicollis and A. sylvaticus): Duration and enhancement of infectivity for Ixodes ricinus ticks. Eur. J. Epidemiol. 1994, 10, 75–80. [Google Scholar] [CrossRef]
- Horowitz, R.I.; Freeman, P.R. Efficacy of Double-Dose Dapsone Combination Therapy in the Treatment of Chronic Lyme Disease/Post-Treatment Lyme Disease Syndrome (PTLDS) and Associated Co-infections: A Report of Three Cases and Retrospective Chart Review. Antibiotics 2020, 9, 725. [Google Scholar] [CrossRef]
- Kubiak, K.; Szczotko, M.; Dmitryjuk, M. Borrelia miyamotoi—An Emerging Human Tick-Borne Pathogen in Europe. Microorganisms 2021, 9, 154. [Google Scholar] [CrossRef]
- Barbour, A.G.; Bunikis, J.; Travinsky, B.; Hoen, A.G.; Diuk-Wasser, M.A.; Fish, D.; Tsao, J.I. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg. 2009, 81, 1120–1131. [Google Scholar] [CrossRef] [Green Version]
- Maggi, R.G.; Reichelt, S.; Toliver, M.; Engber, B. Borrelia species in Ixodes affinis and Ixodes scapularis ticks collected from the coastal plain of North Carolina. Ticks Tick Borne Dis. 2010, 1, 168–171. [Google Scholar] [CrossRef]
- Michalski, M.M.; Kubiak, K.; Szczotko, M.; Chajecka, M.; Dmitryjuk, M. Molecular Detection of Borrelia burgdorferi Sensu Lato and Anaplasma phagocytophilum in Ticks Collected from Dogs in Urban Areas of North-Eastern Poland. Pathogens 2020, 9, 455. [Google Scholar] [CrossRef]
- Manilla, G.; Iori, A. Illustrated key to the ticks of Italy. I. Larval stages of the species of the Ixodinae subfamily (Acari, Ixodoidea, Ixodidae). Parassitologia 1992, 34, 83–95. [Google Scholar]
- Rimoldi, S.G.; Merli, S.; Bestetti, G.; Giacomet, V.; Cislaghi, G.; Grande, R.; Zanzani, S.; Pagani, C.; Trevisan, G.; De Faveri, E.; et al. Occurrence of Lyme disease infection in a non-endemic area in Northern Italy. G. Ital. Dermatol. Venereol. 2020, 155, 320–324. [Google Scholar] [CrossRef]
- Trevisan, G.; Crovato, F.; Marcuccio, C.; Fumarola, D.; Scarpa, C. Lyme disease in Italy. Zentralbl. Bakteriol. Mikrobiol. Hyg. Abt. 1 Orig. A. 1987, 263, 459–463. [Google Scholar] [CrossRef]
- Barbour, A.G.; Hayes, S.F. Biology of Borrelia species. Microbiol. Rev. 1986, 50, 381–400. [Google Scholar] [CrossRef]
- Richter, D.; Spielman, A.; Komar, N.; Matuschka, F.R. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 2000, 6, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Gern, L. Borrelia burgdorferi sensu lato, the agent of lyme borreliosis: Life in the wilds. Parasite 2008, 15, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Kurtenbach, K.; Schafer, S.M.; Sewell, H.S.; Peacey, M.; Hoodless, A.; Nuttall, P.A.; Randolph, S.E. Differential survival of Lyme borreliosis spirochetes in ticks that feed on birds. Infect. Immun. 2002, 70, 5893–5895. [Google Scholar] [CrossRef] [Green Version]
- Voordouw, M.J. Co-feeding transmission in Lyme disease pathogens. Parasitology 2015, 142, 290–302. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.D.; Anderson, J.F.; Durden, L.A. First detection of Lyme disease spirochete Borrelia burgdorferi in ticks collected from a raptor in Canada. J. Wild. Rehabil. 2014, 34, 11–16. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A.; Anderson, J.F. Infection Prevalence of Borrelia burgdorferi in Ticks Collected from Songbirds in Far-Western Canada. Open J. Anim. Sci. 2015, 5, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Anderson, J.F.; Bierman, B.C.; Durden, L.A. Extensive Distribution of the Lyme Disease Bacterium, Borrelia burgdorferi Sensu Lato, in Multiple Tick Species Parasitizing Avian and Mammalian Hosts across Canada. Healthcare 2018, 6, 131. [Google Scholar] [CrossRef] [Green Version]
- Slatculescu, A.M.; Clow, K.M.; McKay, R.; Talbot, B.; Logan, J.J.; Thickstun, C.R.; Jardine, C.M.; Ogden, N.H.; Knudby, A.J.; Kulkarni, M.A. Species distribution models for the eastern blacklegged tick, Ixodes scapularis, and the Lyme disease pathogen, Borrelia burgdorferi, in Ontario, Canada. PLoS ONE 2020, 15, e0238126. [Google Scholar] [CrossRef]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Anderson, J.F.; Durden, L.A.; Manord, J.M.; Smith, M.L. Detection of Borrelia Genomospecies 2 in Ixodes spinipalpis Ticks Collected from a Rabbit in Canada. J. Parasitol. 2017, 103, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.D.; Anderson, J.F.; Durden, L.A. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J. Parasitol. 2012, 98, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Beauchamp, G.; Nguon, S.; Trudel, L.; Milord, F.; Lindsay, L.R.; Belanger, D.; Ogden, N.H. Associations between Ixodes scapularis ticks and small mammal hosts in a newly endemic zone in southeastern Canada: Implications for Borrelia burgdorferi transmission. Ticks Tick Borne Dis. 2011, 2, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Arsnoe, I.; Tsao, J.I.; Hickling, G.J. Nymphal Ixodes scapularis questing behavior explains geographic variation in Lyme borreliosis risk in the eastern United States. Ticks Tick Borne Dis. 2019, 10, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Rose, I.; Yoshimizu, M.H.; Bonilla, D.L.; Fedorova, N.; Lane, R.S.; Padgett, K.A. Phylogeography of Borrelia spirochetes in Ixodes pacificus and Ixodes spinipalpis ticks highlights differential acarological risk of tick-borne disease transmission in northern versus southern California. PLoS ONE 2019, 14, e0214726. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Leonhard, S.; Girard, Y.A.; Hahn, N.; Mun, J.; Padgett, K.A.; Lane, R.S. Identifying the reservoir hosts of the Lyme disease spirochete Borrelia burgdorferi in California: The role of the western gray squirrel (Sciurus griseus). Am. J. Trop. Med. Hyg. 2008, 79, 535–540. [Google Scholar] [CrossRef]
- Xu, G.; Pearson, P.; Dykstra, E.; Andrews, E.S.; Rich, S.M. Human-Biting Ixodes Ticks and Pathogen Prevalence from California, Oregon, and Washington. Vector Borne Zoonotic Dis. 2019, 19, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Perez, A.M.; Sanchez-Montes, S.; Foley, J.; Guzman-Cornejo, C.; Colunga-Salas, P.; Pascoe, E.; Becker, I.; Delgado-de la Mora, J.; Licona-Enriquez, J.D.; Suzan, G. Molecular evidence of Borrelia burgdorferi sensu stricto and Rickettsia massiliae in ticks collected from a domestic-wild carnivore interface in Chihuahua, Mexico. Ticks Tick Borne Dis. 2019, 10, 1118–1123. [Google Scholar] [CrossRef]
- Feria-Arroyo, T.P.; Castro-Arellano, I.; Gordillo-Perez, G.; Cavazos, A.L.; Vargas-Sandoval, M.; Grover, A.; Torres, J.; Medina, R.F.; de Leon, A.A.; Esteve-Gassent, M.D. Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texas-Mexico transboundary region. Parasites Vectors 2014, 7, 199. [Google Scholar] [CrossRef] [Green Version]
- Dall’Agnol, B.; Michel, T.; Weck, B.; Souza, U.A.; Webster, A.; Leal, B.F.; Klafke, G.M.; Martins, J.R.; Ott, R.; Venzal, J.M.; et al. Borrelia burgdorferi sensu lato in Ixodes longiscutatus ticks from Brazilian Pampa. Ticks Tick Borne Dis. 2017, 8, 928–932. [Google Scholar] [CrossRef]
- Munoz-Leal, S.; Ramirez, D.G.; Luz, H.R.; Faccini, J.L.H.; Labruna, M.B. “Candidatus Borrelia ibitipoquensis”, a Borrelia valaisiana-Related Genospecies Characterized from Ixodes paranaensis in Brazil. Microb. Ecol. 2020, 80, 682–689. [Google Scholar] [CrossRef]
