Skull Morphology, Bite Force, and Diet in Insectivorous Bats from Tropical Dry Forests in Colombia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Derived from Field Work
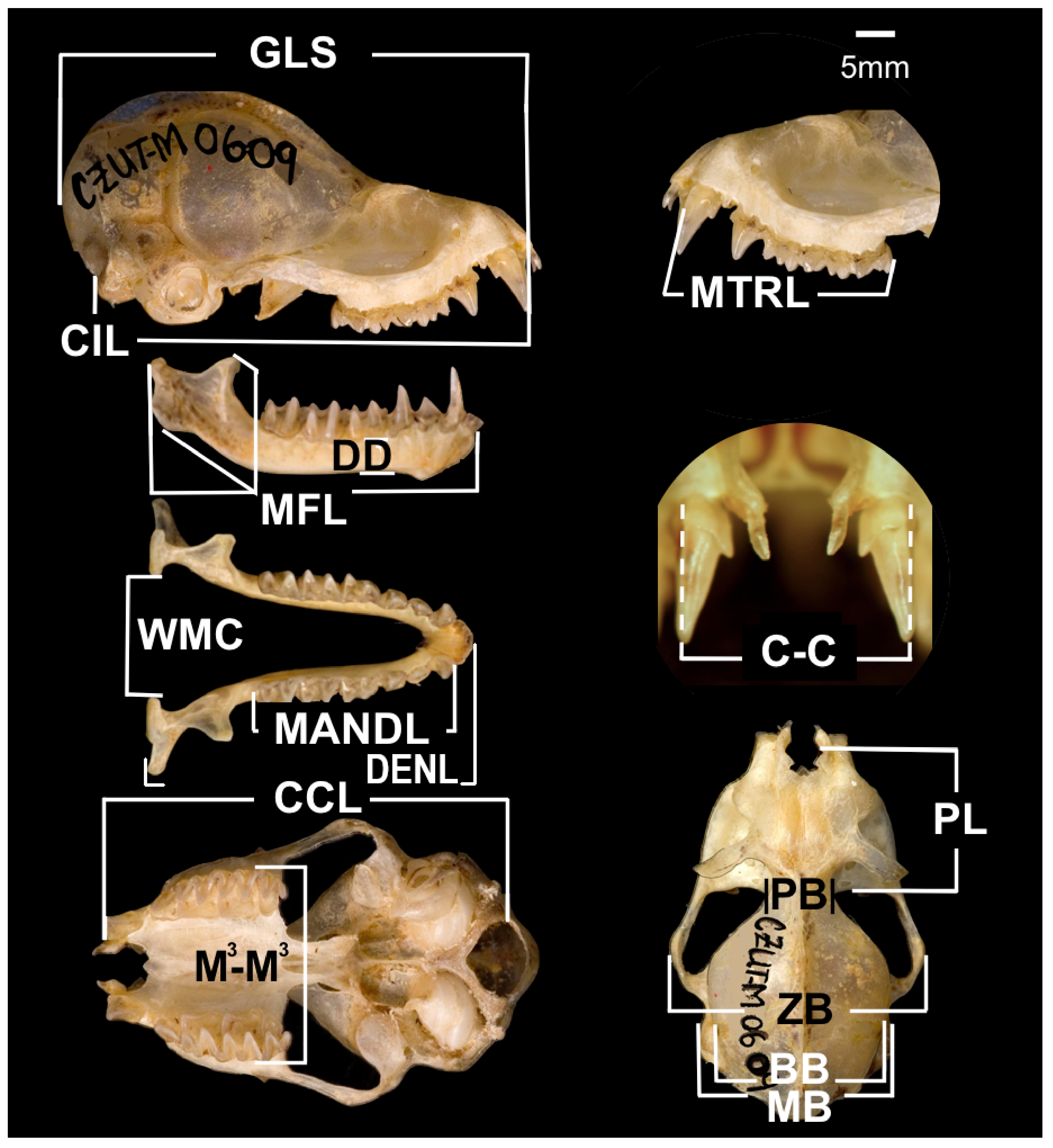
2.2. Skull Morphology
2.3. Statistical Analyses
2.3.1. Variation in Skull Morphology
2.3.2. Bite Force between Bat Species
2.3.3. Bite Force, Skull Morphology, and Diet of Bats
3. Results
3.1. Morphological Variation of the Skull
3.2. Bite Force between Bat Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Trait | Abbreviation | Variable | Description | Unit | Key Reference |
|---|---|---|---|---|---|
| Head | GLS | Greatest skull length | Distance between the most posterior point of the occiput to the most anterior point of the premaxilla, including the incisors | mm | Cisneros et al. [91] |
| Head | CIL | Condyloincisive length | Distance between the most anterior point of the premaxilla and the most posterior point of the occipital condyles | mm | Cisneros et al. [91] |
| Head | CCL | Condylocanine length | Distance taken from the anterior edge of the alveolus of the first maxillary tooth to the most posterior point of the occipital condyles | mm | Cisneros et al. [91] |
| Head | BB | Braincase breadth | Maximum width of the cranium measured from the dorsal side and posterior to the zygomatic arches | mm | Cisneros et al. [91] |
| Head | ZB | Zygomatic breadth | Greatest distance between the external extremities of the zygomatic arches | mm | Cisneros et al. [91] |
| Head | MB | Mastoid breadth | Greatest width of the skull across the mastoids | mm | Murillo-García and De la Vega [92] |
| Head | PL | Rostrum length | Distance between the posterior palatal notch and the anterior border of the incisor alveolus | mm | Murillo-García and De la Vega [92] |
| Head | PB | Interorbital length | Least constriction of the skull measured behind the orbital processes | mm | Murillo-García and De la Vega [92] |
| Head | MTRL | Maxillary tooth row length | Distance from the anterior edge of the alveolus of the first maxillary tooth to the posterior edge of the last molar | mm | Murillo-García and De la Vega [92] |
| Head | M3–M3 | Width at M3 | Distance between the outer margins of the upper third molars | mm | Murillo-García and De la Vega [92] |
| Head | C-C | Palatal width at canines | Distance between the width of the palate between the cingula of the upper canines | mm | Murillo-García and De la Vega [92] |
| Head | DENL | Dentary length | Distance from the midpoint of the mandibular condyle to the most anterior margin of the dentary | mm | Murillo-García and De la Vega [92] |
| Head | DD | Dentary depth under the protoconid of m2 | Perpendicular height from the ventral surface of the mandible to below the m2 protoconid | mm | Ospina-Garcés et al. [33] |
| Head | MFL | Masseteric fossa length | Distance across the masseteric fossa | mm | Ospina-Garcés et al. [33] |
| Head | MANDL | Mandibular tooth row length | Distance from the most anterior surface of the lower canine to the most posterior surface of m3 | mm | Murillo-García and De la Vega [92] |
| Head | WMC | Width at mandibular condyles | Widest distance between inner margins of mandibular condyles | mm | Murillo-García and De la Vega [92] |
| Head | BF | Bite force | Maximum bite force produced by molars | N/g | Shi et al. [28] |
| Body | BL | Body length | Distance between the end of the snout to tip of the tail | mm | Díaz et al. [93] |
| Body | HBL | Head and body length | Distance between the end of the snout and the insertion of the tail to the body | mm | Díaz et al. [93] |
| Body | TL | Tail length | Distance between the insertion of the tail to the body and the last caudal vertebra | mm | Díaz et al. [93] |
| Body | Ll | Hindfoot length | Distance from heel to end of longest toe including nail | mm | Díaz et al. [93] |
| Body | cL | Calcar length | Distance of the cartilage extending from the tarsus, where the uropatagium originates | mm | Díaz et al. [93] |
| Body | Lur | Length of uropathy | Membrane distance between the legs | mm | Díaz et al. [93] |
| Body | LE | Ear length | Distance between the basal notch and the distal end of the pinna | mm | Díaz et al. [93] |
| Body | tL | tragus length | Distance of the small skin prominence located in front of the external auditory canal | mm | Díaz et al. [93] |
| Body | FA | Length of forearm | Distance between elbow and wrist with folded wing | mm | Velazco and Gardner [94] |
| Body | 3ML | Third metacarpal length | Distance from the third metacarpal between the wrist bones to the phalange | mm | Díaz et al. [93] |
| Body | LTb | Length of the tibia | Distance between the end of the knee and the ankle | mm | Díaz et al. [93] |
| Body | Mas | Mass | Weight of the body | g | Cisneros et al. [91] |
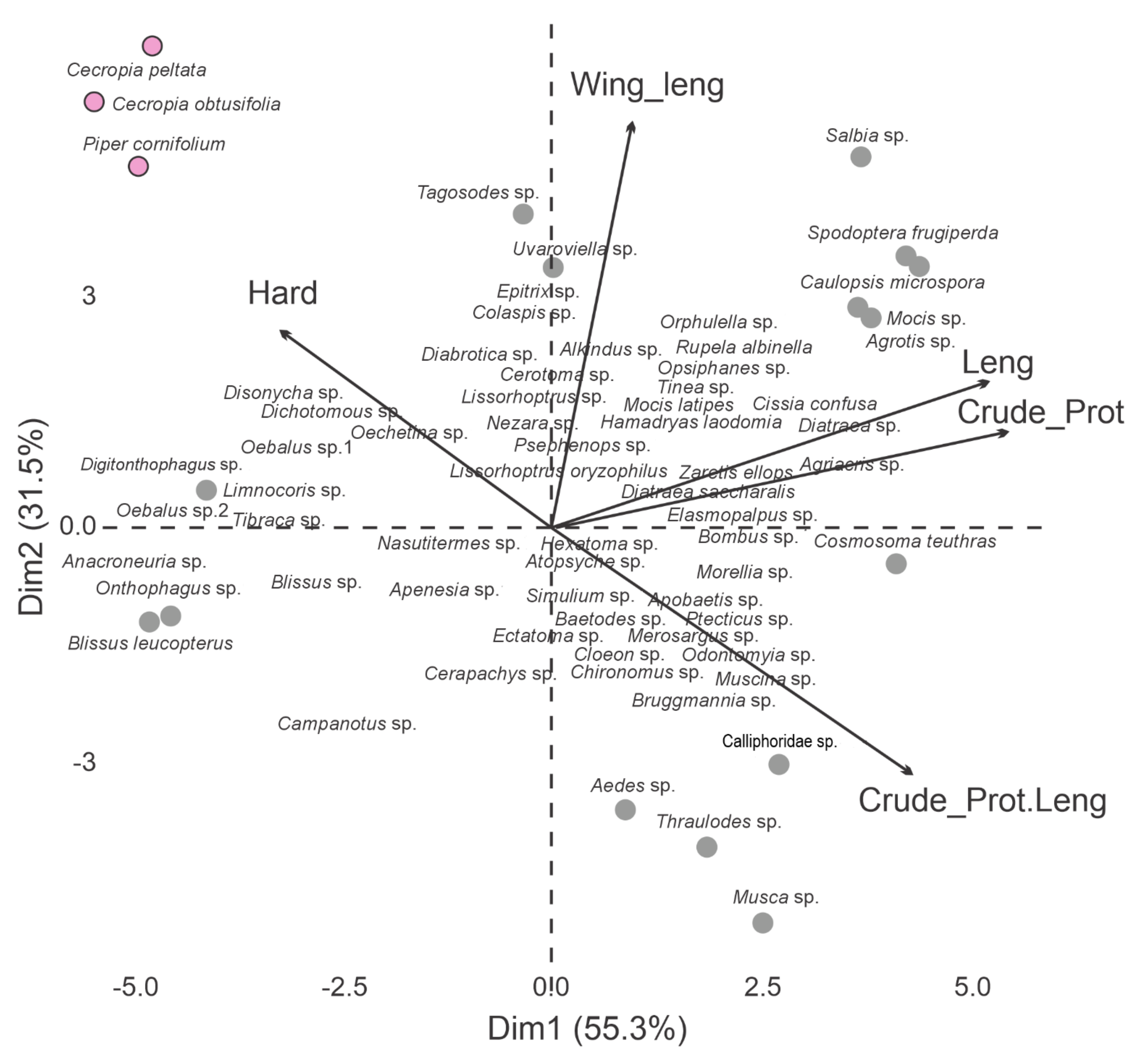
| Order | Genus | n | Hardness (N/g) | Total Length (mm) | Width across Wings or Fruit (mm) | Crude Protein (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coleoptera | Cerotoma sp. | 22 | 0.73 | ±0.49 | 0.95 | ±0.77 | 1.78 | ±1.82 | 19.56 | ±24.41 |
| Digitonthophagus sp. | 15 | 0.72 | ±0.48 | 1.17 | ±1.05 | 1.70 | ±1.72 | 20.50 | ±25.60 | |
| Onthophagus sp. | 20 | 0.67 | ±0.41 | 1.11 | ±0.97 | 2.22 | ±2.38 | 19.33 | ±24.12 | |
| Dichotomous sp. | 12 | 0.60 | ±0.32 | 1.60 | ±1.59 | 1.23 | ±1.128 | 20.67 | ±25.82 | |
| Limnocoris sp. | 20 | 0.57 | ±0.28 | 1.71 | ±1.73 | 1.67 | ±1.68 | 19.56 | ±24.41 | |
| Lissorhoptrus sp. | 15 | 0.56 | ±0.27 | 1.34 | ±1.26 | 1.50 | ±1.47 | 19.75 | ±24.65 | |
| Psephenops sp. | 15 | 0.55 | ±0.26 | 1.54 | ±1.52 | 1.24 | ±1.14 | 19.60 | ±24.46 | |
| Diabrotica sp. | 15 | 0.55 | ±0.26 | 1.20 | ±1.09 | 1.36 | ±1.29 | 19.54 | ±24.38 | |
| Oechetina sp. | 16 | 0.55 | ±0.26 | 2.20 | ±2.36 | 1.80 | ±1.85 | 19.64 | ±24.51 | |
| Colaspis sp. | 15 | 0.50 | ±0.20 | 1.50 | ±1.47 | 1.56 | ±1.54 | 19.50 | ±24.33 | |
| Lissorhoptrus oryzophilus | 22 | 0.47 | ±0.16 | 1.60 | ±1.59 | 1.33 | ±1.25 | 18.53 | ±23.10 | |
| Epitrix sp. | 15 | 0.45 | ±0.13 | 1.30 | ±1.21 | 1.21 | ±1.10 | 19.75 | ±24.65 | |
| Disonycha sp. | 16 | 0.45 | ±0.13 | 1.10 | ±0.96 | 1.27 | ±1.17 | 19.64 | ±24.51 | |
| Diptera | Aedes sp. | 30 | 0.05 | ±0.37 | 0.67 | ±0.41 | 0.75 | ±0.51 | 9.46 | ±11.58 |
| Bruggmannia sp. | 25 | 0.04 | ±0.38 | 0.56 | ±0.27 | 0.98 | ±0.81 | 11.00 | ±13.54 | |
| Calliphoridae | 25 | 0.03 | ±0.39 | 0.91 | ±0.72 | 1.12 | ±0.98 | 10.20 | ±12.52 | |
| Chironomus sp. | 30 | 0.05 | ±0.37 | 0.65 | ±0.39 | 1.15 | ±1.02 | 10.56 | ±12.98 | |
| Hexatoma sp. | 15 | 0.07 | ±0.34 | 0.68 | ±0.42 | 1.3 | ±1.21 | 9.23 | ±11.29 | |
| Morellia sp. | 20 | 0.05 | ±0.37 | 0.91 | ±0.72 | 1.45 | ±1.40 | 9.37 | ±11.46 | |
| Simulium sp. | 15 | 0.05 | ±0.37 | 0.56 | ±0.27 | 1.47 | ±1.43 | 7.60 | ±9.22 | |
| Merosargus sp. | 16 | 0.03 | ±0.39 | 1.12 | ±0.98 | 1.60 | ±1.59 | 6.20 | ±7.44 | |
| Odontomyia sp. | 20 | 0.04 | ±0.38 | 1.50 | ±1.47 | 1.72 | ±1.75 | 5.23 | ±6.20 | |
| Ptecticus sp. | 20 | 0.05 | ±0.37 | 1.60 | ±1.59 | 1.85 | ±1.91 | 6.30 | ±7.56 | |
| Musca sp. | 20 | 0.06 | ±0.35 | 1.50 | ±1.47 | 2.40 | ±2.61 | 9.45 | ±11.57 | |
| Muscina sp. | 20 | 0.70 | ±0.45 | 1.50 | ±1.47 | 2.50 | ±2.74 | 9.40 | ±11.50 | |
| Ephemeroptera | Thraulodes sp. | 15 | 0.46 | ±0.15 | 1.23 | ±1.12 | 1.10 | ±0.96 | 16.00 | ±19.89 |
| Apobaetis sp. | 16 | 0.40 | ±0.07 | 1.40 | ±1.34 | 1.50 | ±1.47 | 16.50 | ±20.52 | |
| Baetodes sp. | 15 | 0.37 | ±0.03 | 1.55 | ±1.53 | 1.55 | ±1.53 | 16.20 | ±20.14 | |
| Cloeon sp. | 16 | 0.40 | ±0.07 | 1.70 | ±1.72 | 1.60 | ±1.59 | 15.40 | ±19.12 | |
| Hemiptera | Oebalus sp.1 | 20 | 0.06 | ±0.35 | 0.95 | ±0.77 | 0.53 | ±0.23 | 15.30 | ±19.00 |
| Oebalus sp.2 | 20 | 0.06 | ±0.35 | 1.12 | ±0.98 | 0.97 | ±0.79 | 15.20 | ±18.87 | |
| Blissus leucopterus | 20 | 0.05 | ±0.37 | 1.16 | ±1.03 | 1.11 | ±0.97 | 15.80 | ±19.63 | |
| Tibraca sp. | 15 | 0.05 | ±0.37 | 0.55 | ±0.26 | 0.42 | ±0.09 | 15.40 | ±19.12 | |
| Nezara sp. | 15 | 0.04 | ±0.38 | 0.80 | ±0.58 | 0.75 | ±0.51 | 15.70 | ±19.51 | |
| Alkindus sp. | 15 | 0.03 | ±0.39 | 0.51 | ±0.21 | 0.46 | ±0.15 | 1.20 | ±1.09 | |
| Blissus sp. | 16 | 0.03 | ±0.39 | 0.57 | ±0.28 | 0.43 | ±0.11 | 16.23 | ±20.18 | |
| Tagosodes sp. | 15 | 0.01 | ±0.42 | 0.80 | ±0.58 | 0.76 | ±0.53 | 16.50 | ±20.52 | |
| Hymenoptera | Campanotus sp. | 16 | 0.05 | ±0.37 | 0.60 | ±0.32 | 0.43 | ±0.43 | 21.90 | ±27.38 |
| Cerapachys sp. | 20 | 0.05 | ±0.37 | 0.55 | ±0.26 | 0.50 | ±0.20 | 20.80 | ±25.99 | |
| Apenesia sp. | 20 | 0.05 | ±0.37 | 0.50 | ±0.20 | 0.60 | ±0.32 | 21.60 | ±27.00 | |
| Bombus sp. | 22 | 0.06 | ±0.35 | 1.40 | ±1.34 | 0.80 | ±0.58 | 21.70 | ±27.13 | |
| Ectatoma sp. | 20 | 0.04 | ±0.38 | 1.20 | ±1.09 | 0.90 | ±0.70 | 21.20 | ±26.49 | |
| Isoptera | Nasutitermes sp. | 16 | 0.03 | ±0.39 | 2.05 | ±2.16 | 0.45 | ±0.43 | 16.40 | ±20.40 |
| Lepidoptera | Mocis sp. | 25 | 0.01 | ±0.42 | 2.25 | ±2.42 | 1.35 | ±1.28 | 26.90 | ±33.73 |
| Agrotis sp. | 25 | 0.01 | ±0.42 | 1.87 | ±1.94 | 1.25 | ±1.15 | 26.61 | ±33.37 | |
| Spodoptera frugiperda | 25 | 0.01 | ±0.42 | 2.50 | ±2.74 | 1.30 | ±1.21 | 26.60 | ±33.35 | |
| Salbia sp. | 25 | 0.01 | ±0.42 | 2.40 | ±2.61 | 1.30 | ±1.21 | 27.30 | ±34.24 | |
| Mocis latipes | 30 | 0.01 | ±0.42 | 2.04 | ±2.15 | 1.50 | ±1.47 | 26.40 | ±33.10 | |
| Diatraea saccharalis | 30 | 0.01 | ±0.42 | 2.16 | ±2.30 | 1.40 | ±1.34 | 26.20 | ±32.85 | |
| Cissia confusa | 26 | 0.01 | ±0.42 | 2.50 | ±2.74 | 1.30 | ±1.21 | 25.40 | ±31.83 | |
| Elasmopalpus sp. | 25 | 0.02 | ±0.40 | 2.87 | ±3.21 | 1.25 | ±1.15 | 26.80 | ±33.61 | |
| Hamadryas laodomia | 25 | 0.01 | ±0.42 | 1.52 | ±1.49 | 1.24 | ±1.14 | 26.50 | ±33.23 | |
| Diatraea sp. | 25 | 0.01 | ±0.42 | 2.70 | ±2.99 | 1.23 | ±1.12 | 26.28 | ±32.95 | |
| Tinea sp. | 25 | 0.01 | ±0.42 | 2.70 | ±2.99 | 1.23 | ±1.12 | 26.70 | ±33.48 | |
| Zaretis ellops | 25 | 0.01 | ±0.42 | 2.26 | ±2.43 | 1.23 | ±1.12 | 27.30 | ±34.24 | |
| Cosmosoma teuthras | 25 | 0.02 | ±0.40 | 1.60 | ±1.59 | 1.20 | ±1.09 | 26.50 | ±33.23 | |
| Rupela albinella | 26 | 0.02 | ±0.40 | 1.94 | ±2.03 | 1.12 | ±0.98 | 25.03 | ±31.36 | |
| Opsiphanes sp. | 25 | 0.02 | ±0.40 | 2.26 | ±2.43 | 1.10 | ±0.96 | 26.40 | ±33.10 | |
| Orthoptera | Orphulella sp. | 15 | 0.01 | ±0.42 | 2.25 | ±2.44 | 1.22 | ±1.11 | 25.60 | ±32.08 |
| Caulopsis microspora | 16 | 0.01 | ±0.42 | 2.40 | ±2.61 | 1.50 | ±1.47 | 25.70 | ±32.21 | |
| Uvaroviella sp. | 15 | 0.02 | ±0.40 | 2.70 | ±2.99 | 2.10 | ±2.23 | 25.90 | ±32.46 | |
| Agriacris sp. | 15 | 0.02 | ±0.40 | 2.91 | ±3.26 | 1.80 | ±1.85 | 21.40 | ±26.75 | |
| Plecoptera | Anacroneuria sp. | 20 | 0.6 | ±0.32 | 3.30 | ±3.75 | 1.50 | ±1.47 | 15.20 | ±18.87 |
| Trichoptera | Atopsyche sp. | 20 | 0.02 | ±0.40 | 1.20 | ±1.09 | 0.50 | ±0.20 | 16.10 | ±20.01 |
| Rosales | Cecropia peltata | 157 | 4.20 | ±4.90 | 49.99 | ±63.07 | 14.20 | ±17.60 | ||
| Cecropia obtusifolia | 112 | 3.70 | ±4.26 | 5.60 | ±6.67 | ---- | 14.66 | ±18.18 | ||
| Piperales | Piper cornifolium | 87 | 3.50 | ±4.01 | 50.20 | ±63.34 | 9.10 | ±11.12 | ||