- Flores, F.S.; Saracho-Bottero, M.N.; Sebastian, P.S.; Venzal, J.M.; Mangold, A.J.; Nava, S. Borrelia genospecies in Ixodes sp. cf. Ixodes affinis (Acari: Ixodidae) from Argentina. Ticks Tick Borne Dis. 2020, 11, 101546. [Google Scholar] [CrossRef]
- Labruna, M.B.; Onofrio, V.C.; Barros-Battesti, D.M.; Gianizella, S.L.; Venzal, J.M.; Guglielmone, A.A. Synonymy of Ixodes aragaoi with Ixodes fuscipes, and reinstatement of Ixodes spinosus (Acari: Ixodidae). Ticks Tick Borne Dis. 2020, 11, 101349. [Google Scholar] [CrossRef]
- Carvalho, L.A.; Maya, L.; Armua-Fernandez, M.T.; Felix, M.L.; Bazzano, V.; Barbieri, A.M.; Gonzalez, E.M.; Lado, P.; Colina, R.; Diaz, P.; et al. Borrelia burgdorferi sensu lato infecting Ixodes auritulus ticks in Uruguay. Exp. Appl. Acarol. 2020, 80, 109–125. [Google Scholar] [CrossRef]
- Verdugo, C.; Jimenez, O.; Hernandez, C.; Alvarez, P.; Espinoza, A.; Gonzalez-Acuna, D. Infection with Borrelia chilensis in Ixodes stilesi ticks collected from Pudu puda deer. Ticks Tick Borne Dis. 2017, 8, 733–740. [Google Scholar] [CrossRef]
- Hvidsten, D.; Frafjord, K.; Gray, J.S.; Henningsson, A.J.; Jenkins, A.; Kristiansen, B.E.; Lager, M.; Rognerud, B.; Slatsve, A.M.; Stordal, F.; et al. The distribution limit of the common tick, Ixodes ricinus, and some associated pathogens in north-western Europe. Ticks Tick Borne Dis. 2020, 11, 101388. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Wilhelmsson, P. First records of tick-borne pathogens in populations of the taiga tick Ixodes persulcatus in Sweden. Parasites Vectors 2019, 12, 559. [Google Scholar] [CrossRef] [Green Version]
- Gylfe, Å.; Olsen, B.; Strasevicius, D.; Marti Ras, N.; Weihe, P.; Noppa, L.; Ostberg, Y.; Baranton, G.; Bergstrom, S. Isolation of Lyme disease Borrelia from puffins (Fratercula arctica) and seabird ticks (Ixodes uriae) on the Faeroe Islands. J. Clin. Microbiol. 1999, 37, 890–896. [Google Scholar] [CrossRef] [Green Version]
- Shpynov, S. Ixodes persulcatus, a major vector of Alphaproteobacteria in Russia. Ticks Tick Borne Dis. 2012, 3, 305–307. [Google Scholar] [CrossRef]
- Mukhacheva, T.A.; Kovalev, S.Y. Borrelia spirochetes in Russia: Genospecies differentiation by real-time PCR. Ticks Tick Borne Dis. 2014, 5, 722–726. [Google Scholar] [CrossRef]
- Masuzawa, T.; Takada, N.; Kudeken, M.; Fukui, T.; Yano, Y.; Ishiguro, F.; Kawamura, Y.; Imai, Y.; Ezaki, T. Borrelia sinica sp. nov., a lyme disease-related Borrelia species isolated in China. Int. J. Syst. Evol. Microbiol. 2001, 51, 1817–1824. [Google Scholar] [CrossRef] [Green Version]
- Margos, G.; Chu, C.Y.; Takano, A.; Jiang, B.G.; Liu, W.; Kurtenbach, K.; Masuzawa, T.; Fingerle, V.; Cao, W.C.; Kawabata, H. Borrelia yangtzensis sp. nov., a rodent-associated species in Asia, is related to Borrelia valaisiana. Int. J. Syst. Evol. Microbiol. 2015, 65, 3836–3840. [Google Scholar] [CrossRef]
- Murase, Y.; Konnai, S.; Githaka, N.; Hidano, A.; Taylor, K.; Ito, T.; Takano, A.; Ando, S.; Kawabata, H.; Tsubota, T.; et al. Prevalence of Lyme Borrelia in Ixodes persulcatus ticks from an area with a confirmed case of Lyme disease. J. Vet. Med. Sci. 2013, 75, 215–218. [Google Scholar] [CrossRef] [Green Version]
- Fukunaga, M.; Hamase, A.; Okada, K.; Inoue, H.; Tsuruta, Y.; Miyamoto, K.; Nakao, M. Characterization of spirochetes isolated from ticks (Ixodes tanuki, Ixodes turdus, and Ixodes columnae) and comparison of the sequences with those of Borrelia burgdorferi sensu lato strains. Appl. Environ. Microbiol. 1996, 62, 2338–2344. [Google Scholar] [CrossRef] [Green Version]
- Masuzawa, T.; Hashimoto, N.; Kudeken, M.; Kadosaka, T.; Nakamura, M.; Kawabata, H.; Koizumi, N.; Imai, Y. New genomospecies related to Borrelia valaisiana, isolated from mammals in Okinawa archipelago, Japan. J. Med. Microbiol. 2004, 53, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Masuzawa, T.; Fukui, T.; Miyake, M.; Oh, H.B.; Cho, M.K.; Chang, W.H.; Imai, Y.; Yanagihara, Y. Determination of members of a Borrelia afzelii-related group isolated from Ixodes nipponensis in Korea as Borrelia valaisiana. Int. J. Syst. Bacteriol. 1999, 49 Pt 4, 1409–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Goo, Y.K.; Geraldino, P.J.L.; Kwon, O.D.; Kwak, D. Molecular Detection and Characterization of Borrelia garinii (Spirochaetales: Borreliaceae) in Ixodes nipponensis (Ixodida: Ixodidae) Parasitizing a Dog in Korea. Pathogens 2019, 8, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, Y.; Kim, S.Y.; Lee, Y.S.; Kim, D.W.; Kwon, T.; Hwang, K.J. Whole-Genome Sequence of Borrelia garinii Strain 935T Isolated from Ixodes persulcatus in South Korea. Genome Announc. 2014, 2, e01298-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Guleria, S.; Sharma, R.; Sharma, A. Lyme Disease: A Case Report with Typical and Atypical Lesions. Indian Dermatol. Online J. 2017, 8, 124–127. [Google Scholar] [CrossRef]
- Khoo, J.J.; Ishak, S.N.; Lim, F.S.; Mohd-Taib, F.S.; Khor, C.S.; Loong, S.K.; AbuBakar, S. Detection of a Borrelia sp. from Ixodes granulatus Ticks Collected from Rodents in Malaysia. J. Med. Entomol. 2018, 55, 1642–1647. [Google Scholar] [CrossRef] [Green Version]
- Younsi, H.; Sarih, M.; Jouda, F.; Godfroid, E.; Gern, L.; Bouattour, A.; Baranton, G.; Postic, D. Characterization of Borrelia lusitaniae isolates collected in Tunisia and Morocco. J. Clin. Microbiol. 2005, 43, 1587–1593. [Google Scholar] [CrossRef] [Green Version]
- Drehmann, M.; Chitimia-Dobler, L.; Lindau, A.; Frank, A.; Mai, S.; Fachet, K.; Hauck, D.; Knoll, S.; Strube, C.; Luhken, R.; et al. Ixodes frontalis: A neglected but ubiquitous tick species in Germany. Exp. Appl. Acarol. 2019, 78, 79–91. [Google Scholar] [CrossRef]
- Apanaskevich, D.A.; Horak, I.G.; Matthee, C.A.; Matthee, S. A new species of Ixodes (Acari: Ixodidae) from South African mammals. J. Parasitol. 2011, 97, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Maurizi, L.; Marie, J.L.; Aoun, O.; Courtin, C.; Gorsane, S.; Chal, D.; Davoust, B. Seroprevalence survey of equine Lyme borreliosis in France and in sub-Saharan Africa. Vector Borne Zoonotic Dis. 2010, 10, 535–537. [Google Scholar] [CrossRef]
- Hussain-Yusuf, H.; Stenos, J.; Vincent, G.; Shima, A.; Abell, S.; Preece, N.D.; Tadepalli, M.; Hii, S.F.; Bowie, N.; Mitram, K.; et al. Screening for Rickettsia, Coxiella and Borrelia Species in Ticks from Queensland, Australia. Pathogens 2020, 9, 1016. [Google Scholar] [CrossRef]
- Margos, G.; Vollmer, S.A.; Ogden, N.H.; Fish, D. Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect. Genet. Evol. 2011, 11, 1545–1563. [Google Scholar] [CrossRef] [Green Version]
- Millins, C.; Magierecka, A.; Gilbert, L.; Edoff, A.; Brereton, A.; Kilbride, E.; Denwood, M.; Birtles, R.; Biek, R. An Invasive Mammal (the Gray Squirrel, Sciurus carolinensis) Commonly Hosts Diverse and Atypical Genotypes of the Zoonotic Pathogen Borrelia burgdorferi Sensu Lato. Appl. Environ. Microbiol. 2015, 81, 4236–4245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.P., Jr.; Muzaffar, S.B.; Lavers, J.; Lacombe, E.H.; Cahill, B.K.; Lubelczyk, C.B.; Kinsler, A.; Mathers, A.J.; Rand, P.W. Borrelia garinii in seabird ticks (Ixodes uriae), Atlantic Coast, North America. Emerg. Infect. Dis. 2006, 12, 1909–1912. [Google Scholar] [CrossRef] [Green Version]
- Lane, R.S.; Quistad, G.B. Borreliacidal factor in the blood of the western fence lizard (Sceloporus occidentalis). J. Parasitol. 1998, 84, 29–34. [Google Scholar] [CrossRef]