| Variables | F-Value | p-Value |
|---|---|---|
| GLS | 4.79 | <0.01 |
| CIL | 12.45 | <0.02 |
| CCL | 11.60 | <0.02 |
| BB | 7.00 | <0.04 |
| ZB | 9.27 | <0.03 |
| MB | 2.34 | >0.82 |
| PL | 2.51 | >0.13 |
| PB | 0.32 | >0.34 |
| MTRL | 1.21 | >0.32 |
| M3-M3 | 1.13 | >0.95 |
| C-C | 1.28 | >0.26 |
| DENL | 10.06 | <0.03 |
| DD | 2.35 | >0.77 |
| MFL | 2.30 | >0.65 |
| MANDL | 2.40 | >0.66 |
| WMC | 0.51 | >0.33 |
| BF | 7.39 | <0.01 |
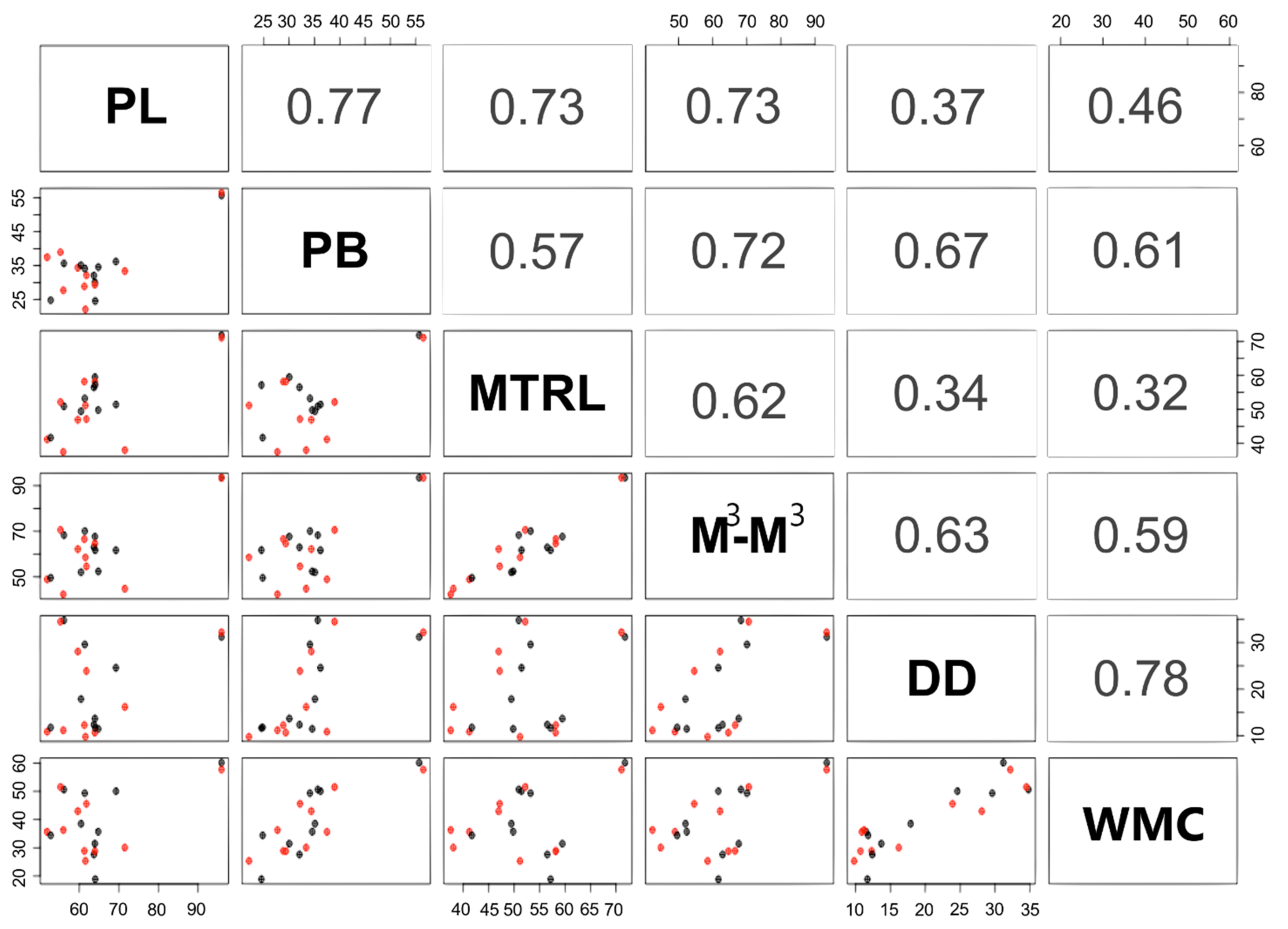
| GLS | CIL | CCL | BB | ZB | MB | PL | PB | MTRL | M3-M3 | C-C | DENL | DD | MFL | MANDL | WMC | BS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GLS | 1 | ||||||||||||||||
| CIL | 0.937 | 1 | |||||||||||||||
| CCL | 0.972 | 0.959 | 1 | ||||||||||||||
| BB | 0.900 | 0.884 | 0.857 | 1 | |||||||||||||
| ZB | 0.959 | 0.932 | 0.945 | 0.924 | 1 | ||||||||||||
| MB | 0.906 | 0.862 | 0.874 | 0.973 | 0.911 | 1 | |||||||||||
| PL | 0.783 | 0.769 | 0.731 | 0.718 | 0.841 | 0.639 | 1 | ||||||||||
| PB | 0.791 | 0.713 | 0.726 | 0.764 | 0.821 | 0.798 | 0.779 | 1 | |||||||||
| MTRL | 0.894 | 0.880 | 0.876 | 0.814 | 0.863 | 0.756 | 0.761 | 0.601 | 1 | ||||||||
| M3-M3 | 0.967 | 0.896 | 0.927 | 0.936 | 0.941 | 0.928 | 0.739 | 0.734 | 0.919 | 1 | |||||||
| C-C | 0.867 | 0.782 | 0.806 | 0.822 | 0.881 | 0.838 | 0.801 | 0.913 | 0.692 | 0.843 | 1 | ||||||
| DENL | 0.954 | 0.901 | 0.945 | 0.854 | 0.901 | 0.865 | 0.712 | 0.717 | 0.914 | 0.941 | 0.759 | 1 | |||||
| DD | 0.604 | 0.502 | 0.522 | 0.728 | 0.646 | 0.795 | 0.362 | 0.676 | 0.338 | 0.632 | 0.658 | 0.501 | 1 | ||||
| MFL | 0.544 | 0.442 | 0.462 | 0.668 | 0.586 | 0.735 | 0.302 | 0.616 | 0.278 | 0.572 | 0.598 | 0.441 | 0.466 | 1 | |||
| MANDL | 0.811 | 0.724 | 0.761 | 0.737 | 0.749 | 0.771 | 0.604 | 0.788 | 0.720 | 0.804 | 0.813 | 0.841 | 0.495 | 0.457 | 1 | ||
| WMC | 0.570 | 0.427 | 0.456 | 0.674 | 0.613 | 0.744 | 0.459 | 0.812 | 0.322 | 0.595 | 0.758 | 0.496 | 0.895 | 0.357 | 0.619 | 1 | |
| BS | 0.581 | 0.547 | 0.546 | 0.670 | 0.607 | 0.721 | 0.451 | 0.648 | 0.308 | 0.555 | 0.729 | 0.497 | 0.621 | 0.307 | 0.482 | 0.692 | 1 |
| Group | Dim.1 | Dim.2 |
|---|---|---|
| GLS | 0.975 | −0.126 |
| CIL | 0.920 | −0.241 |
| CCL | 0.932 | −0.211 |
| BB | 0.947 | 0.036 |
| ZB | 0.973 | −0.072 |
| MB | 0.947 | 0.147 |
| PL | 0.815 | −0.170 |
| PB | 0.859 | 0.299 |
| MTRL | 0.871 | −0.458 |
| M3-M3 | 0.971 | −0.124 |
| C-C | 0.920 | 0.232 |
| DENL | 0.927 | −0.234 |
| DD | 0.683 | 0.569 |
| MFL | 0.683 | 0.601 |
| MANDL | 0.830 | 0.048 |
| WMC | 0.692 | 0.669 |
| BF | 0.654 | 0.504 |
| Variables | CVA 1 | CVA 2 |
|---|---|---|
| M. temminckii (F) | 10.19 | 3.08 |
| M. temminckii (M) | 8.93 | 3.43 |
| M. coibensis (F) | 33.92 | 1.40 |
| M. coibensis (M) | 33.27 | 2.25 |
| M. molossus (F) | 22.17 | 0.55 |
| M. molossus (M) | 19.27 | 0.61 |
| M. nigricans (F) | −19.70 | 2.71 |
| M. nigricans (M) | −20.86 | 2.64 |
| M. riparius (F) | −5.94 | 3.69 |
| M. riparius (M) | −9.08 | 3.33 |
| R. io (F) | −18.74 | 3.06 |
| R. io (M) | −19.52 | 3.15 |
| N. albiventris (F) | 29.18 | −7.04 |
| N. albiventris (M) | 27.25 | −7.40 |
| P. macrotis (F) | −14.32 | −1.11 |
| P. macrotis (M) | −16.62 | −1.65 |
| S. bilineata (F) | −16.55 | −4.55 |
| S. bilineata (M) | −20.43 | −4.96 |
| S. leptura (F) | −18.63 | −2.01 |
| S. leptura (M) | −24.19 | −2.49 |
Appendix B
References
- Senawi, J.; Schmieder, D.; Siemers, B.; Kingston, T. Beyond size-morphological predictors of bite force in a diverse insectivorous bat assemblage from Malaysia. Funct. Ecol. 2015, 29, 1411–1420. [Google Scholar] [CrossRef]
- Herrel, A.; Podos, J.; Huber, S.K.; Hendry, A.P. Bite performance and morphology in a population of Darwin’s finches: Implications for the evolution of beak shape. Funct. Ecol. 2005, 19, 43–48. [Google Scholar] [CrossRef]
- Gannon, W.L.; Rácz, G.R. Character displacement and ecomorphological analysis of two long-eared Myotis (M. auriculus and M. evotis). J. Mammal. 2006, 87, 171–179. [Google Scholar] [CrossRef]
- Anderson, R.A.; Macbrayer, L.D.; Herrel, A. Bite force in vertebrates: Opportunities and caveats for use of a nonpareil whole-animal performance measure. Biol. J. Linn. Soc. Lond. 2008, 93, 709–720. [Google Scholar] [CrossRef]
- Freeman, P.W. Macroevolution in Microchiroptera: Recoupling morphology and ecology with phylogeny. Evol. Ecol. Res. 2000, 2, 317–335. [Google Scholar]
- Van Cakenberghe, V.; Herrel, A.; Aguirre, L.F. Evolutionary relationships between cranial shape and diet in bats (Mammalia: Chiroptera). In Topics in Functional and Ecological Vertebrate Morphology; Aerts, P., D’aout, K., Herrel, A., van Damme, R., Eds.; Shaker Publishing: Maastricht, The Netherlands, 2002; pp. 205–236. [Google Scholar]
- Wake, D.B.; Roth, G. Evolution and adaptation. (Book reviews: Complex organismal functions. Integration and evolution in vertebrates). Science 1990, 247, 1350–1351. [Google Scholar]
- Pedersen, S.C. Skull growth and the acoustical axis of the head in bats. In Ontogeny, Functional Ecology and Evolution of Bats; Adams, R.A., Pedersen, S.C., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 174–213. [Google Scholar]
- Marroig, G.; Cheverud, J.M. A comparison of phenotypic variation and covariation patterns and the role of phylogeny, ecology, and ontogeny during cranial evolution of new world monkeys. Evolution 2010, 55, 2576–2600. [Google Scholar] [CrossRef]
- Santana, S.E.; Dumont, E.R.; Davis, J.L. Mechanics of bite force production and its relationship to diet in bats. Funct. Ecol. 2010, 24, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Santana, S.E.; Grosse, I.R.; Dumont, E.R. Dietary hardness, loading behavior, and the evolution of skull form in bats. Evolution 2012, 66, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Thorpe, R.S. Size and shape variation in a lesser Antillean anole, Anolis oculatus (Sauria: Iguanidae) in relation to habitat. Biol. J. Linn. Soc. Lond. 1997, 60, 53–72. [Google Scholar]
- Maestri, R.; Patterson, B.D.; Fornel, R.; Monteiro, L.R.; De Freitas, T.R.O. Diet, bite force and skull morphology in the generalist rodent morphotype. J. Evol. Biol. Res. 2016, 29, 2191–2204. [Google Scholar] [CrossRef]
- Sosa, M.; De Asençâo, A.; Soriano, P.J. Dieta y patrón reproductivo de Rhogeessa minutilla (Chiroptera: Vespertilionidae) en una zona árida de los Andes de Venezuela. Rev. Biol Trop. 1996, 44, 867–875. [Google Scholar]
- Aguirre, L.F.; Herrel, A.; Van Damme, R.; Matthysen, E. The implications of food hardness for diet in bats. Funct. Ecol. 2003, 17, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Cooper, N.; Jetz, W.; Freckleton, R.P. Phylogenetic comparative approaches for studying niche conservatism. J. Evol. Biol. Res. 2010, 23, 2529–2539. [Google Scholar] [CrossRef]
- Gray, J.A.; Sherratt, E.; Hutchinson, M.N.; Jones, M.E.H. Changes in ontogenetic patterns facilitate diversification in skull shape of Australian agamid lizards. BMC Evol. Biol. 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, B.K.; Engstrom, M.D. Species diversity of bats (Mammalia: Chiroptera) in Iwokrama forest, Guyana and the Guianan subregion: Implications for conservation. Biodivers. Conserv. 2001, 10, 613–657. [Google Scholar] [CrossRef]
- Ramírez-Chaves, H.; Suárez-Castro, F.; González-Maya, J.F. Cambios recientes a la lista de mamíferos de Colombia. Mammal. Notes 2016, 3, 1–20. [Google Scholar] [CrossRef]
- García-Herrera, L.V.; Ramírez-Fráncel, L.A.; Reinoso-Flórez, G. Mamíferos del departamento del Tolima: distribución y estado de conservación. Rev. U.D.C.A Act. Div. Cient. 2019, 22, e1100. [Google Scholar]
- Hernández-Leal, O.F.; Sánchez, F.; Lizcano, D.J. Murciélagos insectívoros aéreos en un paisaje ganadero del piedemonte llanero colombiano. Biota Colombiana 2021, 22, 164–183. [Google Scholar] [CrossRef]
- Aguirre, L.F.; Herrel, A.; Van Damme, R.; Matthysen, E. Ecomorphological analysis of trophic niche partitioning in a tropical savannah bat community. Proc. R. Soc. Lond. 2002, 269, 1271–1278. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, M.R.; Peracchi, A.L.; Monteiro, L.R. Morphological correlates of bite force and diet in the skull and mandible of Phyllostomid bats. Funct. Ecol. 2009, 23, 715–723. [Google Scholar] [CrossRef]
- Norberg, U.M.; Rayner, J.M.V. Ecological morphology and flight in bats (Mammalia; Chiroptera): Wing adaptations, flight performance, foraging strategy and echolocation. Philos. Trans. R. Soc. A 1987, 316, 335–427. [Google Scholar]
- Santana, S.E.; Cheung, E. Go big or go fish: Morphological specializations in carnivorous bats. Proc. R. Soc. Lond. 2016, 283, 20160615. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, P.; Wroe, S. Bite forces and evolutionary adaptations to feeding ecology in carnivores. Ecology 2007, 88, 347–358. [Google Scholar] [CrossRef]
- Santana, S.E.; Dumont, E.R. Connecting behaviour and performance: The evolution of biting behaviour and bite performance in bats. J. Evol. Biol. 2009, 22, 2131–2145. [Google Scholar] [CrossRef] [Green Version]
- Shi, B.; Wang, Y.; Gong, L.; Chang, Y.; Liu, T.; Zhao, X.; Lin, A.; Feng, J.; Jiang, T. Correlation of skull morphology and bite force in a bird-eating bat (Ia io; Vespertilionidae). Front. Zool. 2020, 17, 8. [Google Scholar] [CrossRef] [Green Version]
- Santana, S.E.; Geipel, I.; Dumont, E.R.; Kalka, M.B.; Kalko, E.K.V. All you can eat: High performance capacity and plasticity in the common big-eared bat, Micronycteris microtis (Chiroptera: Phyllostomidae). PLoS ONE 2011, 6, e28584. [Google Scholar] [CrossRef] [Green Version]
- Giménez, A.L.; Giannini, N.P. Morphofunctional segregation in molossid bat species (Chiroptera: Molossidae) from the South American Southern Cone. Hystrix SCI J. 2016, 27, 170–180. [Google Scholar]
- Siemers, B.M.; Güttinger, R. Prey conspicuousness can explain apparent prey selectivity. Curr. Biol. 2006, 16, R157–R159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunz, T.H. (Ed.) Methods of assessing the availability of prey to insectivorous bats. In Ecological and Behavioral Methods for the Study of Bats, 2nd ed.; Smithsonian Institution Press: Washington, DC, USA, 1988; pp. 191–210. [Google Scholar]
- Ospina-Garcés, S.M.; De Luna, E.; Herrera, M.L.G.; Arroyo-Cabrales, J.; Flores-Martínez, J.J. Bite force, cranial morphometrics and size in the fishing bat Myotis vivesi (Chiroptera: Vespertilionidae). Rev. Biol. Trop. 2018, 66, 1614–1628. [Google Scholar]
- Schulz, M. Diet and Foraging Behavior of the Golden-Tipped Bat, Kerivoula papuensis: A Spider Specialist? J. Mammal. 2000, 81, 948–957. [Google Scholar] [CrossRef] [Green Version]
- Braun de Torrez, E.C.; Brown, V.A.; McCracken, G.F.; Kunz, T.H. Sympatric Bat Species Prey Opportunistically on a Major Moth Pest of Pecans. Sustainability 2019, 11, 6365. [Google Scholar] [CrossRef] [Green Version]
- Murphy, P.G.; Lugo, A.E. Ecology of tropical dry forest. Ann. Rev. Ecol. Syst. 1986, 17, 67–88. [Google Scholar] [CrossRef]