- Kuo, M.M.; Lane, R.S.; Giclas, P.C. A comparative study of mammalian and reptilian alternative pathway of complement-mediated killing of the Lyme disease spirochete (Borrelia burgdorferi). J. Parasitol. 2000, 86, 1223–1228. [Google Scholar] [CrossRef]
- Michalik, J.; Wodecka, B.; Skoracki, M.; Sikora, B.; Stańczak, J. Prevalence of avian-associated Borrelia burgdorferi s.l. genospecies in Ixodes ricinus ticks collected from blackbirds (Turdus merula) and song thrushes (T. philomelos). Int. J. Med. Microbiol. 2008, 298, 129–138. [Google Scholar] [CrossRef]
- Olsen, B.; Jaenson, T.G.; Bergstrom, S. Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl. Environ. Microbiol. 1995, 61, 3082–3087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primers 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed]
- Winslow, C.; Coburn, J. Recent discoveries and advancements in research on the Lyme disease spirochete Borrelia burgdorferi. F1000Research 2019, 8, 763. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, S.F.; Buttle, K.F.; Charon, N.W. Structural analysis of the Leptospiraceae and Borrelia burgdorferi by high-voltage electron microscopy. J. Bacteriol. 1996, 178, 6539–6545. [Google Scholar] [CrossRef] [Green Version]
- Cinco, M.; Banfi, E.; Trevisan, G.; Stanek, G. Characterization of the first tick isolate of Borrelia burgdorferi from Italy. APMIS 1989, 97, 381–382. [Google Scholar] [CrossRef]
- Fraser, C.M.; Casjens, S.; Huang, W.M.; Sutton, G.G.; Clayton, R.; Lathigra, R.; White, O.; Ketchum, K.A.; Dodson, R.; Hickey, E.K.; et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 1997, 390, 580–586. [Google Scholar] [CrossRef]
- Casjens, S.; Palmer, N.; van Vugt, R.; Huang, W.M.; Stevenson, B.; Rosa, P.; Lathigra, R.; Sutton, G.; Peterson, J.; Dodson, R.J.; et al. A bacterial genome in flux: The twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 2000, 35, 490–516. [Google Scholar] [CrossRef]
- Le Fleche, A.; Postic, D.; Girardet, K.; Peter, O.; Baranton, G. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 1997, 47, 921–925. [Google Scholar] [CrossRef] [Green Version]
- Marconi, R.T.; Garon, C.F. Phylogenetic analysis of the genus Borrelia: A comparison of North American and European isolates of Borrelia burgdorferi. J. Bacteriol. 1992, 174, 241–244. [Google Scholar] [CrossRef] [Green Version]
- Elbaum-Garfinkle, S. Close to home: A history of Yale and Lyme disease. Yale J. Biol. Med. 2011, 84, 103–108. [Google Scholar]
- Qiu, W.G.; Bruno, J.F.; McCaig, W.D.; Xu, Y.; Livey, I.; Schriefer, M.E.; Luft, B.J. Wide distribution of a high-virulence Borrelia burgdorferi clone in Europe and North America. Emerg. Infect. Dis. 2008, 14, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Baril, C.; Richaud, C.; Baranton, G.; Girons, I.S. Linear chromosome of Borrelia burgdorferi. Res. Microbiol. 1989, 140, 507–516. [Google Scholar] [CrossRef]
- Casjens, S.R.; Mongodin, E.F.; Qiu, W.G.; Luft, B.J.; Schutzer, S.E.; Gilcrease, E.B.; Huang, W.M.; Vujadinovic, M.; Aron, J.K.; Vargas, L.C.; et al. Genome stability of Lyme disease spirochetes: Comparative genomics of Borrelia burgdorferi plasmids. PLoS ONE 2012, 7, e33280. [Google Scholar] [CrossRef] [Green Version]
- Mongodin, E.F.; Casjens, S.R.; Bruno, J.F.; Xu, Y.; Drabek, E.F.; Riley, D.R.; Cantarel, B.L.; Pagan, P.E.; Hernandez, Y.A.; Vargas, L.C.; et al. Inter- and intra-specific pan-genomes of Borrelia burgdorferi sensu lato: Genome stability and adaptive radiation. BMC Genom. 2013, 14, 693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuler, W.; Bunikis, I.; Weber-Lehman, J.; Comstedt, P.; Kutschan-Bunikis, S.; Stanek, G.; Huber, J.; Meinke, A.; Bergstrom, S.; Lundberg, U. Complete genome sequence of Borrelia afzelii K78 and comparative genome analysis. PLoS ONE 2015, 10, e0120548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, J.J.; Gazumyan, A.; Schwartz, I. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J. Bacteriol. 1992, 174, 3757–3765. [Google Scholar] [CrossRef] [Green Version]
- Strle, K.; Jones, K.L.; Drouin, E.E.; Li, X.; Steere, A.C. Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am. J. Pathol. 2011, 178, 2726–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ružić-Sabljić, E.; Cerar, T. Borrelia genotyping in lyme disease. Open Dermatol. J. 2016, 10, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Barbour, A.G.; Cook, V.J. Genotyping Strains of Lyme Disease Agents Directly from Ticks, Blood, or Tissue. Methods Mol. Biol. 2018, 1690, 1–11. [Google Scholar] [CrossRef]
- Casselli, T.; Divan, A.; Vomhof-DeKrey, E.E.; Tourand, Y.; Pecoraro, H.L.; Brissette, C.A. A murine model of Lyme disease demonstrates that Borrelia burgdorferi colonizes the dura mater and induces inflammation in the central nervous system. PLoS Pathog. 2021, 17, e1009256. [Google Scholar] [CrossRef]
- Jaulhac, B.; Heller, R.; Limbach, F.X.; Hansmann, Y.; Lipsker, D.; Monteil, H.; Sibilia, J.; Piemont, Y. Direct molecular typing of Borrelia burgdorferi sensu lato species in synovial samples from patients with lyme arthritis. J. Clin. Microbiol. 2000, 38, 1895–1900. [Google Scholar] [CrossRef] [Green Version]
- Rauer, S.; Spohn, N.; Rasiah, C.; Neubert, U.; Vogt, A. Enzyme-linked immunosorbent assay using recombinant OspC and the internal 14-kDa flagellin fragment for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 1998, 36, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Rasiah, C.; Schiltz, E.; Reichert, J.; Vogt, A. Purification and characterization of a tryptic peptide of Borrelia burgdorferi flagellin, which reduces cross-reactivity in immunoblots and ELISA. J. Gen. Microbiol. 1992, 138, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Coburn, J.; Medrano, M.; Cugini, C. Borrelia burgdorferi and its tropisms for adhesion molecules in the joint. Curr. Opin. Rheumatol. 2002, 14, 394–398. [Google Scholar] [CrossRef]
- Coburn, J. Adhesion mechanisms of the Lyme disease spirochete, Borrelia burgdorferi. Curr. Drug. Targets Infect. Disord. 2001, 1, 171–179. [Google Scholar] [CrossRef]
- Zoller, L.; Cremer, J.; Faulde, M. Western blot as a tool in the diagnosis of Lyme borreliosis. Electrophoresis 1993, 14, 937–944. [Google Scholar] [CrossRef]
- Probert, W.S.; Johnson, B.J. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol. 1998, 30, 1003–1015. [Google Scholar] [CrossRef] [Green Version]
- Wilske, B.; Preac-Mursic, V.; Gobel, U.B.; Graf, B.; Jauris, S.; Soutschek, E.; Schwab, E.; Zumstein, G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J. Clin. Microbiol. 1993, 31, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Wilske, B.; Preac-Mursic, V.; Jauris, S.; Hofmann, A.; Pradel, I.; Soutschek, E.; Schwab, E.; Will, G.; Wanner, G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect. Immun. 1993, 61, 2182–2191. [Google Scholar] [CrossRef] [Green Version]
- Grimm, D.; Tilly, K.; Byram, R.; Stewart, P.E.; Krum, J.G.; Bueschel, D.M.; Schwan, T.G.; Policastro, P.F.; Elias, A.F.; Rosa, P.A. Outer-surface protein C of the Lyme disease spirochete: A protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 2004, 101, 3142–3147. [Google Scholar] [CrossRef] [Green Version]
- Tilly, K.; Krum, J.G.; Bestor, A.; Jewett, M.W.; Grimm, D.; Bueschel, D.; Byram, R.; Dorward, D.; Vanraden, M.J.; Stewart, P.; et al. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 2006, 74, 3554–3564. [Google Scholar] [CrossRef] [Green Version]
- Dulipati, V.; Meri, S.; Panelius, J. Complement evasion strategies of Borrelia burgdorferi sensu lato. FEBS Lett. 2020, 594, 2645–2656. [Google Scholar] [CrossRef]
- Pulzova, L.; Bhide, M. Outer surface proteins of Borrelia: Peerless immune evasion tools. Curr. Protein Pept. Sci. 2014, 15, 75–88. [Google Scholar] [CrossRef]
- Li, X.; Neelakanta, G.; Liu, X.; Beck, D.S.; Kantor, F.S.; Fish, D.; Anderson, J.F.; Fikrig, E. Role of outer surface protein D in the Borrelia burgdorferi life cycle. Infect. Immun. 2007, 75, 4237–4244. [Google Scholar] [CrossRef] [Green Version]
- Luft, B.J.; Jiang, W.; Munoz, P.; Dattwyler, R.J.; Gorevic, P.D. Biochemical and immunological characterization of the surface proteins of Borrelia burgdorferi. Infect. Immun. 1989, 57, 3637–3645. [Google Scholar] [CrossRef] [Green Version]
- Bergstrom, S.; Bundoc, V.G.; Barbour, A.G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol. Microbiol. 1989, 3, 479–486. [Google Scholar] [CrossRef]
- Masuzawa, T.; Wilske, B.; Komikado, T.; Suzuki, H.; Kawabata, H.; Sato, N.; Muramatsu, K.; Sato, N.; Isogai, E.; Isogai, H.; et al. Comparison of OspA serotypes for Borrelia burgdorferi sensu lato from Japan, Europe and North America. Microbiol. Immunol. 1996, 40, 539–545. [Google Scholar] [CrossRef]
- Mason, L.M.; Herkes, E.A.; Krupna-Gaylord, M.A.; Oei, A.; van der Poll, T.; Wormser, G.P.; Schwartz, I.; Petzke, M.M.; Hovius, J.W. Borrelia burgdorferi clinical isolates induce human innate immune responses that are not dependent on genotype. Immunobiology 2015, 220, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; McHugh, G.A.; Glickstein, L.J.; Steere, A.C. Analysis of Borrelia burgdorferi genotypes in patients with Lyme arthritis: High frequency of ribosomal RNA intergenic spacer type 1 strains in antibiotic-refractory arthritis. Arthritis Rheum. 2009, 60, 2174–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norris, S.J.; Carter, C.J.; Howell, J.K.; Barbour, A.G. Low-passage-associated proteins of Borrelia burgdorferi B31: Characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect. Immun. 1992, 60, 4662–4672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.C.; Stevenson, B. Borrelia burgdorferi erp genes are expressed at different levels within tissues of chronically infected mammalian hosts. Int. J. Med. Microbiol. 2006, 296 (Suppl. S40), 185–194. [Google Scholar] [CrossRef]
- Jutras, B.L.; Chenail, A.M.; Stevenson, B. Changes in bacterial growth rate govern expression of the Borrelia burgdorferi OspC and Erp infection-associated surface proteins. J. Bacteriol. 2013, 195, 757–764. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, B.; El-Hage, N.; Hines, M.A.; Miller, J.C.; Babb, K. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: A possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 2002, 70, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Burns, L.H.; Adams, C.A.; Riley, S.P.; Jutras, B.L.; Bowman, A.; Chenail, A.M.; Cooley, A.E.; Haselhorst, L.A.; Moore, A.M.; Babb, K.; et al. BpaB, a novel protein encoded by the Lyme disease spirochete’s cp32 prophages, binds to erp Operator 2 DNA. Nucleic Acids Res. 2010, 38, 5443–5455. [Google Scholar] [CrossRef]
- Brissette, C.A.; Haupt, K.; Barthel, D.; Cooley, A.E.; Bowman, A.; Skerka, C.; Wallich, R.; Zipfel, P.F.; Kraiczy, P.; Stevenson, B. Borrelia burgdorferi infection-associated surface proteins ErpP, ErpA, and ErpC bind human plasminogen. Infect. Immun. 2009, 77, 300–306. [Google Scholar] [CrossRef] [Green Version]
- von Lackum, K.; Ollison, K.M.; Bykowski, T.; Nowalk, A.J.; Hughes, J.L.; Carroll, J.A.; Zuckert, W.R.; Stevenson, B. Regulated synthesis of the Borrelia burgdorferi inner-membrane lipoprotein IpLA7 (P22, P22-A) during the Lyme disease spirochaete’s mammal-tick infectious cycle. Microbiology 2007, 153, 1361–1371. [Google Scholar] [CrossRef] [Green Version]
- Alitalo, A.; Meri, T.; Comstedt, P.; Jeffery, L.; Tornberg, J.; Strandin, T.; Lankinen, H.; Bergstrom, S.; Cinco, M.; Vuppala, S.R.; et al. Expression of complement factor H binding immunoevasion proteins in Borrelia garinii isolated from patients with neuroborreliosis. Eur. J. Immunol. 2005, 35, 3043–3053. [Google Scholar] [CrossRef]
- Lam, T.T.; Nguyen, T.P.; Montgomery, R.R.; Kantor, F.S.; Fikrig, E.; Flavell, R.A. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect. Immun. 1994, 62, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Luft, B.J.; Gorevic, P.D.; Jiang, W.; Munoz, P.; Dattwyler, R.J. Immunologic and structural characterization of the dominant 66- to 73-kDa antigens of Borrelia burgdorferi. J. Immunol. 1991, 146, 2776–2782. [Google Scholar]
- Ristow, L.C.; Bonde, M.; Lin, Y.P.; Sato, H.; Curtis, M.; Wesley, E.; Hahn, B.L.; Fang, J.; Wilcox, D.A.; Leong, J.M.; et al. Integrin binding by Borrelia burgdorferi P66 facilitates dissemination but is not required for infectivity. Cell. Microbiol. 2015, 17, 1021–1036. [Google Scholar] [CrossRef] [Green Version]
- Carreiro, M.M.; Laux, D.C.; Nelson, D.R. Characterization of the heat shock response and identification of heat shock protein antigens of Borrelia burgdorferi. Infect. Immun. 1990, 58, 2186–2191. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Brissette, C.A.; Bowman, A.; Stevenson, B. Borrelia burgdorferi BmpA is a laminin-binding protein. Infect. Immun. 2009, 77, 4940–4946. [Google Scholar] [CrossRef] [Green Version]
- Ditton, H.J.; Neuss, M.; Zoller, L. Evidence that Borrelia burgdorferi immunodominant proteins p100, p94 and p83 are identical. FEMS Microbiol. Lett. 1992, 73, 217–220. [Google Scholar] [CrossRef]
- Jauris-Heipke, S.; Fuchs, R.; Hofmann, A.; Lottspeich, F.; Preac-Mursic, V.; Soutschek, E.; Will, G.; Wilske, B. Molecular characterization of the p100 gene of Borrelia burgdorferi strain PKo. FEMS Microbiol. Lett. 1993, 114, 235–241. [Google Scholar] [CrossRef]
- Chaconas, G.; Castellanos, M.; Verhey, T.B. Changing of the guard: How the Lyme disease spirochete subverts the host immune response. J. Biol. Chem. 2020, 295, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Burgdorfer, W.; Barbour, A.G.; Hayes, S.F.; Benach, J.L.; Grunwaldt, E.; Davis, J.P. Lyme disease-a tick-borne spirochetosis? Science 1982, 216, 1317–1319. [Google Scholar] [CrossRef] [Green Version]
- Canica, M.M.; Nato, F.; du Merle, L.; Mazie, J.C.; Baranton, G.; Postic, D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand. J. Infect. Dis. 1993, 25, 441–448. [Google Scholar] [CrossRef]
- Nakao, M.; Miyamoto, K.; Fukunaga, M.; Hashimoto, Y.; Takahashi, H. Comparative studies on Borrelia afzelii isolated from a patient of Lyme disease, Ixodes persulcatus ticks, and Apodemus speciosus rodents in Japan. Microbiol. Immunol. 1994, 38, 413–420. [Google Scholar] [CrossRef]
- Rudenko, N.; Golovchenko, M.; Lin, T.; Gao, L.; Grubhoffer, L.; Oliver, J.H., Jr. Delineation of a new species of the Borrelia burgdorferi Sensu Lato Complex, Borrelia americana sp. nov. J. Clin. Microbiol. 2009, 47, 3875–3880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunaj, J.; Drewnowska, J.; Moniuszko-Malinowska, A.; Swiecicka, I.; Pancewicz, S. First metagenomic report of Borrelia americana and Borrelia carolinensis in Poland—A preliminary study. Ann. Agric. Environ. Med. 2021, 28, 49–55. [Google Scholar] [CrossRef]