- Gast, F.; Escobar-Sarria, F.; Repizzo, A.; Alvarez, M.; Mendoza-Cifuentes, H.; Villarreal-Leal, H.F. El bosque seco tropical (Bs-T) en Colombia; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH): Bogotá, Colombia, 1997; p. 350. [Google Scholar]
- Forero-Medina, G.; Joppa, L. Representation of Global and National Conservation Priorities by Colombia’s Protected Area Network. PLoS ONE 2010, 5, e13210. [Google Scholar] [CrossRef]
- Portillo-Quintero, C.; Sánchez-Azofeifa, G.A. Extent and conservation of tropical dry forests in the Americas. Biol. Conserv. 2010, 143, 144–155. [Google Scholar] [CrossRef]
- Ávila-Gómez, E.S.; Moreno, C.E.; García-Morales, R.; Zuria, I.; Sánchez-Rojas, G.; Briones-Salas, M. Deforestation thresholds for phyllostomid bat populations in tropical landscapes in the Huasteca region, Mexico. Trop. Conserv. Sci. 2015, 8, 646–661. [Google Scholar] [CrossRef]
- Sikes, R.S.; Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef]
- Ariza, A.; Isaacs, P.; González, M.R. Mapa de Coberturas de Bosque seco Tropical en Colombia (escala 1:800.000,2.0v); Instituto de Investigaciones de Recursos Biológicos “Alexander von Humboldt”-Ministerio de Ambiente y Desarrollo: Bogotá, Columbia, 2014; p. 1. [Google Scholar]
- Lim, B.K.; Loureiro, L.O.; Upham, N.S.; Brocca, J.L. Phylogeography of Dominican Republic bats and implications for systematic relationships in the Neotropics. J. Mammal. 2017, 98, 986–993. [Google Scholar] [CrossRef]
- Kunz, T.H.; Anthony, E.L.P. Age estimation and postnatal growth in the bat Myotis lucifugus. J. Mammal. 1982, 63, 23–32. [Google Scholar] [CrossRef]
- Brunet-Rossinni, A.J.; Wilkinson, G.S. Methods for age estimation and the study of senescence in Bats. In Ecological and Behavioral Methods for the Study of Bats, 2nd ed.; Kunz, T.H., Parsons, S., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2009; p. 901. [Google Scholar]
- Racey, P.A. Ageing and assessment of reproductive status of Pipistrelle bats, Pipistrellus pipistrellus. J. Zool. 1974, 173, 264–271. [Google Scholar] [CrossRef]
- Racey, P.A. Reproductive Assessment of Bats. In Ecological and Behavioral Methods for the Study of Bats, 2nd ed.; Kunz, T.H., Parsons, S., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2009; p. 901. [Google Scholar]
- Freeman, P.W.; Lemen, C.A. Measuring bite force in small mammals with a Piezo-resistive sensor. J. Mammal. 2008, 89, 513–517. [Google Scholar] [CrossRef]
- Shiel, C.B.; Mcaney, C.M.; Fairley, J.S. Analysis of the diet of Natterer’s Bat Myotis Nattereri and the common long-eared bat Plecotus Auritus. In the West of Ireland. J. Zool. 1991, 223, 299–305. [Google Scholar] [CrossRef]
- Clare, E.L.; Fraser, E.E.; Braid, H.E.; Fenton, B.M.; Hebert, P.D.N. Species on the menu of a generalist predator, the eastern red bat (Lasiurus borealis): Using a molecular approach to detect arthropod prey. Molecular Ecology. 2009, 18, 2532–2542. [Google Scholar] [CrossRef]
- Whitaker, J.O., Jr.; Mccrackern, G.F.; Siemers, B.M. Food Habits Analysis of Insectivorous Bats. In Ecological and Behavioral Methods for the Study of Bats, 2nd ed.; Kunz, T.H., Parsons, S., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2009; p. 901. [Google Scholar]
- McCracken, G.F.; Westbrook, J.K.; Brown, V.A.; Eldridge, M.; Federico, P.; Kunz, T.H. Bats Track and Exploit Changes in Insect Pest Populations. PLoS ONE 2012, 7, e43839. [Google Scholar] [CrossRef] [Green Version]
- Maas, B.; Karp, D.S.; Bumrungsri, S.; Darras, K.; Gonthier, D.; Huang, J.C.C.; Lindell, C.A.; Maine, J.J.; Mestre, L.; Michel, N.L.; et al. Bird and bat predation services in tropical forests and agroforestry landscapes. Biol. Rev. 2015, 91, 1081–1101. [Google Scholar] [CrossRef] [Green Version]
- Kolkert, H.; Andrew, R.; Smith, R.; Rader, R.; Reid, N. Insectivorous bats selectively source moths and eat mostly pest insects on dryland and irrigated cotton farms. Ecol. Evol. 2020, 10, 371–388. [Google Scholar] [CrossRef] [Green Version]
- Instituto Adolfo Lutz. Normas Analíticas: Métodos Químicos e Físicos de Composição de Alimentos; IAL: São Paulo, Brazil, 2005; p. 60. [Google Scholar]
- Emerson, S.B.; Radinsky, A. Functional Analysis of Sabertooth Cranial Morphology. Paleobiology 1980, 6, 295–312. [Google Scholar] [CrossRef]
- Freeman, P.W. Functional cranial analysis of large animalivorous bats (Microchiroptera). Biol. J. Linn. Soc. Lond. 1984, 21, 387–408. [Google Scholar] [CrossRef] [Green Version]
- Dumont, E.R.; Herrel, A. The effects of gape angle and bite point on bite force in bats. J. Exp. Biol. 2003, 206, 2117–2123. [Google Scholar] [CrossRef] [Green Version]
- Kalko, E.K.V.; Handley, C.O., Jr.; Handley, D. Organization, diversity and long-term dynamics of a neotropical bat community. In Long-Term Studies of Vertebrate Communities; Cody, M.L., Smallwood, J.A., Eds.; Academic Press Inc. Book Department: San Diego, CA, USA, 1996; pp. 503–553. [Google Scholar]
- Marinello, M.M.; Bernard, E. Wing morphology of Neotropical bats: A quantitative and qualitative analysis with implications for habitat use. Can. J. Zool. 2014, 92, 141–147. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Palacio, F.X.; Apodaca, M.J.; Crisci, J.V. Análisis Multivariado para datos Biológicos: Teoría y su Aplicación Utilizando el Lenguaje R, 1st ed.; Fundación de História Natural Félix de Azara: Buenos Aires, Argentina, 2020; p. 268. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.5.3; R Foundation for Statistical Computing: Vienna, Austria; Available online: www.R-project.org (accessed on 5 May 2021).