- Anderson, J.F.; Magnarelli, L.A.; McAninch, J.B. New Borrelia burgdorferi antigenic variant isolated from Ixodes dammini from upstate New York. J. Clin. Microbiol. 1988, 26, 2209–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margos, G.; Vollmer, S.A.; Cornet, M.; Garnier, M.; Fingerle, V.; Wilske, B.; Bormane, A.; Vitorino, L.; Collares-Pereira, M.; Drancourt, M.; et al. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl. Environ. Microbiol. 2009, 75, 5410–5416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postic, D.; Ras, N.M.; Lane, R.S.; Hendson, M.; Baranton, G. Expanded diversity among Californian Borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127). J. Clin. Microbiol. 1998, 36, 3497–3504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, B.S.; Zeidner, N.S.; Burkot, T.R.; Maupin, G.O.; Piesman, J. Borrelia isolates in Northern Colorado identified as Borrelia bissettii. J. Clin. Microbiol. 2000, 38, 3103–3105. [Google Scholar] [CrossRef] [Green Version]
- Postic, D.; Garnier, M.; Baranton, G. Multilocus sequence analysis of atypical Borrelia burgdorferi sensu lato isolates--description of Borrelia californiensis sp. nov., and genomospecies 1 and 2. Int. J. Med. Microbiol. 2007, 297, 263–271. [Google Scholar] [CrossRef]
- Margos, G.; Lane, R.S.; Fedorova, N.; Koloczek, J.; Piesman, J.; Hojgaard, A.; Sing, A.; Fingerle, V. Borrelia bissettiae sp. nov. and Borrelia californiensis sp. nov. prevail in diverse enzootic transmission cycles. Int. J. Syst. Evol. Microbiol. 2016, 66, 1447–1452. [Google Scholar] [CrossRef]
- Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Oliver, J.H., Jr. Borrelia carolinensis sp. nov., a new (14th) member of the Borrelia burgdorferi Sensu Lato complex from the southeastern region of the United States. J. Clin. Microbiol. 2009, 47, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Foley, J.; Ott-Conn, C.; Worth, J.; Poulsen, A.; Clifford, D. An Ixodes minor and Borrelia carolinensis enzootic cycle involving a critically endangered Mojave Desert rodent. Ecol. Evol. 2014, 4, 576–581. [Google Scholar] [CrossRef]
- Springer, A.; Raulf, M.K.; Fingerle, V.; Strube, C. Borrelia prevalence and species distribution in ticks removed from humans in Germany, 2013–2017. Ticks Tick Borne Dis. 2020, 11, 101363. [Google Scholar] [CrossRef]
- Ivanova, L.B.; Tomova, A.; Gonzalez-Acuna, D.; Murua, R.; Moreno, C.X.; Hernandez, C.; Cabello, J.; Cabello, C.; Daniels, T.J.; Godfrey, H.P.; et al. Borrelia chilensis, a new member of the Borrelia burgdorferi sensu lato complex that extends the range of this genospecies in the Southern Hemisphere. Environ. Microbiol. 2014, 16, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Casjens, S.R.; Fraser-Liggett, C.M.; Mongodin, E.F.; Qiu, W.G.; Dunn, J.J.; Luft, B.J.; Schutzer, S.E. Whole genome sequence of an unusual Borrelia burgdorferi sensu lato isolate. J. Bacteriol. 2011, 193, 1489–1490. [Google Scholar] [CrossRef] [Green Version]
- Baranton, G.; Postic, D.; Saint Girons, I.; Boerlin, P.; Piffaretti, J.C.; Assous, M.; Grimont, P.A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 1992, 42, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, H.; Masuzawa, T.; Yanagihara, Y. Genomic analysis of Borrelia japonica sp. nov. isolated from Ixodes ovatus in Japan. Microbiol. Immunol. 1993, 37, 843–848. [Google Scholar] [CrossRef]
- Masuzawa, T.; Suzuki, H.; Kawabata, H.; Ishiguro, F.; Takada, N.; Yano, Y.; Yanagihara, Y. Identification of spirochetes isolated from wild rodents in Japan as Borrelia japonica. J. Clin. Microbiol. 1995, 33, 1392–1394. [Google Scholar] [CrossRef] [Green Version]
- Margos, G.; Hojgaard, A.; Lane, R.S.; Cornet, M.; Fingerle, V.; Rudenko, N.; Ogden, N.; Aanensen, D.M.; Fish, D.; Piesman, J. Multilocus sequence analysis of Borrelia bissettii strains from North America reveals a new Borrelia species, Borrelia kurtenbachii. Ticks Tick Borne Dis. 2010, 1, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Margos, G.; Fedorova, N.; Kleinjan, J.E.; Hartberger, C.; Schwan, T.G.; Sing, A.; Fingerle, V. Borrelia lanei sp. nov. extends the diversity of Borrelia species in California. Int. J. Syst. Evol. Microbiol. 2017, 67, 3872–3876. [Google Scholar] [CrossRef] [Green Version]
- Richter, D.; Matuschka, F.R. Perpetuation of the Lyme disease spirochete Borrelia lusitaniae by lizards. Appl. Environ. Microbiol. 2006, 72, 4627–4632. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.L.; Graham, C.B.; Hojgaard, A.; Breuner, N.E.; Maes, S.E.; Boegler, K.A.; Replogle, A.J.; Kingry, L.C.; Petersen, J.M.; Eisen, L.; et al. Isolation of the Lyme Disease Spirochete Borrelia mayonii from Naturally Infected Rodents in Minnesota. J. Med. Entomol. 2017, 54, 1088–1092. [Google Scholar] [CrossRef]
- Pritt, B.S.; Mead, P.S.; Johnson, D.K.H.; Neitzel, D.F.; Respicio-Kingry, L.B.; Davis, J.P.; Schiffman, E.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: A descriptive study. Lancet Infect. Dis. 2016, 16, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; van Dam, A.P.; Dankert, J. Phenotypic and genetic characterization of a novel Borrelia burgdorferi sensu lato isolate from a patient with lyme borreliosis. J. Clin. Microbiol. 1999, 37, 3025–3028. [Google Scholar] [CrossRef] [Green Version]
- Richter, D.; Schlee, D.B.; Allgower, R.; Matuschka, F.R. Relationships of a novel Lyme disease spirochete, Borrelia spielmani sp. nov., with its hosts in Central Europe. Appl. Environ. Microbiol. 2004, 70, 6414–6419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foldvari, G.; Farkas, R.; Lakos, A. Borrelia spielmanii erythema migrans, Hungary. Emerg. Infect. Dis. 2005, 11, 1794–1795. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, H.; Shimada, Y.; Sakata, Y.; Watanabe, M.; Itamoto, K.; Okuda, M.; Masuzawa, T.; Inokuma, H. Detection of Borrelia garinii, Borrelia tanukii and Borrelia sp. closely related to Borrelia valaisiana in Ixodes ticks removed from dogs and cats in Japan. Vet. Parasitol. 2007, 144, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Norte, A.C.; Araujo, P.M.; da Silva, L.P.; Tenreiro, P.Q.; Ramos, J.A.; Nuncio, M.S.; Ze-Ze, L.; de Carvalho, I.L. Characterization Through Multilocus Sequence Analysis of Borrelia turdi Isolates from Portugal. Microb. Ecol. 2016, 72, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Margos, G.; Becker, N.S.; Fingerle, V.; Sing, A.; Ramos, J.A.; Carvalho, I.L.; Norte, A.C. Core genome phylogenetic analysis of the avian associated Borrelia turdi indicates a close relationship to Borrelia garinii. Mol. Phylogenet. Evol. 2019, 131, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Masuzawa, T.; Pan, M.J.; Kadosaka, T.; Kudeken, M.; Takada, N.; Yano, Y.; Imai, Y.; Yanagihara, Y. Characterization and identification of Borrelia isolates as Borrelia valaisiana in Taiwan and Kinmen Islands. Microbiol. Immunol. 2000, 44, 1003–1009. [Google Scholar] [CrossRef] [Green Version]
- Hanincova, K.; Taragelova, V.; Koci, J.; Schafer, S.M.; Hails, R.; Ullmann, A.J.; Piesman, J.; Labuda, M.; Kurtenbach, K. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl. Environ. Microbiol. 2003, 69, 2825–2830. [Google Scholar] [CrossRef] [Green Version]