- Dumont, E.R. Feeding mechanisms in bats: Variation within the constraints of flight. Integr. Comp. Biol. 2007, 47, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Dumont, E.R. The effect of food hardness on feeding behaviour in frugivorous bats (Phyllostomidae): An experimental study. J. Zool. 1999, 248, 219–229. [Google Scholar] [CrossRef]
- Dumont, E.R. Bats and fruit: An ecomorphological approach. In Bat Ecology; Kunz, T.H., Fenton, M.B., Eds.; The University of Chicago Press: Chicago, IL, USA, 2003; pp. 398–429. [Google Scholar]
- Dumont, E.R.; Davalos, L.M.; Goldberg, A.; Santana, S.E.; Rex, K.; Voigt, C.C. Morphological innovation, diversification and invasion of a new adaptive zone. Proc. R. Soc. B Biol. Sci. 2012, 279, 1797–1805. [Google Scholar] [CrossRef] [Green Version]
- McLellan, L.J. A morphometric analysis of three species of Carollia (Chiroptera: Phyllostomidae). Am. Mus. Novit. 1984, 48, 85–94. [Google Scholar]
- Myers, P. Sexual dimorphism in size of vespertilionid bats. Am. Nat. 1978, 112, 701–711. [Google Scholar] [CrossRef]
- Williams, D.F.; Findley, J.S. Sexual size dimorphism in Vespertilionidae bats. Am. Midl. Nat. 1979, 102, 113–126. [Google Scholar] [CrossRef]
- Bradbury, J.W.; Emmons, L.H. Social organization of some Trinidad bats. 1. Emballonuridae. Z. Tierpsychol. 1974, 36, 137–183. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, M.E.; Suttie, J.M. The sheath-tailed bat Coleura seychellensis (Chiroptera: Emballonuridae) in the Seychelles Islands. J. Zool. 1982, 197, 421–426. [Google Scholar] [CrossRef]
- Herrel, A.; Petrochic, S.; Draud, M. Sexual dimorphism, bite force and diet in the diamondback terrapin. J. Zool. 2017, 304, 217–224. [Google Scholar] [CrossRef]
- Herrel, A.; Van Damme, R.; Vanhooydonck, B.; De Vree, F. The implications of bite performance for diet in two species of lacertid lizards. Can. J. Zool. 2001, 79, 662–670. [Google Scholar] [CrossRef]
- Nogueira, R.M.; Monteiro, L.; Peracchi, A.L.; De Araújo, A.F.B. Ecomorphological analysis of the masticatory apparatus in the seed-eating bats, genus Chiroderma (Chiroptera, Phyllostomidae). J. Zool. 2005, 266, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Herrel, A.; Holanova, V. Cranial morphology and bite force in Chamaeleolis lizards-adaptations to molluscivory? Zoology 2008, 111, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Freeman, P.W.; Lemen, C.A. Simple predictors of bite force in bats: The good, the better, and the better still. J. Zool. 2010, 282, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Aranguren, C.I.; González-Carcacía, J.A.; Martínez, H.; Nassar, J.M. Noctilio albiventris (Noctilionidae), a potential seed disperser in disturbed tropical dry forest habitats. Acta Chiropt. 2011, 13, 189–194. [Google Scholar] [CrossRef]
- Ospina-Garcés, S.M.; De Luna, E.D.; Herrera, M.L.G.; Flores-Martínez, J.J. Cranial shape and diet variation in Myotis species (Chiroptera, Vespertilionidae), testing the relationship between form and function. Acta Chiropt. 2016, 8, 163–180. [Google Scholar] [CrossRef]
- Salinas-Ramos, V.B.; Ancillotto, L.; Bosso, L.; Sánchez-Cordero, V.; Russo, D. Interspecific competition in bats: State of knowledge and research challenges. Mammal. Rev. 2020, 50, 68–81. [Google Scholar] [CrossRef]
- Vleut, I.; Galindo-Gonzalez, J.; De Boer, W.F.; Levy-Tacher, S.I.; Vazquez, L.-B. Niche differentiation and its relationship with food abundance and vegetation complexity in four Frugivorous bat species in southern Mexico. Biotropica 2015, 47, 606–615. [Google Scholar] [CrossRef]
- Perry, J.M.G.; Hartstone-Rose, A.; Wall, C.E. The jaw adductors of Strepsirrhines in relation to body size, diet, and ingested food size. Anat. Rec. 2011, 294, 712–728. [Google Scholar] [CrossRef]
- Santana, S.E. Comparative anatomy of bat jaw musculature via diffusible iodine-based contrast-enhanced computed tomography. Anat. Rec. 2018, 301, 267–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loureiro, L.O.; Gregorin, R.; Perini, F.A. Diversity, morphological phylogeny, and distribution of bats of the genus Molossus E. Geoffroy, 1805 (Chiroptera, Molossidae) in Brazil. Zoosystema 2018, 40, 425–452. [Google Scholar] [CrossRef]
- Loureiro, L.O.; Engstrom, M.D.; Lim, B.K. Not all Molossus are created equal: Genetic variation in the mas- tiff bat reveals diversity masked by conservative morphology. Acta Chiropterologica 2019, 21, 51–64. [Google Scholar] [CrossRef]
- Herring, S.W.; Herring, S.E. The superficial masseter and gape in mammals. Am. Naturalist. 1974, 108, 561–576. [Google Scholar] [CrossRef]
- Reduker, D.W. Functional analysis of the masticatory apparatus in two species of Myotis. J. Mammal. 1983, 64, 277–286. [Google Scholar] [CrossRef]
- Freeman, P.W. Frugivorous and Animalivorous bats (Microchiroptera) dental and cranial adaptations. Biol. J. Linn. Soc. Lond. 1988, 33, 249–272. [Google Scholar] [CrossRef] [Green Version]
- Dumont, E.R.; Herrel, A.; Medellín, R.A.; Vargas-Contreras, J.A.; Santana, S.E. Built to bite: Cranial design and function in the wrinkle-faced bat. Proc. Zool. Soc. Lond. 2009, 279, 329–337. [Google Scholar] [CrossRef]
- Mancina, C.A.; Herrera, M.L.G. Disparate feeding strategies used by syntopic Antillean nectarivorous bats to obtain dietary protein. J. Mammal. 2010, 91, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Cisneros, L.M.; Fagan, M.E.; Willig, M.R. Effects of human-modified landscapes on taxonomic, functional and phylogenetic dimensions of bat biodiversity. Divers. Distrib. 2015, 21, 523–533. [Google Scholar] [CrossRef]
- Murillo-García, O.E.; De la Vega, M.E. Divergence, convergence and phenotypic diversity of neotropical frugivorous bats. Diversity 2018, 10, 100. [Google Scholar] [CrossRef] [Green Version]
- Díaz, M.M.; Solari, S.; Aguirre, L.F.; Aguiar, L.M.S.; Barquez, R.M. Clave de Identificación de los Murciélagos de Sudamérica; Publicación Especial No. 2; PCMA, Programa de Conservación de los Murciélagos de Argentina: Tucumán, Argentina, 2016; pp. 1–160. [Google Scholar]
- Velazco, P.M.; Gardner, A.L. A new species of Platyrrhinus (Chiroptera: Phyllostomidae) from western Colombia and Ecuador, with emended diagnoses of P. aquilus, P. dorsalis, and P. umbratus. Proc. Biol. Soc. Wash. 2009, 122, 249–281. [Google Scholar] [CrossRef]


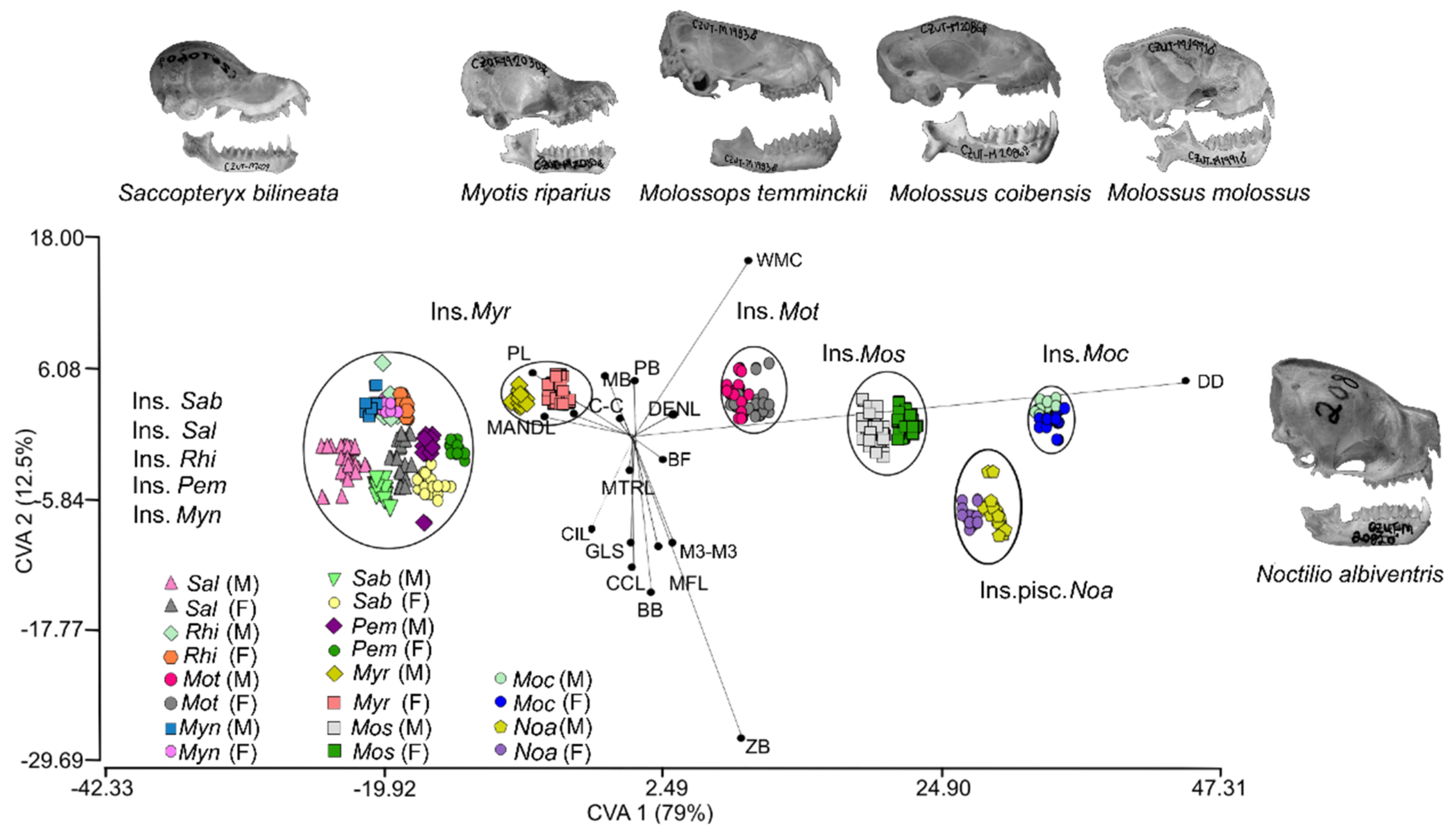


| Species | Sex | Pem | Sab | Sal | Noa | Mot | Moc | Mom | Myn | Myr | Rhi | Significant ANOVA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 30 | 30 | 23 | 24 | 22 | 24 | 30 | 30 | 30 | 21 | ||||||||||||
| GLS | F | 15.55 | ±0.28 | 15.13 | ±0.07 | 14.43 | ±0.30 | 19.67 | ±2.39 | 14.58 | ±0.21 | 15.46 | ±0.23 | 15.8 | ±0.41 | 13.48 | ±0.78 | 13.41 | ±0.81 | 12.53 | ±1.27 | F = 4.79 |
| M | 15.05 | ±0.21 | 15.90 | ±0.58 | 13.16 | ±0.63 | 19.56 | ±2.20 | 13.09 | ±0.66 | 15.3 | ±0.32 | 15.62 | ±0.41 | 13.46 | ±0.50 | 13.36 | ±0.54 | 11.33 | ±1.44 | p < 0.01 | |
| CIL | F | 13.58 | ±0.52 | 13.34 | ±0.38 | 12.82 | ±0.09 | 16.33 | ±2.05 | 12.05 | ±0.33 | 12.32 | ±0.18 | 13.44 | ±0.44 | 11.38 | ±0.71 | 11.89 | ±0.43 | 9.39 | ±1.83 | F = 12.45 |
| M | 13.37 | ±0.59 | 11.92 | ±0.17 | 11.63 | ±0.32 | 16.39 | ±2.16 | 0.73 | ±0.79 | 12.1 | ±0.07 | 13.9 | ±0.87 | 11.31 | ±0.49 | 11.63 | ±0.32 | 9.45 | ±1.46 | p < 0.02 | |
| CCL | F | 12.35 | ±0.28 | 13.06 | ±0.71 | 11.70 | ±0.11 | 15.28 | ±2.07 | 11.12 | ±0.47 | 12.02 | ±0.08 | 12.83 | ±0.58 | 10.55 | ±0.82 | 10.44 | ±0.88 | 9.5 | ±1.45 | F = 11.60 |
| M | 12.02 | ±0.39 | 12.08 | ±0.42 | 10.18 | ±0.62 | 15.25 | ±2.16 | 9.37 | ±1.07 | 11.8 | ±0.27 | 12.39 | ±0.59 | 10.58 | ±0.40 | 10.52 | ±0.44 | 8.95 | ±1.30 | p < 0.02 | |
| BB | F | 7.09 | ±0.02 | 6.07 | ±0.68 | 7.30 | ±0.12 | 10.55 | ±2.22 | 6.89 | ±0.15 | 7.89 | ±0.50 | 8.11 | ±0.65 | 6.23 | ±0.57 | 6.22 | ±0.58 | 4.79 | ±1.50 | F = 7.00 |
| M | 6.9 | ±0.01 | 6.01 | ±0.54 | 6.94 | ±0.03 | 10.58 | ±2.26 | 6.16 | ±0.45 | 7.91 | ±0.62 | 8.1 | ±0.74 | 5.72 | ±0.71 | 5.21 | ±1.03 | 5.35 | ±0.94 | p < 0.04 | |
| ZB | F | 9.26 | ±0.05 | 10.02 | ±0.31 | 8.88 | ±0.23 | 14.36 | ±2.33 | 8.92 | ±0.21 | 9.77 | ±0.19 | 10.28 | ±0.43 | 7.5 | ±0.87 | 8.43 | ±0.43 | 6.18 | ±1.48 | F = 9.27 |
| M | 8.86 | ±0.14 | 9.31 | ±0.05 | 8.31 | ±0.38 | 15.02 | ±2.54 | 7.81 | ±0.60 | 9.86 | ±0.29 | 10.12 | ±0.40 | 7.06 | ±0.93 | 8.38 | ±0.35 | 7.14 | ±0.89 | p < 0.03 | |
| MB | F | 7.35 | ±0.06 | 6.93 | ±0.34 | 7.09 | ±0.23 | 10.7 | ±2.10 | 7.2 | ±0.2 | 8.66 | ±0.78 | 8.76 | ±0.84 | 6.35 | ±0.71 | 6.16 | ±0.84 | 5.33 | ±1.37 | |
| M | 7.178 | ±0.08 | 6.33 | ±0.57 | 6.55 | ±0.44 | 11.08 | ±2.16 | 6.56 | ±0.43 | 8.84 | ±0.87 | 8.76 | ±0.83 | 6.8 | ±0.30 | 5.42 | ±1.09 | 5.65 | ±0.96 | ||
| PL | F | 6.39 | ±0.11 | 6.37 | ±0.13 | 6.40 | ±0.10 | 9.59 | ±2.61 | 6.93 | ±0.34 | 5.61 | ±0.78 | 6.14 | ±0.83 | 6.48 | ±0.04 | 6.04 | ±0.41 | 5.28 | ±1.06 | |
| M | 6.13 | ±0.21 | 6.39 | ±0.01 | 6.15 | ±0.19 | 9.59 | ±2.57 | 6.18 | ±0.17 | 5.52 | ±0.69 | 5.96 | ±0.34 | 5.19 | ±0.96 | 7.15 | ±0.61 | 5.60 | ±0.63 | ||
| PB | F | 3.00 | ±0.49 | 3.21 | ±0.26 | 2.45 | ±1.13 | 5.57 | ±2.48 | 3.62 | ±0.22 | 3.57 | ±0.16 | 3.41 | ±0.02 | 3.46 | ±0.03 | 3.51 | ±0.10 | 2.48 | ±1.10 | |
| M | 2.88 | ±0.56 | 2.93 | ±0.51 | 2.21 | ±1.29 | 5.65 | ±2.41 | 3.22 | ±0.21 | 3.9 | ±0.53 | 3.44 | ±0.03 | 3.75 | ±0.36 | 3.34 | ±0.08 | 2.77 | ±0.69 | ||
| MTRL | F | 5.95 | ±0.67 | 5.65 | ±0.30 | 5.72 | ±0.38 | 7.18 | ±2.22 | 5.14 | ±0.34 | 5.09 | ±0.41 | 5.32 | ±0.12 | 4.98 | ±0.54 | 4.94 | ±0.59 | 4.17 | ±1.57 | |
| M | 5.82 | ±0.77 | 5.81 | ±0.77 | 5.12 | ±0.10 | 7.11 | ±2.01 | 4.72 | ±0.29 | 5.22 | ±0.19 | 4.7 | ±0.31 | 4.12 | ±0.86 | 3.8 | −1.16 | 3.75 | ±1.21 | ||
| M3-M3 | F | 6.77 | ±0.29 | 6.29 | ±0.08 | 6.17 | ±0.18 | 9.35 | ±2.33 | 6.17 | ±0.18 | 6.83 | ±0.34 | 7.01 | ±0.48 | 5.24 | ±0.92 | 5.2 | ±0.95 | 4.96 | ±1.14 | |
| M | 6.66 | ±0.40 | 6.46 | ±0.27 | 5.85 | ±0.14 | 9.34 | ±2.20 | 5.46 | ±0.41 | 7.06 | ±0.67 | 6.21 | ±0.10 | 4.89 | ±0.79 | 4.48 | −1.07 | 4.23 | ±1.23 | ||
| C-C | F | 3.88 | ±0.09 | 3.22 | ±0.48 | 2.30 | ±1.30 | 6.61 | ±2.49 | 3.42 | ±0.31 | 4.04 | ±0.23 | 4.3 | ±0.46 | 3.13 | ±0.57 | 3.57 | ±0.18 | 3.30 | ±0.41 | |
| M | 3.68 | ±0.05 | 3.2 | ±0.38 | 2.62 | ±0.90 | 6.59 | ±2.67 | 3.06 | ±0.50 | 3.88 | ±0.23 | 3.6 | ±0.02 | 3.25 | ±0.33 | 3.48 | ±0.12 | 2.84 | ±0.70 | ||
| Biometric features (mm) of the jaw | ||||||||||||||||||||||
| DENL | F | 10.47 | ±0.06 | 11.24 | ±0.58 | 10.07 | ±0.21 | 13.67 | ±2.21 | 10.19 | ±0.13 | 10.01 | ±0.25 | 11.33 | ±0.64 | 9.69 | ±0.47 | 8.89 | ±1.00 | 8.28 | ±1.42 | F = 10.06 |
| M | 10.13 | ±0.22 | 11.07 | ±0.76 | 9.27 | ±0.27 | 13.2 | ±1.98 | 9.01 | ±0.43 | 10.38 | ±0.37 | 10.31 | ±0.33 | 9.42 | ±0.19 | 7.61 | ±1.22 | 7.02 | ±1.55 | p < 0.03 | |
| DD | F | 1.37 | ±0.67 | 1.24 | ±0.81 | 1.17 | ±0.89 | 3.12 | ±1.22 | 2.46 | ±0.51 | 3.48 | ±1.61 | 2.96 | ±1.05 | 1.15 | ±0.91 | 1.79 | ±0.22 | 1.18 | ±0.88 | |
| M | 1.23 | ±0.68 | 1.07 | ±0.86 | 0.97 | ±0.95 | 3.22 | ±1.36 | 2.39 | ±0.51 | 3.45 | ±1.59 | 2.81 | ±0.94 | 1.09 | ±0.83 | 1.62 | ±0.28 | 1.12 | ±0.80 | ||