- Stanek, G.; Reiter, M. The expanding Lyme Borrelia complex--clinical significance of genomic species? Clin. Microbiol. Infect. 2011, 17, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.M.; Lee, W.G.; Ryou, J.; Yang, S.C.; Park, S.W.; Roh, J.Y.; Lee, Y.J.; Park, C.; Han, M.G. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg. Infect. Dis. 2014, 20, 1358–1361. [Google Scholar] [CrossRef]
- Tanskul, P.; Stark, H.E.; Inlao, I. A checklist of ticks of Thailand (Acari: Metastigmata: Ixodoidea). J. Med. Entomol. 1983, 20, 330–341. [Google Scholar] [CrossRef]
- Lau, A.C.C.; Qiu, Y.; Moustafa, M.A.M.; Nakao, R.; Shimozuru, M.; Onuma, M.; Mohd-Azlan, J.; Tsubota, T. Detection of Borrelia burgdorferi Sensu Lato and Relapsing Fever Borrelia in Feeding Ixodes Ticks and Rodents in Sarawak, Malaysia: New Geographical Records of Borrelia yangtzensis and Borrelia miyamotoi. Pathogens 2020, 9, 846. [Google Scholar] [CrossRef]
- Burioni, R.; Grillo, R.; Magaro, M.; Dettori, G. Lyme disease in Italy: Isolation of Borrelia burgdorferi from a patient. Eur. J. Epidemiol. 1988, 4, 506–510. [Google Scholar] [CrossRef]
- Cinco, M.; Trevisan, G.; Agolzer, A. Isolation of Borrelia burgdorferi from a Lyme seronegative patient in northern Italy: Expression of OspB immunodominant proteins on the isolated strain. Microbiologica 1992, 15, 95–98. [Google Scholar]
- Lardieri, G.; Salvi, A.; Camerini, F.; Cinco, M.; Trevisan, G. Isolation of Borrelia burgdorferi from myocardium. Lancet 1993, 342, 490. [Google Scholar] [CrossRef]
- Cinco, M.; De Giovannini, R. Protein and antigenic analysis of Borrelia burgdorferi isolated in northern Italy: Computerized analysis of phenotypic characteristics. J. Clin. Microbiol. 1993, 31, 440–443. [Google Scholar] [CrossRef] [Green Version]
- Stefanelli, S.; Paladini, A.; Conforti, P.L.; Leoncini, F.; Vigano, S.; De Giovannini, R.; Cinco, M. Isolation of Borrelia burgdorferi in Tuscany (Italy). New Microbiol. 1994, 17, 333–336. [Google Scholar]
- Trevisan, G.; Stinco, G.; Cinco, M. Neonatal skin lesions due to a spirochetal infection: A case of congenital Lyme borreliosis? Int. J. Dermatol. 1997, 36, 677–680. [Google Scholar] [CrossRef]
- Ciceroni, L.; Ciarrocchi, S.; Simeoni, J. Antigenic and genomic analysis of a Borrelia burgdorferi sensu stricto strain isolated from Ixodes ricinus ticks in Alto Adige-South Tyrol, Italy. Eur. J. Epidemiol. 1998, 14, 511–517. [Google Scholar] [CrossRef]
- Trevisan, G.; Padovan, C.; Scaini, M.T.; Cinco, M.; Floris, R.; Bonin, S. Anetoderma associated with lyme disease: A case report. Acta Derm. Venereol. 2008, 88, 536–538. [Google Scholar] [CrossRef]
- Van Dam, A.P.; Kuiper, H.; Vos, K.; Widjojokusumo, A.; de Jongh, B.M.; Spanjaard, L.; Ramselaar, A.C.; Kramer, M.D.; Dankert, J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 1993, 17, 708–717. [Google Scholar] [CrossRef]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Pauluzzi, P.; Bonin, S.; Gonzalez Inchaurraga, M.A.; Stanta, G.; Trevisan, G. Detection of spirochaetal DNA simultaneously in skin biopsies, peripheral blood and urine from patients with erythema migrans. Acta Derm. Venereol. 2004, 84, 106–110. [Google Scholar] [CrossRef]
- Trevisan, G. Skin manifestations of Lyme borreliosis. Alpe Adria Microbiol. J. 1994, 3, 261–262. [Google Scholar]
- Mullegger, R.R. Dermatological manifestations of Lyme borreliosis. Eur. J. Dermatol. 2004, 14, 296–309. [Google Scholar]
- Aberer, E.; Wutte, N. Atrophosclerodermic manifestations of Lyme borreliosis. Open Dermatol. J. 2016, 10, 27–43. [Google Scholar] [CrossRef] [Green Version]
- Ogrinc, K.; Maraspin, V. Nervous system involvement in lyme borreliosis. Open Dermatol. J. 2016, 10, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Walter, L.; Surth, V.; Rottgerding, F.; Zipfel, P.F.; Fritz-Wolf, K.; Kraiczy, P. Elucidating the Immune Evasion Mechanisms of Borrelia mayonii, the Causative Agent of Lyme Disease. Front. Immunol. 2019, 10, 2722. [Google Scholar] [CrossRef]
- Buchan, B.W.; Jobe, D.A.; Mashock, M.; Gerstbrein, D.; Faron, M.L.; Ledeboer, N.A.; Callister, S.M. Evaluation of a Novel Multiplex High-Definition PCR Assay for Detection of Tick-Borne Pathogens in Whole-Blood Specimens. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef] [Green Version]
- Yoshinari, N.H.; Oyafuso, L.K.; Monteiro, F.G.; de Barros, P.J.; da Cruz, F.C.; Ferreira, L.G.; Bonasser, F.; Baggio, D.; Cossermelli, W. Lyme disease. Report of a case observed in Brazil. Rev. Hosp. Clin. 1993, 48, 170–174. [Google Scholar]
- Gouveia, E.A.; Alves, M.F.; Mantovani, E.; Oyafuso, L.K.; Bonoldi, V.L.; Yoshinari, N.H. Profile of patients with Baggio-Yoshinari Syndrome admitted at “Instituto de Infectologia Emilio Ribas”. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Lino, A.M.M.; Spera, R.R.; de Campos, F.P.F.; Freitas, C.H.A.; Garcia, M.R.T.; Lopes, L.D.C.; Prokopowitsch, A.S. Adult-onset opsoclonus-myoclonus-ataxia syndrome as a manifestation of brazilian lyme disease-like syndrome: A case report and review of literature. Autops. Case. Rep. 2014, 4, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshinari, N.H.; Mantovani, E.; Bonoldi, V.L.N.; Marangoni, R.G.; Gauditano, G. Brazilian lyme-like disease or Baggio-Yoshinari syndrome: Exotic and emerging Brazilian tick-borne zoonosis. Rev. Assoc. Med. Bras. 2010, 56, 363–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basile, R.C.; Yoshinari, N.H.; Mantovani, E.; Bonoldi, V.N.; Macoris, D.D.; Queiroz-Neto, A. Brazilian borreliosis with special emphasis on humans and horses. Braz. J. Microbiol. 2017, 48, 167–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spolidorio, M.G.; Labruna, M.B.; Machado, R.Z.; Moraes-Filho, J.; Zago, A.M.; Donatele, D.M.; Pinheiro, S.R.; Silveira, I.; Caliari, K.M.; Yoshinari, N.H. Survey for tick-borne zoonoses in the state of Espirito Santo, southeastern Brazil. Am. J. Trop. Med. Hyg. 2010, 83, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Colunga-Salas, P.; Sanchez-Montes, S.; Volkow, P.; Ruiz-Remigio, A.; Becker, I. Lyme disease and relapsing fever in Mexico: An overview of human and wildlife infections. PLoS ONE 2020, 15, e0238496. [Google Scholar] [CrossRef]
- Rodriguez, I.; Fernandez, C.; Cinco, M.; Pedroso, R.; Fuentes, O. Do antiborrelial antibodies suggest Lyme disease in Cuba? Emerg. Infect. Dis. 2004, 10, 1698–1700. [Google Scholar] [CrossRef]
- Mantovani, E.; Marangoni, R.G.; Gauditano, G.; Bonoldi, V.L.; Yoshinari, N.H. Amplification of the flgE gene provides evidence for the existence of a Brazilian borreliosis. Rev. Inst. Med. Trop. Sao Paulo 2012, 54, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Lopes, F.A.; Rezende, J.; Silva, D.; Alves, F.C.G.; Oliveira, C.E.; Costa, I.P.D. Molecular evidence of Borrelia burgdorferi sensu lato in patients in Brazilian central-western region. Rev. Bras. Reumatol. Engl. Ed. 2017, 57, 641–645. [Google Scholar] [CrossRef]
- Murgia, R.; Cinco, M. Induction of cystic forms by different stress conditions in Borrelia burgdorferi. APMIS 2004, 112, 57–62. [Google Scholar] [CrossRef]
- Miziara, C.; Gelmeti Serrano, V.A.; Yoshinari, N. Passage of Borrelia burgdorferi through diverse Ixodid hard ticks causes distinct diseases: Lyme borreliosis and Baggio-Yoshinari syndrome. Clinics 2018, 73, e394. [Google Scholar] [CrossRef]
- Oliveira, A.; Fonseca, A.H.; Costa, C.M.; Mantovani, E.; Yoshinari, N.H. Growth, cysts and kinetics of Borrelia garinii (Spirochaetales: Spirochaetacea) in different culture media. Mem. Inst. Oswaldo. Cruz. 2010, 105, 717–719. [Google Scholar] [CrossRef] [Green Version]
- Rosa Neto, N.S.; Gauditano, G.; Yoshinari, N.H. Chronic lymphomonocytic meningoencephalitis, oligoarthritis and erythema nodosum: Report of Baggio-Yoshinari syndrome of long and relapsing evolution. Rev. Bras. Reumatol. 2014, 54, 148–151. [Google Scholar] [CrossRef] [Green Version]
- Hepner, S.; Fingerle, V.; Duscher, G.G.; Felsberger, G.; Marosevic, D.; Rollins, R.E.; Okeyo, M.; Sing, A.; Margos, G. Population structure of Borrelia turcica from Greece and Turkey. Infect. Genet. Evol. 2020, 77, 104050. [Google Scholar] [CrossRef]
- Pacheco, A.; Cordeiro, M.D.; Cepeda, M.B.; Luz, H.R.; Cardozo, S.V.; Berto, B.P.; Guterres, A.; Fonseca, A.H.D. Hemoparasites in ticks of wild birds of Serra dos Orgaos National Park, state of Rio de Janeiro, Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 238–244. [Google Scholar] [CrossRef]
- Cicuttin, G.L.; De Salvo, M.N.; Venzal, J.M.; Nava, S. Borrelia spp. in ticks and birds from a protected urban area in Buenos Aires city, Argentina. Ticks Tick Borne Dis. 2019, 10, 101282. [Google Scholar] [CrossRef]
- Morales-Diaz, J.; Colunga-Salas, P.; Romero-Salas, D.; Sanchez-Montes, S.; Estrada-Souza, I.M.; Ochoa-Ochoa, L.M.; Becker, I.; Flores-Primo, A.; Cruz-Romero, A. Molecular detection of reptile-associated Borrelia in Boa constrictor (Squamata: Boidae) from Veracruz, Mexico. Acta Trop. 2020, 205, 105422. [Google Scholar] [CrossRef]
- Guner, E.S.; Hashimoto, N.; Kadosaka, T.; Imai, Y.; Masuzawa, T. A novel, fast-growing Borrelia sp. isolated from the hard tick Hyalomma aegyptium in Turkey. Microbiology 2003, 149, 2539–2544. [Google Scholar] [CrossRef] [Green Version]
- Kalmar, Z.; Cozma, V.; Sprong, H.; Jahfari, S.; D’Amico, G.; Marcutan, D.I.; Ionica, A.M.; Magdas, C.; Modry, D.; Mihalca, A.D. Transstadial transmission of Borrelia turcica in Hyalomma aegyptium ticks. PLoS ONE 2015, 10, e0115520. [Google Scholar] [CrossRef]
- Hepner, S.; Fingerle, V.; Heylen, D.; Marosevic, D.; Ghaffari, K.; Okeyo, M.; Sing, A.; Margos, G. First investigations on serum resistance and sensitivity of Borrelia turcica. Ticks Tick Borne Dis. 2019, 10, 1157–1161. [Google Scholar] [CrossRef]
- Loh, S.M.; Gillett, A.; Ryan, U.; Irwin, P.; Oskam, C. Molecular characterization of ‘Candidatus Borrelia tachyglossi’ (family Spirochaetaceae) in echidna ticks, Bothriocroton concolor. Int. J. Syst. Evol. Microbiol. 2017, 67, 1075–1080. [Google Scholar] [CrossRef] [Green Version]
- Augee, M.; Gooden, B.; Musser, A. Echidna: Extraordinary Egg.-Laying Mammal; CSIRO Publishing: Collingwood, VIC, Australia, 2006. [Google Scholar]
- Oorebeek, M.; Rismiller, P. Bothriocroton concolor (Acari: Ixodidae) on the Kangaroo Island kangaroo: A new host-parasite relationship. J. Med. Entomol. 2007, 44, 901–902. [Google Scholar] [CrossRef]
- Carley, J.G.; Pope, J.H. A new species of Borrelia (B. queenslandica) from Rattus villosissimus in Queensland. Aust. J. Exp. Biol. Med. Sci. 1962, 40, 255–261. [Google Scholar] [CrossRef]
- Lee, J.K.; Smith, W.C.; McIntosh, C.; Ferrari, F.G.; Moore-Henderson, B.; Varela-Stokes, A. Detection of a Borrelia species in questing Gulf Coast ticks, Amblyomma maculatum. Ticks Tick Borne Dis. 2014, 5, 449–452. [Google Scholar] [CrossRef] [Green Version]
- Teel, P.D.; Ketchum, H.R.; Mock, D.E.; Wright, R.E.; Strey, O.F. The Gulf Coast tick: A review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J. Med. Entomol. 2010, 47, 707–722. [Google Scholar] [CrossRef]
- Nava, S.; Velazco, P.M.; Guglielmone, A.A. First record of Amblyomma longirostre (Koch, 1844) (Acari: Ixodidae) from Peru, with a review of this tick’s host relationships. Syst. Appl. Acarol. 2010, 15, 21–30. [Google Scholar] [CrossRef]
- Colunga-Salas, P.; Sanchez-Montes, S.; Ochoa-Ochoa, L.M.; Grostieta, E.; Becker, I. Molecular detection of the reptile-associated Borrelia group in Amblyomma dissimile, Mexico. Med. Vet. Entomol. 2021, 35, 202–206. [Google Scholar] [CrossRef]
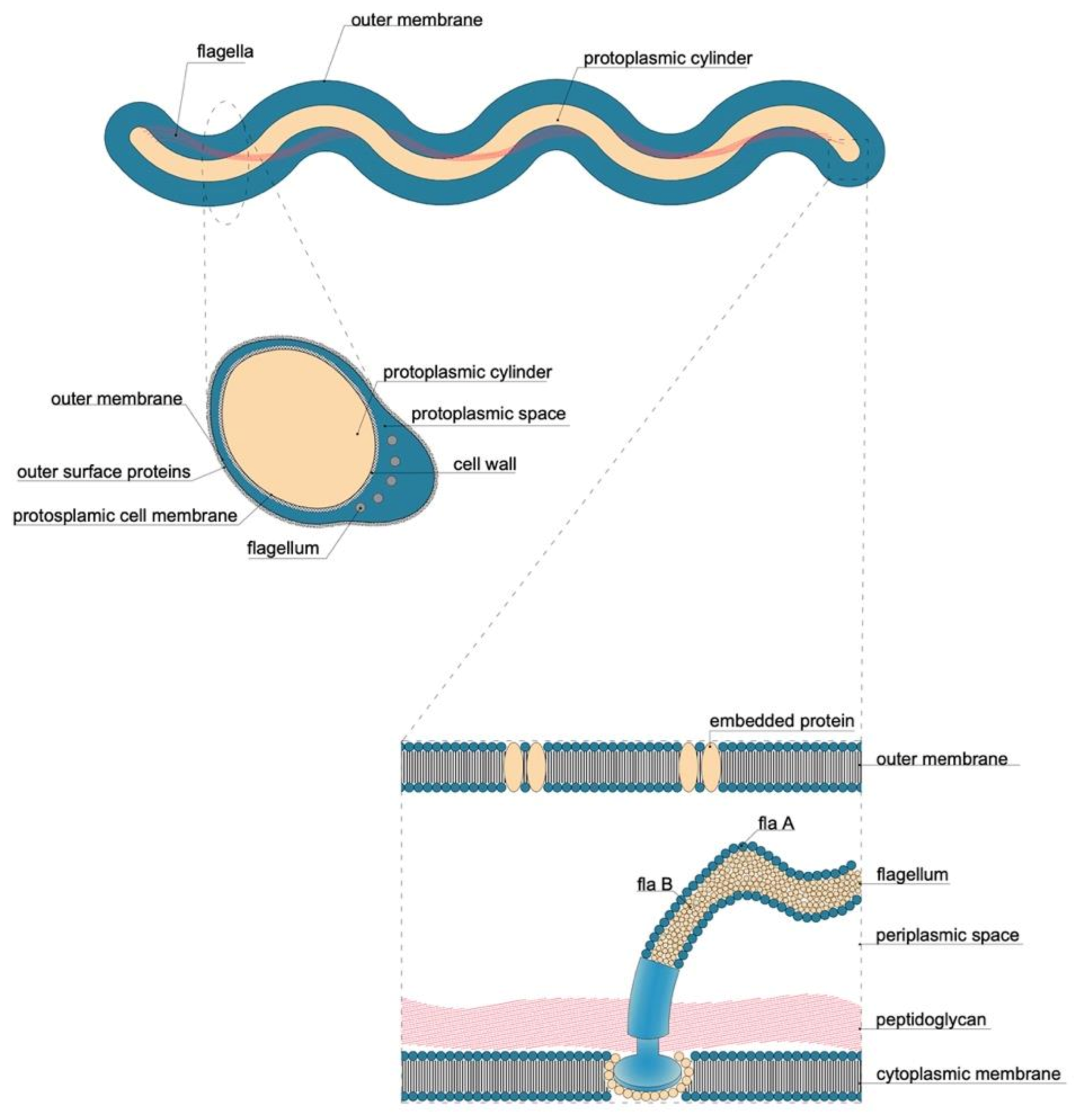
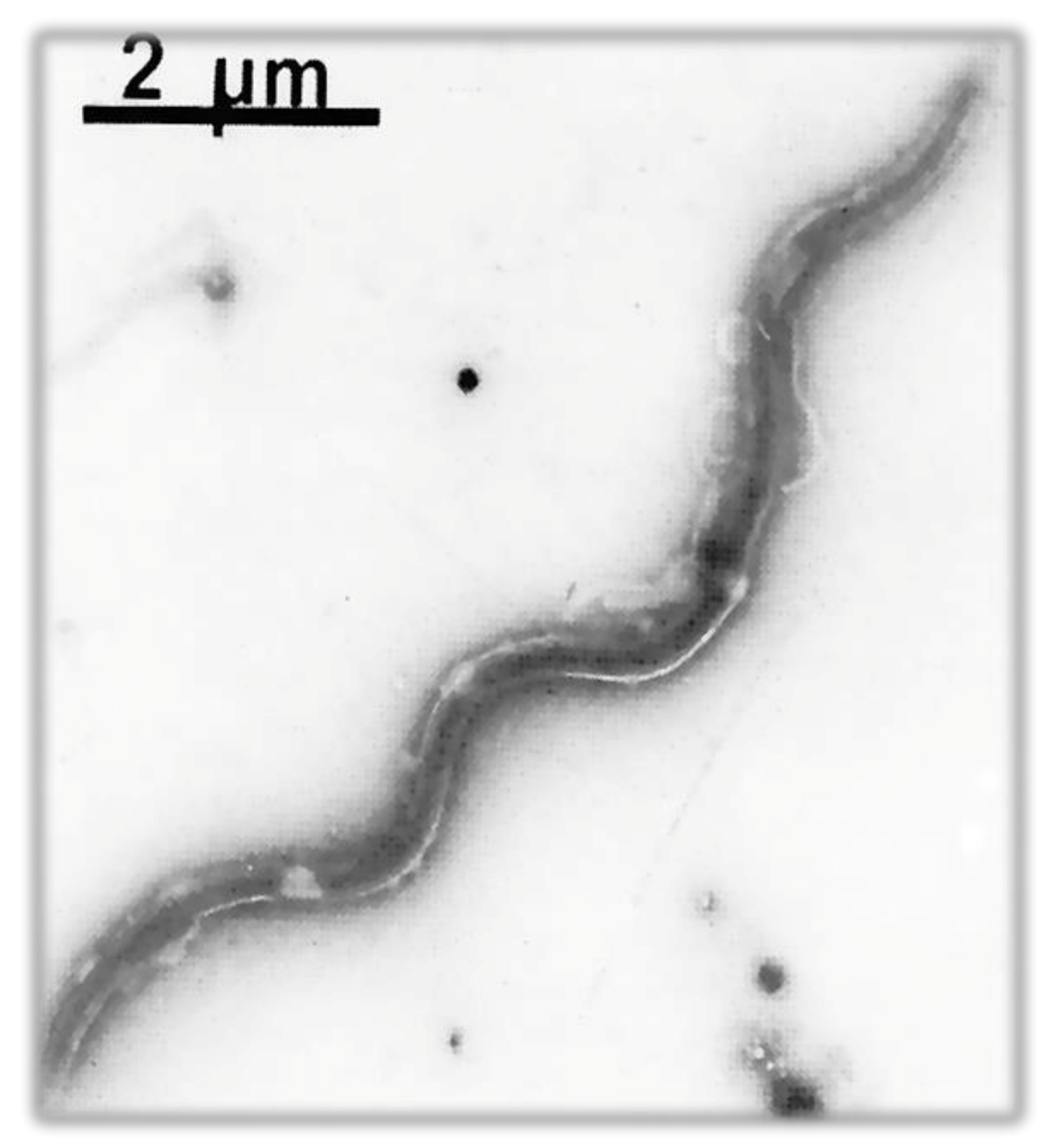
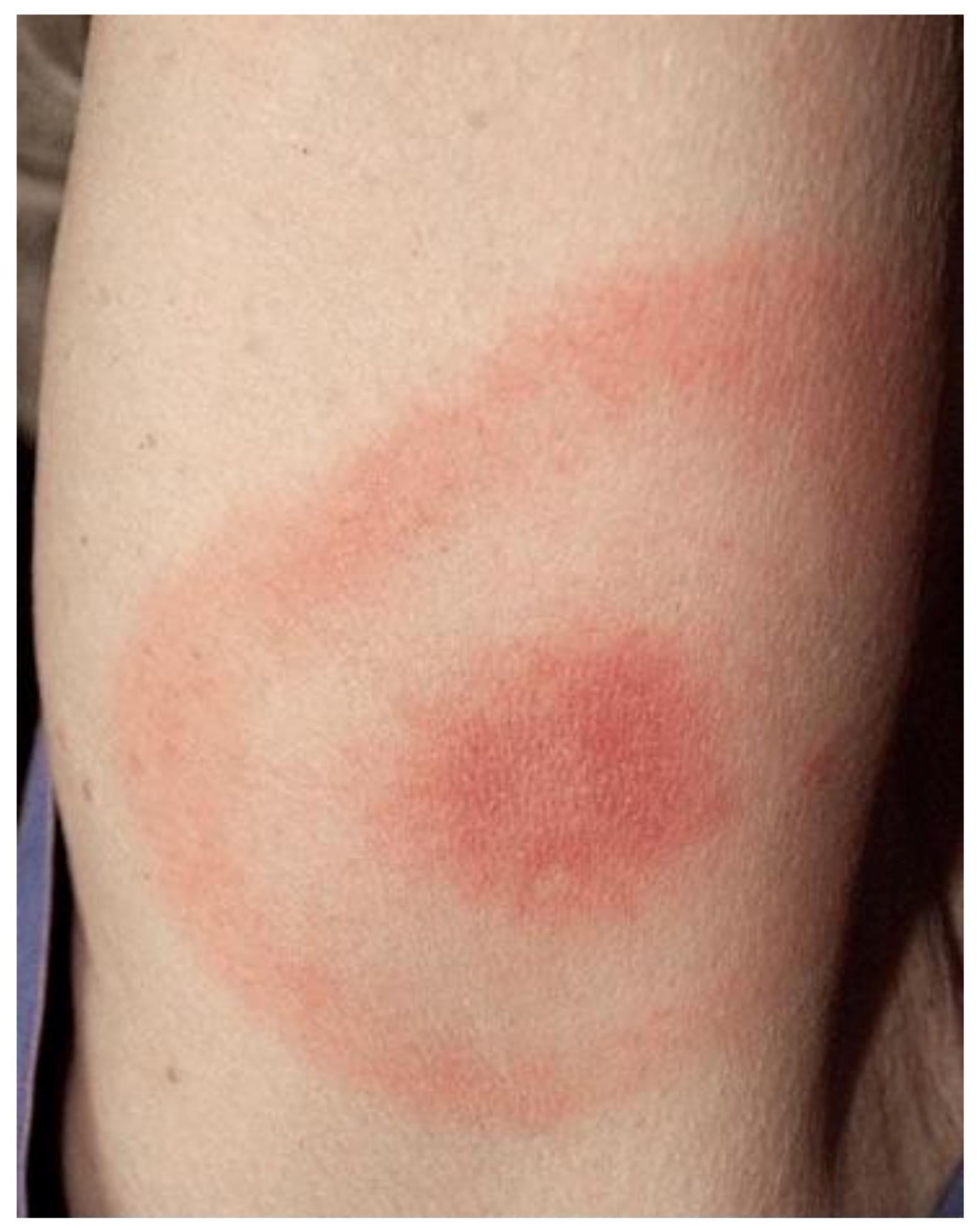
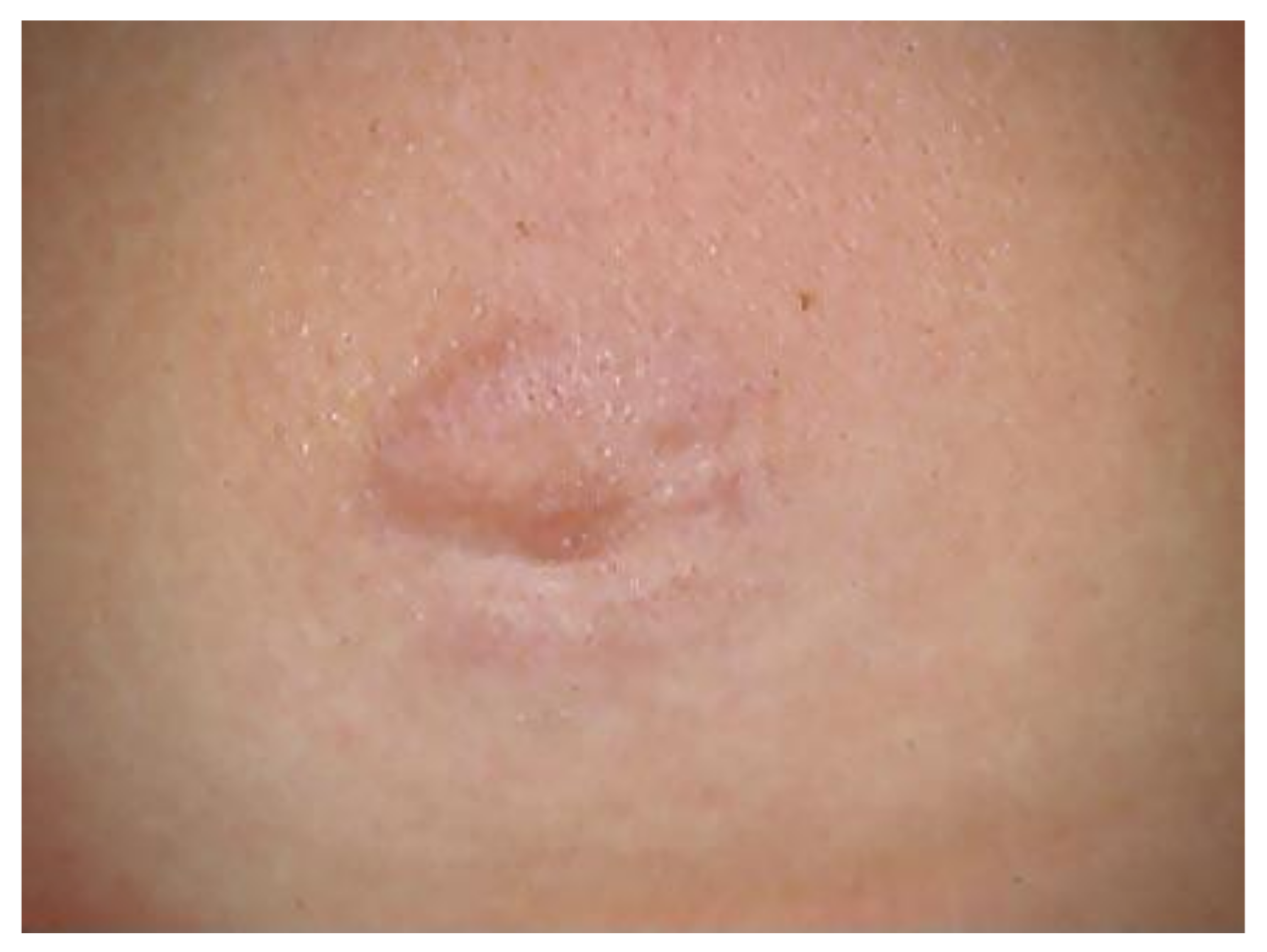
| Order | Family | Genus | Species/Groups |
|---|---|---|---|
| Brachispirales | Brachyspiraceae | Brachispira | Brachispira aalborgi |
| Brachispira pilosicoli | |||
| Brevinematales | Brevinemataceae | Brevinema | Brevinema andersoni |
| Leptospirales | Leptospiraceae | Leptonema | Leptonema illini |
| Leptospira | Leptospira interrogans | ||
| Turneriella | Turneriella parva | ||
| Spirochetales | Spirochaetaceae | Marispirochaeta | Marispirochaeta aestuarii |
| Spirochaeta | Spirochaeta dissipatitropha | ||
| Treponema | Treponema pallidum | ||
| Borreliaceae | Cristispira | Cristispira pectinis | |
| Borrelia | Lyme Group * | ||
| Echidna-Reptile Group * | |||
| Relapsing Fever Group |
| Disease | Treponema Species | Symptoms | Reference |
|---|---|---|---|
| Bejel (or endemic syphilis) | Treponema pallidum sp. endemicum | Mouth ulcers, mutilating nodules in bone | [21] |
| Yaws | Treponema. pallidum sp. pertenue | Ulcers and papilloma, mainly children | [22] |
| Pinta | Treponema carateum | Itchy patches, skin pigmentary changes | [23] |
| Noma (Cancrum oris) | Treponema (Borrelia) vincentii and Fusobacterium necrophorum and others | Orofacial gangrene, mainly children | [24,25,26] |
| Groups | Subgroups | Humans | Clinical Aspects | Host Reservoirs | Vector Ticks | ||
|---|---|---|---|---|---|---|---|
| EM 1 | Fever | Hard Ticks | Soft Ticks/Lice | ||||
| Lyme Group | Organotropism | Yes | Yes | No | Rodents | Ixodes sp. | |
| High Spirochaetemia | Yes | Yes | Yes | Rodents | Ixodes sp. | ||
| Baggio–Yoshinari | Yes | Yes | Yes (78%) | Amblyomma sp. | |||
| Echidna-Reptile Group | Unknown | Echidna, Reptile | Hyalomma sp., Bothriocroton sp., Amblyomma sp. | ||||
| Relapsing Fever (RF) Group | STBRF High Spirochaetemia | Yes | No | Yes | Rodents Birds Insectivorous Ornithodoros moubata | Ornithodoros sp. | |
| HTBRF High Spirochaetemia | Yes | No | Yes | Rodents Birds Cervi (Odocoileus virginiatus) | Ixodes, Amblyomma sp. | ||
| Louse Fever High Spirochaetemia | Yes | No | Yes | Pediculus sp. | |||
| Avian Worldwide RF High Spirochaetemia | Unknown | Birds Bats | Argas sp. Carios kelleyi | ||||
| Geographical Area | Hard Ticks | Reservoirs | Borrelia Species | |
|---|---|---|---|---|
| America | ||||
| Canada | Ixodes scapularis [100], Ixodes cookie [98], Ixodes spinipalpis [101], Ixodes angustus, Ixodes auritulus [102] and Ixodes scapularis | Peromyscus leucopus, Peromyscus maniculatus, Tamias striatus, Tamiasciurus hudsonicus [103], Geothlypis trichas | B. burgdorferi, B. bissettii (Lewis), B. andersoni, B. lanei | |
| USA | Atlantic Coast | Ixodes scapularis [104] | Peromyscus leucopus, Tamiasciurus hudsonicus | B. burgdorferi s.s., B. bissettii, B. carolinensis, B. kurtenbachii, B. mayoni |
| Northwest | Ixodes pacificus, Ixodes spinipalpis [105] | Sciurus criseus [106], Sciurus carolinensis | B. burgdorferi, B. carolinensis, B. lanei | |
| West Coast | Ixodes pacificus, Ixodes spinipalpis, Ixodes angustus [107] | Peromyscus maniculatus, Peromyscus boylii, Neotoma fuscipes, Melospiza melodia | B. burgdorferi, B. carolinensis, B. lanei | |