| MFL | F | 4.11 | ±0.02 | 2.38 | ±0.78 | 2.26 | ±0.84 | 7.1 | ±1.40 | 6.28 | ±1.02 | 5.39 | ±0.61 | 6.87 | ±1.29 | 2.45 | ±0.75 | 2.62 | ±0.67 | 1.31 | ±1.28 | |
| M | 4.07 | ±0.04 | 2.22 | ±0.81 | 2.16 | ±0.84 | 7.06 | ±1.42 | 6.15 | ±1.00 | 5.27 | ±0.60 | 6.75 | ±1.28 | 2.33 | ±0.76 | 2.53 | ±0.67 | 1.27 | ±1.25 | ||
| MANDL | F | 6.2 | ±0.06 | 6.24 | ±0.09 | 4.16 | ±1.84 | 8.40 | ±2.10 | 6.04 | ±0.09 | 6.42 | ±0.26 | 6.58 | ±0.41 | 6.37 | ±0.22 | 5.83 | ±0.29 | 5.15 | ±0.91 | |
| M | 6.16 | ±0.28 | 6.15 | ±0.27 | 6.01 | ±0.13 | 7.88 | ±1.96 | 5.28 | ±0.57 | 6.28 | ±0.39 | 5.69 | ±0.18 | 6.47 | ±0.58 | 4.56 | ±1.28 | 4.25 | ±1.58 | ||
| WMC | F | 3.15 | ±0.65 | 2.76 | ±0.95 | 1.89 | ±1.65 | 6.01 | ±1.62 | 5.01 | ±0.82 | 5.06 | ±0.87 | 4.93 | ±0.77 | 3.57 | ±0.31 | 3.85 | ±0.09 | 3.44 | ±0.42 | |
| M | 2.89 | ±0.87 | 2.88 | ±0.88 | 2.53 | ±1.20 | 5.77 | ±1.80 | 4.56 | ±0.68 | 5.15 | ±1.23 | 4.3 | ±0.43 | 3.57 | ±0.24 | 3.01 | ±0.76 | 3.63 | ±0.18 | ||
| BF | F | 0.33 | ±0.31 | 0.25 | ±1.00 | 0.31 | ±0.54 | 0.60 | ±1.79 | 0.32 | ±0.42 | 0.40 | ±0.17 | 0.58 | ±1.65 | 0.23 | ±1.12 | 0.32 | ±0.42 | 0.40 | ±0.21 | F = 7.39 |
| M | 0.30 | ±0.64 | 0.24 | ±1.00 | 0.26 | ±0.94 | 0.64 | ±1.86 | 0.36 | ±0.19 | 0.32 | ±0.52 | 0.58 | ±1.44 | 0.46 | ±0.56 | 0.31 | ±0.57 | 0.40 | ±0.10 | p < 0.01 | |
| Variables | Estimate | Std. Error | t Value | Pr(>|t|) |
|---|---|---|---|---|
| GLS | 14.589 | 0.205 | 11.138 | <2 × 10−16 |
| CIL | −2.346 | 0.235 | −9.992 | <2 × 10−16 |
| CCL | −3.195 | 0.235 | −13.610 | <2 × 10−16 |
| BB | −7.794 | 0.235 | −33.199 | <2 × 10−16 |
| ZB | −5.521 | 0.235 | −23.519 | <2 × 10−16 |
| MB | −7.410 | 0.235 | −31.564 | <2 × 10−16 |
| PL | −8.340 | 0.235 | −35.527 | <2 × 10−16 |
| PB | −11.376 | 0.235 | −48.460 | <2 × 10−16 |
| MTRL | −9.579 | 0.235 | −40.805 | <2 × 10−16 |
| M3-M3 | −8.563 | 0.235 | −36.477 | <2 × 10−16 |
| C-C | −11.096 | 0.235 | −47.268 | <2 × 10−16 |
| DENL | −4.732 | 0.235 | −20.158 | <2 × 10−16 |
| DD | −14.414 | 0.235 | −61.403 | <2 × 10−16 |
| MFL | 0.516 | 0.170 | 76.030 | 0.003 |
| MANDL | −8.789 | 0.235 | −37.438 | <2 × 10−16 |
| WMC | −10.897 | 0.235 | −46.418 | <2 × 10−16 |
| Species | p Value | Species | p Value | Traits | p Value |
|---|---|---|---|---|---|
| M. temminckii-M. coibensis | 0.0156 | M. molossus-S. leptura | <0.0001 | BF-C-C | <0.001 |
| M. temminckii-M. molossus | 0.0006 | M. nigricans-M. riparius | 1.0000 | BF-CCL | <0.001 |
| M. temminckii-M. nigricans | 0.0206 | M. nigricans-R. io | 0.0015 | BF-CIL | <0.001 |
| M. temminckii-M. riparius | 0.0093 | M. nigricans-N. albiventris | <0.0001 | BF-DD | <0.001 |
| M. temminckii-R. io | <0.0001 | M. nigricans-P. macrotis | <0.0001 | BF-DENL | <0.001 |
| M. temminckii-N. albiventris | <0.0001 | M. nigricans-S. bilineata | 0.0076 | BF-GLS | <0.001 |
| M. temminckii-P. macrotis | 0.7338 | M. nigricans-S. leptura | 0.9918 | BF-M3-M3 | <0.001 |
| M. temminckii-S. bilineata | 1.0000 | M. riparius-R. io | 0.0038 | BF-MANDL | <0.001 |
| M. temminckii-S. leptura | 0.2936 | M. riparius-N. albiventris | <0.0001 | BF-MB | <0.001 |
| M. coibensis-M. molossus | 0.9981 | M. riparius-P. macrotis | <0.0001 | BF-MFL | <0.001 |
| M. coibensis-M. nigricans | <0.0001 | M. riparius-S. bilineata | 0.0032 | BF-MTRL | <0.001 |
| M. coibensis-M. riparius | <0.0001 | M. riparius-S. leptura | 0.9657 | BF-PB | <0.001 |
| M. coibensis-R. io | <0.0001 | R. io-N. albiventris | <0.0001 | BF-PL | <0.001 |
| M. coibensis-N. albiventris | <0.0001 | R. io-P. macrotis | <0.0001 | BF-WMC | <0.001 |
| M. coibensis-P. macrotis | 0.7682 | R. io-S. bilineata | <0.0001 | BF-ZB | <0.001 |
| M. coibensis-S. bilineata | 0.0397 | R. io-S. leptura | <0.0001 | BF-FA | 0.425 |
| M. coibensis-S. leptura | <0.0001 | N. albiventris-P. macrotis | <0.0001 | ||
| M. molossus-M. nigricans | <0.0001 | N. albiventris-S. bilineata | <0.0001 | ||
| M. molossus-M. riparius | <0.0001 | N. albiventris-S. leptura | <0.0001 | ||
| M. molossus-R. io | <0.0001 | P. macrotis-S. bilineata | 0.8864 | ||
| M. molossus-N. albiventris | <0.0001 | P. macrotis-S. leptura | 0.0010 | ||
| M. molossus-P. macrotis | 0.2335 | S. bilineata-S. leptura | 0.1583 | ||
| M. molossus-S. bilineata | 0.0021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Fráncel, L.A.; García-Herrera, L.V.; Losada-Prado, S.; Reinoso-Flórez, G.; Lim, B.K.; Sánchez, F.; Sánchez-Hernández, A.; Guevara, G. Skull Morphology, Bite Force, and Diet in Insectivorous Bats from Tropical Dry Forests in Colombia. Biology 2021, 10, 1012. https://doi.org/10.3390/biology10101012
Ramírez-Fráncel LA, García-Herrera LV, Losada-Prado S, Reinoso-Flórez G, Lim BK, Sánchez F, Sánchez-Hernández A, Guevara G. Skull Morphology, Bite Force, and Diet in Insectivorous Bats from Tropical Dry Forests in Colombia. Biology. 2021; 10(10):1012. https://doi.org/10.3390/biology10101012
Chicago/Turabian StyleRamírez-Fráncel, Leidy Azucena, Leidy Viviana García-Herrera, Sergio Losada-Prado, Gladys Reinoso-Flórez, Burton K. Lim, Francisco Sánchez, Alfonso Sánchez-Hernández, and Giovany Guevara. 2021. "Skull Morphology, Bite Force, and Diet in Insectivorous Bats from Tropical Dry Forests in Colombia" Biology 10, no. 10: 1012. https://doi.org/10.3390/biology10101012
APA StyleRamírez-Fráncel, L. A., García-Herrera, L. V., Losada-Prado, S., Reinoso-Flórez, G., Lim, B. K., Sánchez, F., Sánchez-Hernández, A., & Guevara, G. (2021). Skull Morphology, Bite Force, and Diet in Insectivorous Bats from Tropical Dry Forests in Colombia. Biology, 10(10), 1012. https://doi.org/10.3390/biology10101012