| Mexico | Ixodes kingi, Ixodes hearley [108], Ixodes scapularis [109] | Microtus mexicanus, Neotoma mexicana, Neotomodon alstonio, Peromyscus leucopus, Peromyscus maniculatus, Geothlypis trichas | B. burgdorferi s.s., B. lanei | |
| Brazil | Ixodes longiscutatus [110], Ixodes paranaensis [111] | Rodents, Streptoprocne biscutata | Borrelia sp. Aplotipo Pampa, Candidatus B. ibitipoquensis | |
| Argentina | Ixodes pararicinus, Ixodes affinis [112] | Turdus Birds | B. burgdorferi s.l. | |
| Uruguay | Ixodes aragaoi [113], Ixodes auritulus [114] | Rodents, Passerine Birds | B. burgdorferi s.l., B. bissettii, B. americana | |
| Chile | Ixodes stilesi [115] | Southern pudu deer | B. chilensis | |
| Europe | ||||
| Western Europe | Ixodes ricinus [116] | Apodemus flavicollis, Turdus merula, Phasianus colchicus | B. afzelii, B. burgdorferi s.s., B. garinii, B. lusitasnisae | |
| Northern and Eastern Europe | Ixodes ricinus, Ixodes persulcatus [117] | Myodes glareolus | B. garinii, B. afzelii, B. bavariensis | |
| Feroe Island | Ixodes uriae (Borrelia garinii?) [118] | Fratercula arctica | B. garinii, B. uriae | |
| Russia Middle East | Ixodes persulcatus [119], Ixodes pavlovskyi [120], Ixodes tanuki, Ixodes turdus | Myodes glareolus, Apodemus sylvaticus, Tamias sibericus | B. afzelii, B. garinii, B. bavariensis, B. tanuki, B. turdae | |
| Asia | ||||
| China | Ixodes persulcatus, Ixodes granulatus [121,122] | Apodemus speciosus, Niviventer confucianus, Turdus merula | B. garinii, B. sinica, B. valaisiana | |
| Japan | Ixodes persulcatus [123], Ixodes ovatus, Ixodes tanuki, Ixodes turdus [124], Ixodes columnae [125] | Apodemus speciosus, Apodemus ainu | B. garinii, B. tanuki, B. turdae, B. japonica | |
| Korea | Ixodes nipponensis [126,127], Ixodes persulcatus [128] | Wild Rodents, Apodemus agrarius, Migratory Birds | B. afzelii, B. garinii, B. valaisiana | |
| India | Ixodes acutitarsus, Ixodes kashmericus, and Ixodes ovatus [129]. | Rodents (Squirrels, Chipmunks) | B. burgdorferi s.l. | |
| Malaysia | Ixodes granulatus [130]. | B. sinica, B. valaisiana, B. yangtzensis | ||
| Africa | ||||
| North Africa (Tunisia Morocco) | Ixodes ricinus [131], Ixodes frontalis [132] | Turdus merula | B. lusitaniae [131], B. burgdorferi s.l. | |
| South Africa | Unknown: Ixodes rubicundus, Ixodes fynbosensis? [133] | LB in horses (?) [134] | B. burgdorferi s.l. | |
| Australia | ||||
| Unknown: Ixodes olocyclus | Currently undocumented [135] | |||
| Characteristic | Detail |
|---|---|
| Microaerophilic | |
| Shape | Spiral |
| Bacterial body length | 7–24 µm |
| Width | 0.20–0.50 µm |
| Propeller pitch | 1.7–3.3 µm |
| Number of flagella | 7–12 |
| Peripheral sheath | Absent |
| Three-layer surface membrane | It envelops a periplasmic space, containing flagella and the protoplasmic cylinder |
| Cytoplasmic tubules | Absent |
| Species of Borrelia Lyme Group | Reference | Geographical Area | Human Infection | Host Reservoirs | Ticks |
|---|---|---|---|---|---|
| Borrelia burgdorferi sensu stricto | [197] | America, Europe, Asia, Africa | Yes | Rodents, Mammals, Birds | I. scapularis I. pacificus I. ricinus |
| Borrelia afzelii | [198,199] | Europe, Asia | Yes | Rodents | I. ricinus I. persulcatus I. pavlovsky |
| Borrelia americana | [200,201] | South Carolina, California, Poland | Unknown | Rodents, Birds | I. pacificus, I. minor |
| Borrelia andersoni | [202] | US (New York) | Unknown | Cottontail rabbits (Sylvilagus sp.) | I. scapularis I. spinipalpis |
| Borrelia bavariensis | [203] | Europe, Asia | Yes | Rodents, Birds | I. ricinus I. persulcatus |
| Borrelia bissettii | [204,205] | US (Colorado), Europe, China | Yes | Rodents | I. spinipalpis |
| Borrelia californiensis | [206,207] | US (California) | Unknown | Kangoroo Rats (Dipodomys californicus) | I. jellisoni, I. spinipalipis I. pacificus |
| Borrelia carolinensis | [208,209,210] | Southeastern region of the US, California Desert | Unknown | Rodents, Birds | I. minor |
| Borrelia chilensis | [211] | Chile | Unknown | Rodents, Deer | I. stilesi |
| Borrelia finlandensis | [212] | Finland | Unknown | I. ricinus | |
| Borrelia garinii | [213] | Europe and Asia | Yes | Rodents, Birds | I. ricinus I. persulcatus |
| Borrelia ibitipoquensis | [111] | Brazil | Unknown | Birds (Streptoprocne biscutata) | I. paranaensis |
| Borrelia japonica | [214,215] | Japan | Unknown | Rodents (Apodemus speciosus, A argenteus) | I. ovatus |
| Borrelia kurtembachii | [216] | North America | Unknown | Rodents | I. scapularis |
| Borrelia lanei | [139,204,217] | US (California, Oregon, Washington) | Unknown | I. spinipalpis | |
| Borrelia lusitaniae | [149,218] | South Europe, Northern Africa | Yes | Rodents, Lizards | I. ricinus |
| Borrelia mayonii | [219,220] | US, Europe | See Unit Lyme Borrelia Group with Spirochaetemia | ||
| Borrelia sinica | [121] | China | Unknown | Rodents (Niviventer confucianus) | I. granulatus I. ovatus |
| Borrelia spielmani | [221,222,223] | Europe | Yes | Rodents | I. ricinus |
| Borrelia tanukii | [124,224,225] | Japan | Unknown | Unknown (possibly dogs and cats) | I. tanuki |
| Borrelia turdi | [124,225,226] | Japan, Portugal | Unknown | Migratory Birds | I. turdus I. frontalis |
| Borrelia valaisiana | [227,228,229] | Europe, Turkey, Japan | Yes | Rodents (Apodemus agrarius), Song Birds | I. ricinus I. granulatus |
| Borrelia yangtzensis | [230,231,232] | China, Taiwan, Korea, Japan, Malaysia, Thailand | Unknown | Rodents, Migratory Birds | I. granulatus I. nipponensis |
| Year | Genus Species Strain | Isolated From | Geographic Area | References |
|---|---|---|---|---|
| 1989 | B. garinii BITS | I. ricinus Tick | Trieste | [146] |
| 1989 | B. burgdorferi s.s. Alcaide | Polyarthritis | Rome | [233] |
| 1992 | B. afzelii Nancy | Erythema migrans | Trieste | [234] |
| 1992 | B. garinii DA | Roseolar lesion | Trieste | [234] |
| 1993 | B. burgdorferi s.s. Myo I | Heart | Trieste | [235] |
| 1993 | B. burgdorferi s.s. Myo II | Heart | Trieste | |
| 1993 | B. afzelii Gualtieri | Erythema migrans | Trieste | [236] |
| 1994 | B. garinii Versilia | I. ricinus | Versilia | [237] |
| 1997 | B. garinii | Congenital annular erythema | Trieste | [238] |
| 1998 | B. burgdorferi s.s. | Ixodes ricinus | South Tyrol | [239] |
| 2008 | B. afzelii | Atrophic lesion (Anetoderma) | Trieste | [240] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trevisan, G.; Cinco, M.; Trevisini, S.; di Meo, N.; Chersi, K.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group. Biology 2021, 10, 1036. https://doi.org/10.3390/biology10101036
Trevisan G, Cinco M, Trevisini S, di Meo N, Chersi K, Ruscio M, Forgione P, Bonin S. Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group. Biology. 2021; 10(10):1036. https://doi.org/10.3390/biology10101036
Chicago/Turabian StyleTrevisan, Giusto, Marina Cinco, Sara Trevisini, Nicola di Meo, Karin Chersi, Maurizio Ruscio, Patrizia Forgione, and Serena Bonin. 2021. "Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group" Biology 10, no. 10: 1036. https://doi.org/10.3390/biology10101036
APA StyleTrevisan, G., Cinco, M., Trevisini, S., di Meo, N., Chersi, K., Ruscio, M., Forgione, P., & Bonin, S. (2021). Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group. Biology, 10(10), 1036. https://doi.org/10.3390/biology10101036







